Abstract
Electrical activity of fungus Pleurotus ostreatus is characterised by slow (h) irregular waves of baseline potential drift and fast (min) action potential likes spikes of the electrical potential. An exposure of the myceliated substrate to a chloroform vapour lead to several fold decrease of the baseline potential waves and increase of their duration. The chloroform vapour also causes either complete cessation of spiking activity or substantial reduction of the spiking frequency. Removal of the chloroform vapour from the growth containers leads to a gradual restoration of the mycelium electrical activity.
Similar content being viewed by others
Introduction
Most living cells are sensitive to anaesthetics1,2,3. First experiments on anaesthesia of plants have be done by Claude Bernard in late 1800s4. Later experiments on amoeba5 shown that weak concentration of narcotics causes the amoebae to spread out and propagate in a spread condition while narcotic concentrations led to cessation of movements. During last century the experimental evidences mounted up including anaesthesia of yeasts1, various aquatic invertebrates6, plants3,7, protists8, bronchial ciliated cells9. A general consensus now is that any living substrate can be anaesthetised3. The question remains, however, how exactly species without a nervous system would respond to exposure to anaesthetics.
In present paper we focus on fungi anaesthesia. Why fungi? Fungi are the largest, most widely distributed, and oldest group of living organisms10. Smallest fungi are microscopic single cells. The largest (15 hectares) mycelium belongs to Armillaria gallica (synonymous with A. bulbosa, A. inflata, and A. lutea)11 and the largest fruit body belongs to Phellinus ellipsoideus (formerly Fomitiporia ellipsoidea) which weighs half-a-ton12.
Fungi exhibit a high degree of protocognitive abilities. For example, they are capable for efficient exploration of confined spaces13,14,15,16,17. Moreover, optimisation of the mycelial network18 is similar to that of the slime mould Physarum polycephalum19 and transport networks20. Therefore, we can speculate that the fungi can solve the same range of computational problems as P. polycephalum21, including shortest path22,23,24,25,26, Voronoi diagram27, Delaunay triangulation, proximity graphs and spanning tree, concave hull and, possibly, convex hull, and, with some experimental efforts, travelling salesman problem28. The fungi’s protocognitive abilities and computational potential make them fruitful substrates for anaesthesia because they might show us how non-neuron awareness is changing under effects of narcotics.
We use extracellular electrical potential of mycelium as indicator of the fungi activity. Action potential-like spikes of electrical potential have been discovered using intra-cellular recording of mycelium of Neurospora crassa29 and further confirmed in intra-cellular recordings of action potential in hyphae of Pleurotus ostreatus and A. gallica30 and in extra-cellular recordings of fruit bodies of and substrates colonised by mycelium of P. ostreatus31. While the exact nature of the travelling spikes remains uncertain we can speculate, by drawing analogies with oscillations of electrical potential of slime mould Physarum polycephalum32,33,34,35, that the spikes in fungi are triggered by calcium waves, reversing of cytoplasmic flow, translocation of nutrients and metabolites. Studies of electrical activity of higher plants can brings us even more clues. Thus, the plants use the electrical spikes for a long-distance communication aimed to coordinate the activity of their bodies36,37,38. The spikes of electrical potential in plants relate to a motor activity39,40,41,42, responses to changes in temperature43, osmotic environment44, and mechanical stimulation45,46.
The paper is structured as follows. We present the experimental setup in “Methods” section. Analysis of the electrical activity of the intact non-anesthetised and anaesthetised fungi is given in “Results” section. “Discussion” section present some critique and directions for further research.
Methods
A commercial strain of the fungus Pleurotus ostreatus (collection code 21-18, Mogu S.r.l., Italy), previously selected for its superior fitness growing on the targeted substrate, was cultured on sterilised hemp shives contained in plastic (PP5) filter patch microboxes (SacO2, Belgium) that were kept in darkness at ambient room temperature c. 22 \(^{\circ }\)C. After one week of incubation, a hemp brick well colonised by the fungus was manually crumbled and spread on rectangular fragments, c. \(12 \times 12~{\text {cm}}^2\), of moisturised non-woven hemp pads. When these fragments were colonised, as visualised by white and healthy mycelial growth on surface, they were used for experiments.
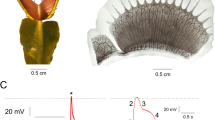
Electrical activity of the colonised hemp pads was recorded using pairs of iridium-coated stainless steel sub-dermal needle electrodes (Spes Medica S.r.l., Italy), with twisted cables and ADC-24 (Pico Technology, UK) high-resolution data logger with a 24-bit A/D converter. To keep electrodes stable we have been placing a polyurethane pad under the fabric. The electrodes were arranged in a line (Fig. 1a,b). The pairs of electrodes were pierced through the fabric and into the polyurethane pad.
The fungal substrates pierced with electrodes was placed into 20 cm by 10 cm by 10 cm plastic boxes with tight lids.
We recorded electrical activity at one sample per second. During the recording, the logger has been doing as many measurements as possible (typically up to 600 per second) and saving the average value. We set the acquisition voltage range to 156 mV with an offset accuracy of 9 \(\upmu \)V at 1 Hz to maintain a gain error of 0.1%. Each electrode pair was considered independent with the noise-free resolution of 17 bits and conversion time of 60 ms. Each pair of electrodes, called channels, reported a difference of the electrical potential between the electrodes. Distance between electrodes was 1-2 cm. In each trial, we recorded eight electrode pairs, channels, simultaneously.
