Abstract
Persistent organic pollutants(POPs) are suggested to be potential risk factors for gestational diabetes mellitus(GDM). We examined the hypothesis that the aryl hydrocarbon receptor trans-activating(AhRT) activity, a potential biomarker for the presence of POPs, could be a GDM risk factor in pregnant women. A total of 390 GDM and 100 normal pregnant(non-GDM) subjects in the Korea National Diabetes Program cohort voluntarily participated. We measured AhRT activity and concentrations of ATP and reactive oxygen in the serum collected at the screening of the participants for GDM using recombinant Hepa1c1c7 cells. Odds ratios(ORs) and 95% confidence intervals(CIs) were estimated using multivariable logistic regression models. The sensitivity and specificity of AhRT activity for GDM diagnostics were measured by receiver operating characteristic(ROC) analysis. Body mass index at pre-pregnancy and delivery and systolic blood pressure were significantly higher in the GDM group. AhRT activity was higher, and ATP concentrations were lower in the GDM group than the non-GDM group(P < 0.0001). AhRT activity was significantly higher in the GDM group(OR 29.3, 95% CI 10.9–79.1) compared with non-GDM(P < 0.0001). Serum glucose concentration at 1 h after a 50 g glucose challenge(glucose-50) was moderately correlated with AhRT activity(r2 = 0.387) and negatively correlated with ATP production(r2 = −0.650). In the ROC curve, AhRT activity had 70.9% sensitivity and 90.0% specificity for glucose-50, a GDM screening method. In conclusion, this study suggests that serum AhRT activity is positively associated with the risk of GDM.
Similar content being viewed by others
Introduction
Gestational diabetes mellitus (GDM) typically develops at the end of the 2nd trimester (24–28 weeks) of pregnancy when insulin secretion cannot compensate for increased insulin resistance. GDM is characterized by the disturbance of glucose metabolism1,2. The global prevalence of GDM varies between 2% and more than 18%. There is an ethnic difference: Asians have higher GDM than Caucasians3,4. In GDM, the pregnant mother experiences a sudden increase in insulin resistance induced by pregnancy, and it also predicts an elevated risk of developing type 2 diabetes later in life2.
Risk factors for GDM include advanced maternal age, high body mass index (BMI) at pre-pregnancy, greater weight gain during pregnancy than the recommendation, and family history of diabetes5. The global increase in GDM prevalence may also be associated with unknown environmental factors. One potential factor is the increased exposure to endocrine-disrupting chemicals (EDCs). Accumulating evidence has demonstrated that exposure to EDCs is linked to impaired glucose metabolism and type 2 diabetes as shown in cross-sectional and prospective studies in humans and experimental in vivo and in vitro studies6. Among EDCs, the exposure to dichlorodiphenyldichloroethylene, a metabolite of the pesticide dichlorodiphenyltrichloroethane, exhibits a moderate relationship with diabetes development in human studies6. Exposure of polychlorinated biphenyls (PCBs), bisphenol A, phthalates, perfluorinated and poly-fluoroalkyl substances (PFAS), polybrominated diphenyl ethers (PBDE) have little research to support their associations with diabetes risk6. Some EDCs are considered persistent organic pollutants (POPs)7. POPs are lipophilic chemicals with long half-lives due to resistance to environmental degradation processes. POPs that are incorporated into the food chain accumulate in the adipocytes of humans over time8. Mitochondrial dysfunction due to POPs subsequently contributes to increased insulin resistance9. Therefore, POPs can be potential risk factors for obesity, type 2 diabetes, and GDM7,8. However, an optimal index for exposure to POPs has not been established. Most studies have used plasma concentrations of 76 POPs as an index, which is determined by standard procedures using gas- and liquid chromatography (LC) with mass spectrophotometry10. Limited numbers of POPs have been identified due to the limited number of facilities available to measure them, the large volume of blood needed, and the high cost. Most POPs bind to aryl hydrocarbon receptors with high affinity and they enter into the cells and bind to dioxin-response elements of their promotors that contribute to transcriptional activation of multiple genes, including cytochrome p450 1A1 (CYP1A1)11. POPs act as potent activators of aryl hydrocarbon receptor transactivating (AhRT) and AhRT bioactivity can be an index of the POPs levels, including dioxin and dioxin-like substances9. However, AhRT is also activated by endogenous indole derivatives such as kynurenine produced by activation of the tryptophan–kynurenine pathway12. Under diabetic conditions, the tryptophan-kynurenine pathway stimulates the increase of endogenous indole derivatives12,13. A highly sensitive cell-based AhR dependent luciferase activity assay has been developed to determine AhRT activity. Serum dioxin levels measured by AhRT activity exhibit a strong correlation with its levels measured by Gas/Liquid chromatography-mass spectrophotometry9. A positive correlation between dioxin and AhRT activity has been shown in the Swedish elderly population, and AhRT activity had a negative association with ATP production in the mitochondria14. Patients with diabetic nephropathy also have increased serum AhRT activity15. Thus, the impact of endogenous indole derivatives may be minimal due to working as weak agonists.
A recent study has demonstrated that serum concentrations of POPs, including mainly chlorinated PCBs and some PFAS and PBDE, have positive associations with GDM risk in US non-obese women in the multi-center prospective cohort study10 and pregnant Greek women11. Here, we examined the hypothesis that AhRT bioactivity in the serum is positively associated with GDM risk in Korean pregnant women at 24–28 weeks of pregnancy. AhRT activity and ATP production in the serum can be biomarkers for the POPs exposure.
Results
Characteristics of GDM and non-GDM participants
At pre-pregnancy, age was not significantly different between the GDM and non-GDM groups. However, height was higher in the non-GDM group than the GDM group (P < 0.001), whereas body weight and BMI were much higher in the GDM group (58.6 ± 10.1 and 23.0 ± 3.8) than the non-GDM group (55.0 ± 5.7 and 20.7 ± 1.9, P < 0.001; Table 1).
At weeks 24–28 of pregnancy screening for GDM, serum glucose concentrations were higher in the GDM group (166.6 ± 23.2 mg/dl) than the non-GDM group (112.8 ± 15.8 mg/dl) at 1 h after 50 g glucose challenge (P < 0.001; Table 1). Systolic blood pressure (SBP) was higher in the GDM group than the non-GDM group (P < 0.001), whereas DBP was not significantly different between the GDM and non-GDM groups. After GDM diagnosis, serum glucose concentrations were maintained in a normal range in pregnant women with GDM by medication and diet therapy. BMI at delivery was higher in the GDM group than the non-GDM group (P = 0.002), although body weight gain was much lower in the GDM group (9.6 kg) than the non-GDM group (14.5 kg; Table 1). Body-weight increased within the recommendations in both GDM and non-GDM groups. The pregnancy duration was shorter in the GDM group than the non-GDM group (Table 1). The birth weights of the infants were not significantly different between the two groups. Apgar at 1 and 5 min were not significantly different between the two groups (Table 1). The results indicated that pregnancy outcomes were not significantly different.
AhRT activity and ATP contents in non-GDM and GDM women
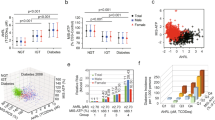
AhRT activity was significantly higher in GDM than non-GDM women (P < 0.0001; Fig. 1A), and ATP concentrations were significantly lower in GDM than the non-GDM women (P < 0.0001; Fig. 1B).
Aryl hydrocarbon receptor transactivating (AhRT) bioactivity and ATP contents of the serum in the non-GDM and GDM groups. (A) AhRT bioactivity. AhRT activity was measured by the modified CALA assay using the serum from 390 GDM and 100 non-GDM participants. AhRT activity was presented as fold induction (FI) over the AhRT of the 10% CS-HS-treated control cells. (B) ATP content. ATP concentration of 10% sample serum-treated cells from the participants was measured by a CellTiter-Glo luciferase kit. It was calculated as % of ATP content in control cells treated with 10% CS-HS-treated control cells. ***Significantly different from the GDM group at P < 0.0001.
Figure 2A shows that non-GDM women had ≤ 2.0 AhRT activity, but about 35% of GDM women also had ≤ 2.0 AhRT activity, although GDM women had higher serum glucose concentrations. However, about 65% of GDM women had much higher AhRT activity (Fig. 2A). Interestingly, ATP contents exhibited a clear separation by GDM: most women with non-GDM included ≥ 80% of the control, but GDM women comprised ≤ 75% of the control group (non-GDM women) in ATP contents (Fig. 2A). In clustering analysis of AhRT activity and ATP contents, GDM and non-GDM women showed a clear separation (Fig. 2A). These results indicated that mitochondrial dysfunction with increased AhRT contents might be involved in GDM risk.
The correlation of serum glucose concentration at 1 h after 50 g glucose challenge, aryl hydrocarbon receptor transactivating (AhRT) bioactivity, and ATP concentration. (A) Relationship between AhRT bioactivity (FI) and ATP contents (%). (B) The proportion of GDM (%), according to the quartiles of AhRT activity (FI) and ATP concentration (%). GDM proportion was calculated by the number of women with GDM divided by the total number of women in each quartile. AhRT bioactivity was measured by the modified CALA assay using the serum from 390 GDM and 100 non-GDM participants. AhRT activity was presented as fold induction (FI) over the AhRT of the 10% CS-HS-treated control cells. ATP concentration of 10% sample serum-treated cells was measured by a CellTiter-Glo luciferase kit. It was calculated as % of ATP content in control cells treated with 10% CS-HS-treated control cells.
When the risk of GDM was analyzed according to the quartiles of AhRT activity or ATP concentrations, most subjects in the upper fourth quartile (Q4) of AhRL (≥ 2.85 FI) and the lower first quartile (Q1) of ATP contents had GDM (Fig. 2B). In contrast, few subjects in the lowest quartile (Q1) of AhRT (< 1.85 FI) and the highest quartile (Q4) of ATP contents (67.1%) had GDM. These results indicate that AhRT activity and ATP contents are the independent parameters for GDM development.
Correlation of AhRT activity, and ATP and reactive oxygen species (ROS) concentrations with GDM-related clinical parameters
AhRT activity was positively correlated with serum glucose concentrations at 1 h after a 50 g challenge and HbA1c (r2 = 0.387 and 0.219, respectively; Table 2). AhRT activity had a strong negative association with ATP contents and a positive association with ROS concentrations (r2 = − 0.400) in GDM women (Table 2). AhRT activity had a weak positive correlation with age, BMI at pre-pregnancy and delivery, SBP, and DBP (Table 2). In contrast to AhRT activity, ATP contents were strongly and negatively correlated with plasma glucose at 1 h after 50 g glucose challenge and weakly negatively associated with SBP and DBP (Table 2). AhRT activity had a positive association with HOMA-IR, an index of insulin resistance, but not with HOMA-B, an indicator of insulin secretion (Table 2). HbA1c contents also exhibited a positive association with AhRT activity and ROS concentrations. ROS concentrations had a weak positive correlation with age and baby Apgar score at 5 min.
Association of age, BMI, and AhRT activity with GDM risk
Age was not associated with the risk of GDM in logistic regression analysis (Table 3). BMI at pre-pregnancy and delivery was highly associated with GDM risk by 1.316 and 3.615-fold (P < 0.0001). Interestingly, AhRT activity was strongly associated with GDM risk by 29.3-fold (95% CI 10.88–79.05, P < 0.0001; Table 3). Therefore, AhRT bioactivity could be used as a diagnostic index and potential risk factor. However, serum glucose concentration at 1 h after 50 g glucose challenge was a better index than AhRT (the difference of their estimate = 0.1468, 90% CI 0.1119–0.1817, P < 0.0001; Table 3).
Sensitivity and specificity of AhRT bioactivity to GDM risk in receiver operating characteristic (ROC) plot
The ROC curve showed that AhRT had 70.9% sensitivity and 90.0% specificity with a 1.979 cutoff value of AhRT for GDM diagnosis (Fig. 3). Area of AhRT and serum glucose concentrations and serum glucose concentrations at 1 h after 50 g glucose challenge was 0.8527 (95% CI 0.8173–0.8873) and 0.9993 (0.9976––1.0000), respectively (Fig. 3). The 95% CI for both parameters did not include the reference value of 0.5. These results indicated that AhRT activity is representative of GDM risk and can be used to assess for GDM risk.
Receiver operating characteristic (ROC) curve for AhRT activity in 390 GDM and 100 non-GDM participants. The ROC curve determined the sensitivity and specificity of AhRT activity for GDM risk. The top and bottom lines in the ROC curves corresponded to GDM predictive tests. The top line represented serum glucose concentration from a 50 g glucose challenge for GDM prediction, and its area under the curve (AUC) was 0.9993. The bottom line indicated AhRT bioactivity to GDM prediction, and its AUC was 0.8527. These results indicated the AhRT bioactivity could be considered as a GDM risk factor. ROC2 was the reference.
Discussion
POPs are reported to be a risk factor for type 2 diabetes and diabetic nephropathy7. AhRT bioactivity is known to be negatively associated with pancreatic β-cell function, and it is a biomarker of exposure to POPs16. AhRT activity can also be a risk factor for GDM16. It is difficult to directly measure POPs in the blood since POPs contain various components. AhRT bioactivity can be an index of POP levels, including dioxin and dioxin-like substances17. In this study, we investigated whether POPs could be risk factors for GDM in Korea by measuring AhRT activity as a potential biomarker of POPs in samples from pregnant women. To the best of our knowledge, the present study is novel research and the first to demonstrate the association of AhRT bioactivity with GDM risk in pregnant women.
GDM develops during the 3rd trimester of pregnancy when elevated insulin resistance in the mother cannot be overcome by compensatory insulin secretion from pancreatic β-cells1. Although the cause of insulin resistance in GDM is different from type 2 diabetes, the etiologies of GDM and type 2 diabetes are similar1. The factors linked to increased insulin resistance in type 2 diabetes are also the primary risk factors for GDM, including weight gain. However, the high incidence of GDM in Asians is related to low insulin secretion capacity, and the inability to compensate for insulin resistance1,18. GDM consequences include short- and long-term maternal and child health problems. GDM increases the risk of preterm birth, preeclampsia, stillbirth, and macrosomia during pregnancy. Women with GDM have an increased risk of subsequent obesity, type 2 diabetes, and cardiovascular diseases, and their children are at risk of childhood obesity2,19. It is important to avoid the modifiable risk factors for GDM, including pre-pregnancy obesity and excessive weight gain before and during pregnancy2. Advanced maternal age is a major unmodifiable risk factor for GDM. However, it is better to investigate modifiable risk factors to prevent GDM.
Exposure to POPs is one of the modifiable risk factors for GDM. Accumulating evidence from experimental studies suggests that pancreatic β-cells can be considered a sensitive target of dioxin toxicity, which reduces β-cell function, thereby increasing the risk of type 2 diabetes and GDM20. Environmental pollution has been markedly increasing in Asian countries over the last 3 decades, and Asians already have lower insulin secretion capacity. These two factors may influence the increase in type 2 diabetes and GDM risk. Exposure to EDCs is one of the environmental and modifiable, but unavoidable risk factors21. The present study showed that AhRT activity was much higher in the GDM group than in the non-GDM group. The sensitivity and specificity of AhRT activity for GDM were 0.8523 and 0.9991, respectively, indicating that AhRT activity could be an independent risk factor for GDM. Therefore, decreasing the accumulation of POPs may be a useful strategy for reducing GDM risk.
The blood is the only sample often used for measuring exposure to POPs. However, serum POPs concentrations may not be a well-representative index of POPs exposure since POPs accumulate in the fat tissues21. Body fat contents may also affect the serum concentrations of POPs. As POPs are composed of various chemicals, it is difficult to measure all POPs in the blood due to the many different types of chemicals comprising POPs, the limited number of available standard compounds for gas- and liquid chromatography/mass spectroscopy, the sensitivity of the equipment, and required amounts of samples. Therefore, another method is necessary for measuring POP exposure. Serum AhRT bioactivity can be an index of serum POP concentrations, including dioxin and dioxin-like substances. Most POPs bind to AHR with a high affinity to activate dioxin-response elements of the promoters, contributing to transcriptional activation of various genes that influence the risk of metabolic diseases, including type 2 diabetes.
EDCs contain some POPs (e.g., PFAS) and non-POPs (i.e., phenols, phthalates, and parabens), and they are reported to be associated with dysregulated glucose metabolism in GDM and type 2 diabetes22,23. Many epidemiological studies have demonstrated an association between POP exposure, metabolic syndrome, and type 2 diabetes7,24. However, the effects of POPs on GDM need more studies to be conclusive25. POPs are lipophilic and hydrophobic pollutants that are stored in fatty tissues. Since POPs are resistant to metabolic degradation, they tend to accumulate in humans26. Previous studies have demonstrated that POPs, including PCBs, are involved in the development of diabetes mellitus (DM) type 2 and insulin resistance27,28. The present study revealed that AhRT bioactivity in the serum is a reliable indicator of POP exposure, and it is correlated with GDM and insulin resistance, but not insulin secretion. EDCs, including POPs, are reported to increase AhRT bioactivity and ROS production and induce mitochondrial dysfunction, which exacerbates metabolic syndrome29. The present study suggests that the activation of serum AhRT bioactivity, as with POP accumulation, contributes to GDM risk by reducing ATP production and increasing ROS production in the mitochondria that are subsequently related to increased insulin resistance.
This study had some limitations. First of all, POPs are not the only AhRT agonists; the endogenous indole derivatives, including tryptophan metabolites, from microbiota production and dietary components, contribute to influencing AhRT activity30. The interaction of endogenous indole derivatives within the ligand-binding domain of AhRT appears to differ from that observed with high-affinity ligands such as dioxin. Therefore, the serum concentrations of endogenous indole derivatives may contribute to a basal level in the participants, although circulating amino acid concentrations are elevated31, and the tryptophan-kynurenine pathway is activated in diabetic patients, including GDM12,13. Since the endogenous indole derivatives have a much lower affinity to AhRT than POPs30, the AhRT activation measured in the present study is more likely to be associated with POP exposure. Second, GDM development is involved in socioeconomic factors and lifestyle factors, including nutrient intake and physical activity32,33. These factors should have used as adjusting covariates, but they were not measured in the present study. Age, BMI, and serum glucose concentrations at 50 g glucose challenge were used as covariates in the present study.
Conclusion
This study suggested that serum AhRT activity has a positive association with GDM risk in Korean women and it is a reliable potential biomarker for exposure to POPs. Exposure to POPs might be a potential risk factor for the development of GDM in pregnant women in Korea and possibly other countries. Further research is needed to evaluate the role of AhRT activation in GDM development.
Methods
Participants
The Korea National Diabetes Program (KNDP) cohort consists of Koreans who have or who at high risk of developing type 2 diabetes who have enrolled at 13 hospitals nationwide since 2006 and were regularly followed up during the 9 years. Among the participants in the KNDP GDM cohort, pregnant women were diagnosed by a universal two-step GDM screening program at 24–28 weeks of gestation. The first step was a 50-g glucose challenge test, and women with a positive result underwent a 3-h 100-g oral glucose tolerance test (OGTT). This study was conducted with the 390 women with GDM among the KNDP cohort whose clinical data and blood samples at 24–28 weeks of gestation were available for AhRT assay and data analysis. Among the pregnant women who were tested for GDM screening, 100 non-GDM women volunteered to participate in the control group. All procedures of the KNDP GDM cohort study were conducted following the guidelines and regulations of the Declaration of Helsinki revised in 2004. This study was approved by the Institutional Review Board (IRB) of Kyung Hee University Hospital (IRB No. KHUH 2015–04-201–016). Informed consent was obtained from all participating subjects.
Laboratory measurements and physiological indexes
Plasma glucose concentrations were measured by the glucose oxidase method using a YSI 2300 STAT (YSI; Yellow Springs, Ohio). Plasma insulin concentrations were measured using a human-specific radioimmunoassay kit (Linco Research, St. Charles, MO). Homeostatic model assessments for insulin resistance (HOMA-IR) and β-cell function (HOMA-B) were calculated using the equations as previously reported34. HbA1c levels were determined at the initial diagnosis of GDM and before delivery by the Variant II HbA1c program (BioRad; Hercules, CA). Gestational hypertension was diagnosed if blood pressure was ≥ 140 for systolic (SBP) and 90 mmHg for diastolic (DBP).
Pregnancy outcomes
Preterm delivery was defined as delivery before 37 weeks of gestation, whereas macrosomia was considered a birth weight of 4000 g or greater1,33. Large for gestational age was defined as a birth weight ≥ 90th percentile based on a Korean reference population. The Apgar score was defined as low if a score of < 7 was recorded 5 min after delivery.
AhRT bioactivity assay
The modified cell-based AhR ligand activity assay was conducted to quantify the dioxin response element (DRE)-dependent luciferase activity (modified CALA assay)9,35,36. Hepa1c1c7, Mouse Hepatoma cells, were cultured in α-minimum essential medium supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin at 37 °C in a 5% CO2 atmosphere. Cells seeded at 1 × 105/well in a 6-well plate. The cells were co-transfected with the pGL4-DRE-Luc (puromycin +) and pRL-mTK Renilla reporter plasmids using Superfect (Qiagen, Valencia, CA, USA). Puromycin (1 μg/ml)-resistant stable colonies were selected after a 3-week incubation. The clones, containing 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD; AhR agonist)-dose-dependent activity, were selected15. The stable cells with a 70% confluent state were treated with heat-inactivated human serum samples or a charcoal-stripped human serum (control serum) in Dulbecco’s modified Eagle medium for 24 h. Luciferase activities were measured using a Dual-Glo Luciferase assay system (Promega, Madison, WI, USA) in a luminometer (Berthold, Bad Wildbad, Germany). The luciferase activity was subsequently normalized against Renilla luciferase activity. AhRT bioactivities of sample serum-treated cells were reported as fold induction over those of the control serum-treated control cells. Standard curves were prepared using luciferase activities (AhRT bioactivity) of the cells exposed to serially diluted TCDD (0–50 pM) for 24 h in the presence of a control serum. When the AhRT bioactivity in a serum sample was converted to TCDD equivalents (pM) using the standard curve (0–10 pM TCDD), a 0.1-fold induction of AhRT bioactivity was equivalent to 0.37 pM TCDD equivalent.
ATP content assay in the serum of pregnant women
The mitochondrial inhibition induced by serum samples was evaluated by measuring intracellular ATP contents9,37. Hepa1c1c7 cells were co-transfected with the pRL-mTK Renilla and pcDNA3.1 (neomycin +) plasmids using Superfect (Qiagen, Valencia, CA, USA). G418-resistant stable colonies were selected for 3 weeks, and then the clones showing stable Renilla luciferase activity were selected. Stable clones (5 × 104/well) were treated with serum samples of pregnant women (10 ul) or control serum in 96-well plates for 48 h. The intracellular ATP contents of the serum-treated cells were measured through the luciferin-luciferase reaction using CellTiter-Glo luciferase kits (Promega, Madison, WI, USA). Renilla luciferase activity was determined by adding an equal amount of Stop & Glo substrate solution from the Dual-Glo Luciferase assay kit (Promega, Madison, WI, USA). ATP concentrations were normalized to Renilla luciferase activity. The dose-dependent effects of four PCBs and TCDD on ATP concentration were also determined in the presence of control serum. The results were presented as % control of ATP content in control cells treated with control serum. Both intra- and inter-assay coefficients of variation were less than 6.0%.
Intracellular ROS assay of the serum of pregnant women
Hepa1c1c7 cells (5X104/well) in a 96-well plate were cultured for 24 h in DMEM containing 10% FBS. After media was switched to 90 μl phenol red-free DMEM, cells were treated for 48 h with 10 μl heat-inactivated human serum samples or charcoal-stripped human serum (CS-hS, control) to obtain a final concentration of 10% serum. We measured ROS using 5-(and-6)-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate, acetyl ester (CM-H2DCFDA, Molecular Probes). ROS intensities were normalized by Hoechst nuclei staining to eliminate the well-to-well seeding error as described9.
Statistical analysis
Statistical analyses were performed using SAS, version 9.1, and data are expressed as means ± standard deviation. The sample size was calculated using the G-power program (α = 0.05, power = 0.95). The effect size was assigned to 0.15 since the standard deviation of blood glucose concentrations in Korean pregnant women was about 15–20%. The sampling ratio between GDM and non-GDM groups was about 0.2. Considering these factors, the total number of samples was about 230, and the number of participants in the present study (GDM: 390 and Non-GDM: 100) was sufficient. The statistical differences between the non-GDM and GDM groups were determined using a two-tailed sample t-test for continuous variables and χ2 test for categorical variables. The association between GDM and AhRT activity was evaluated with multivariate logistic regression analyses after adjusting for age and BMI at pre-pregnancy and 24–28 weeks. Adjusted ORs (95% CI) for the GDM risk to pregnancy outcomes were calculated using a logistic analysis. The correlations between AhRT activity and pregnancy outcomes were analyzed with Pearson correlation coefficients. The ROC curve was measured to determine the sensitivity and specificity of AhRT activity for GDM risk. A value of P < 0.05 was taken to indicate statistical significance.
References
Park, S., Kim, M. H. & Kim, S. H. Early gestational weight gains within current recommendations result in an increased risk of gestational diabetes mellitus among Korean women. Diabetes Metab. Res. Rev. 30(8), 716–725 (2014).
Park, S. & Kim, S. H. Women with rigorously managed overt diabetes during pregnancy do not experience adverse infant outcomes but do remain at serious risk of postpartum diabetes. Endocr. J. 62(4), 319–327 (2015).
Lee, K. W. et al. Prevalence and risk factors of gestational diabetes mellitus in Asia: a systematic review and meta-analysis. BMC Pregnancy Childbirth. 18(1), 494 (2018).
Zhu, Y. & Zhang, C. Prevalence of gestational diabetes and risk of progression to type 2 diabetes: a global perspective. Curr. Diabetes Rep. 16(1), 7 (2016).
Zhang, C., Rawal, S. & Chong, Y. S. Risk factors for gestational diabetes: is prevention possible?. Diabetologia 59(7), 1385–1390 (2016).
Lind, P. M. & Lind, L. Endocrine-disrupting chemicals and risk of diabetes: an evidence-based review. Diabetologia 61(7), 1495–1502 (2018).
Lee, Y. M., Jacobs, D. R. Jr. & Lee, D. H. Persistent organic pollutants and type 2 diabetes: a critical review of review articles. Front. Endocrinol. 9, 712 (2018).
Vassilopoulou, L., Psycharakis, C., Petrakis, D., Tsiaoussis, J. & Tsatsakis, A. M. Obesity, persistent organic pollutants, and related health problems. Adv. Exp. Med. Biol. 960, 81–110 (2017).
Park, W. H. et al. Novel cell-based assay reveals associations of circulating serum AhR-ligands with metabolic syndrome and mitochondrial dysfunction. BioFactors 39(4), 494–504 (2013).
Rahman, M. L. et al. Persistent organic pollutants and gestational diabetes: a multi-center prospective cohort study of healthy US women. Environ. Int. 124, 249–258 (2019).
Nebert, D. W. Aryl hydrocarbon receptor (AHR): “pioneer member” of the basic-helix/loop/helix per-Arnt-sim (bHLH/PAS) family of “sensors” of foreign and endogenous signals. Prog. Lipid Res. 67, 38–57 (2017).
Law, K. P., Han, T.-L., Mao, X. & Zhang, H. Tryptophan and purine metabolites are consistently upregulated in the urinary metabolome of patients diagnosed with gestational diabetes mellitus throughout pregnancy: a longitudinal metabolomics study of Chinese pregnant women part 2. Clin. Chim. Acta 468, 126–139 (2017).
Unluturk, U. & Erbas, T. Diabetes and tryptophan metabolism. In Tryptophan Metabolism: Implications for Biological Processes, Health and Disease (eds Engin, A. & Engin, A. B.) 147–171 (Springer, Cham, 2015).
Ryuk, J. A. et al. Tetragonia tetragonioides (Pall.) Kuntze protects estrogen-deficient rats against disturbances of energy and glucose metabolism and decreases proinflammatory cytokines. Exp. Biol. Med. 242(6), 593–605 (2017).
Kim, J. T. et al. Serum aryl hydrocarbon receptor transactivating activity is elevated in type 2 diabetic patients with diabetic nephropathy. J. Diabetes Investig. 4(5), 483–491 (2013).
Liu, X., Huang, G., Niu, Z., Wei, Y. & Wang, R. Habitual aerobic exercise, gene APOA5 named rs662799 SNP and response of blood lipid and lipoprotein phenotypes among older Chinese adult. Exp. Gerontol. 110, 46–53 (2018).
Liu, X. et al. Relative effect potency estimates for dioxin-like compounds in pregnant women with gestational diabetes mellitus and blood glucose outcomes based on a nested case-control study. Environ. Sci. Technol. 53(13), 7792–7802 (2019).
Kodama, K. et al. Ethnic differences in the relationship between insulin sensitivity and insulin response: a systematic review and meta-analysis. Diabetes Care 36(6), 1789–1796 (2013).
Yang, S. J. et al. Serum magnesium level is associated with type 2 diabetes in women with a history of gestational diabetes mellitus: the Korea National Diabetes Program study. J. Korean Med. Sci. 29(1), 84–89 (2014).
De Tata, V. Association of dioxin and other persistent organic pollutants (POPs) with diabetes: epidemiological evidence and new mechanisms of beta cell dysfunction. Int. J. Mol. Sci. 15(5), 7787–7811 (2014).
Lee, Y. M., Kim, K. S., Jacobs, D. R. Jr. & Lee, D. H. Persistent organic pollutants in adipose tissue should be considered in obesity research. Obes. Rev. 18(2), 129–139 (2017).
Papalou, O., Kandaraki, E. A., Papadakis, G. & Diamanti-Kandarakis, E. Endocrine disrupting chemicals: an occult mediator of metabolic disease. Front. Endocrinol. 10, 112–112 (2019).
Ehrlich, S. et al. Endocrine disruptors: a potential risk factor for gestational diabetes mellitus. Am. J. Perinatol. 33(13), 1313–1318 (2016).
Henriquez-Hernandez, L. A. et al. Persistent organic pollutants and risk of diabetes and obesity on healthy adults: results from a cross-sectional study in Spain. Sci. Total Environ. 607–608, 1096–1102 (2017).
Lee, D. H., Porta, M., Jacobs, D. R. Jr. & Vandenberg, L. N. Chlorinated persistent organic pollutants, obesity, and type 2 diabetes. Endocr. Rev. 35(4), 557–601 (2014).
Heindel, J. J. et al. Metabolism disrupting chemicals and metabolic disorders. Reprod. Toxicol. 68, 3–33 (2017).
Kim, Y. A. et al. Persistent organic pollutant-mediated insulin resistance. Int. J. Environ. Res. Public Health. 16(3), 448 (2019).
Bateman, J. R., Hurley, R. A. & Taber, K. H. Neurodegenerative dementias: improving brain health to decrease risk. J. Neuropsychiatry Clin. Neurosci. 31(1), A4-5 (2019).
Kim, J. T. & Lee, H. K. Metabolic syndrome and the environmental pollutants from mitochondrial perspectives. Rev. Endocr. Metab. Disord. 15(4), 253–262 (2014).
Hubbard, T. D., Murray, I. A. & Perdew, G. H. Indole and tryptophan metabolism: endogenous and dietary routes to ah receptor activation. Drug Metab. Dispos. 43(10), 1522–1535 (2015).
Park, S., Park, J. Y., Lee, J. H. & Kim, S. H. Plasma levels of lysine, tyrosine, and valine during pregnancy are independent risk factors of insulin resistance and gestational diabetes. Metab. Syndr. Relat. Disord. 13(2), 64–70 (2015).
Park, S. et al. Gestational diabetes is associated with high energy and saturated fat intakes and with low plasma visfatin and adiponectin levels independent of pre-pregnancy BMI. Eur. J. Clin. Nutr. 67(2), 196–201 (2013).
Park, S. et al. Maternal vitamin D deficiency in early pregnancy is not associated with gestational diabetes mellitus development or pregnancy outcomes in Korean pregnant women in a prospective study. J. Nutr. Sci. Vitaminol. 60(4), 269–275 (2014).
Wallace, T. M., Levy, J. C. & Matthews, D. R. Use and abuse of HOMA modeling. Diabetes Care 27(6), 1487–1495 (2004).
Henry, E. C., Bemis, J. C., Henry, O., Kende, A. S. & Gasiewicz, T. A. A potential endogenous ligand for the aryl hydrocarbon receptor has potent agonist activity in vitro and in vivo. Arch. Biochem. Biophys. 450(1), 67–77 (2006).
Park, W. H. et al. Relationships between serum-induced AhR bioactivity or mitochondrial inhibition and circulating polychlorinated biphenyls (PCBs). Sci. Rep. 7(1), 9383–9383 (2017).
Anagnostis, P. et al. Type 2 Diabetes mellitus is associated with increased risk of sarcopenia: a systematic review and meta-analysis. Calcif. Tissue Int. https://doi.org/10.1007/s00223-020-00742-y (2020).
Acknowledgment
This research was supported by a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Mistry of Health & Welfare, Republic of Korea (grant No. A102065, HI14C2700) and funded by the Korea Centers for Disease Control and Prevention (grant No. 2020-ER6402-00).
Author information
Authors and Affiliations
Contributions
S.H.K. formulated the research question and interpreted the data. S.P. analyzed the data and wrote the first draft of the manuscript. S.C. designed the study and participated in writing the manuscript. S.Y.P., S.Y., S.H.B., S.Y.R., and J.T.W. participated in designing the study and collecting the data from the participants. Y.K.P. conducted a biochemical assay. All authors read and approved the final draft of this manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Park, S., Chon, S., Park, S.Y. et al. Association of aryl hydrocarbon receptor transactivating activity, a potential biomarker for persistent organic pollutants, with the risk of gestational diabetes mellitus. Sci Rep 11, 3185 (2021). https://doi.org/10.1038/s41598-021-82794-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-82794-0
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.