To study the effect of chloroform we soaked a piece of filter paper c. 4 cm by 4 cm in chloroform (Sigma Aldrich, analytical standard) and placed the piece of paper inside the plastic container with the recorded fungal substrate.
The humidity of the fungal colonies was 70–80% (MerlinLaser Protimeter, UK). The experiments were conducted in a room with ambient temperature 21 \(^{\circ }\)C and in the darkness of protective growing tents (Fig. 1c).
We have conducted ten experiments, in each experiments we recorded electrical activity of the fungi via eight channels, i.e. 80 recordings in total.
Results
Myceliated hemp pad exhibit patterns of electrical activity similar to that of spiking neural tissue. Examples of action potential like spikes, solitary and in trains, are shown in Fig. 2.
Application of the chloroform to the container with fungi substantially affected the electrical activity of the fungi. An example of an extreme, i.e. where almost all electrical activity of mycelium seized, response is shown in Fig. 3. In this example, the introduction of the chloroform leads to the suppression of the spiking activity and reduction of deviation in values of the electrical potential differences recorded on the channels.
The intact, non-anesthetised, mycelium composite (control ) shows median amplitude of the irregular movements of the baseline potential is 0.45 mV (average 0.64 mV, \(\sigma =0.64\)), median duration 29,850 s (average 67,507 s, \(\sigma =29{,}850\)). After exposure to chloroform the baseline potential movements show median amplitude reduced to 0.16 mV (average 0.18 mV, \(\sigma =0.12\)) and median duration increased to 38,507 s (average 38,114 s, \(\sigma =38{,}507\)). For the eight channels (pairs of differential electrodes) recorded exposure to chloroform led to nearly three times decrease in amplitude of the drifts of baseline potential and nearly 1.3 increase in duration of the drifts. Before exposure to chloroform the mycelium composite produced fast (i.e. less than 10–20 min) spikes. Median amplitude of the spikes was 0.48 mV (average 0.52 mV, \(\sigma =0.2\)). Median duration of spikes was 62 s (average 63 s, \(\sigma =18\)), median distance between the spike 214 s (average 189 s, \(\sigma =90\)). After exposure to chloroform the mycelium composite did not show any spiking activity above level of background noise, which was for this particular recording c. 0.05 mV.
In some cases the spiking activity is diminished gradually with decreased frequency and lowered amplitude, as exemplified in Fig. 4. Typically, the intact (control) spiking frequency is a spike per 70 min while after inhalation of chloroform a spike per 254 min in the first 40-50 hours and decreased to nearly zero after. The median amplitude of intact mycelium spikes is 0.51 mV, average 0.74 mV (\(\sigma \)=0.59). Anaesthetised mycelium shows, spikes with median amplitude 0.11 mV, average 0.2 mV (\(\sigma =0.2\)). Spikes are not distributed uniformly but gathered in trains of spikes. In the intact mycelium there is a media of 3 spikes in the train, average number of spikes is 4.2 (\(\sigma =4.4\)). Median duration of a spike train is 84 min, average 112 min (\(\sigma =32\)). Media interval between trains is 53 min, average 55 s (\(\sigma =29\)). Anaesthetised mycelium emits trains with median number of 2 spikes, average 2.5 spikes, average 2.5 spikes (\(\sigma =0.84\)). A median duration of such trains is 29 min, average 51 min (\(\sigma =22\)). The trains appear much more rarely than the trains in the intact mycelium: median interval between trains is 227 min.
Example of electrical activity of mycelium colonised hemp pad before, during and after stimulation with chloroform vapour. Arrow labelled ‘ON’ shows moment when a source of chloroform vapour was added into the enclosure, ‘OFF’ when the source of chloroform was removed. The spiking activity of the mycelium recovering from anaesthesia is zoomed in.
In all ten but one experiment the container remained closed for over 3–4 days. By that time all kinds of electrical activity in mycelium bound substrate extinguished and the mycelium never recovered to a functional state. In experiment illustrated in Fig. 5 we removed a source of chloroform after 16 h and kept the container open and well ventilated for an hour to remove any traces of chloroform from the air. The intact mycelium shows median frequency of spiking as one spike per 27 min, average 24 min. Median amplitude of the spikes is 3.4 mV, average 3.25 mV (\(\sigma =1.45\)). The anaesthetised mycelium demonstrates electrical spiking activity reduced in amplitude: median amplitude of spikes is 0.24 mV, average 0.32 mV (\(\sigma =0.2\)), and low frequency of spiking: median distance between spikes is 38 min, average 40 min. Electrical activity of the mycelium restores to above noise level c. 60 h after the source of the chloroform is removed from the enclosure (insert in Fig. 5). Frequency of spikes is one spike per 82 min (median), average 88 min. The amplitudes of recovering spikes are 0.96 mV in median (average 0.93 mV, \(\sigma =0.08\)) which are three times less than of the spikes in the mycelium before the narcosis but nearly five times higher than of the spike of the anaesthetised mycelium.
Discussion
We demonstrated that the electrical activity of the fungus Pleurotus ostreatus is a reliable indicator of the fungi anaesthesia. When exposed to a chloroform vapour the mycelium reduces frequency and amplitude of its spiking and, in most cases, cease to produce any electrical activity exceeding the noise level (Table 1a). When the chloroform vapour is eliminated from the mycelium enclosure the mycelium electrical activity restores to a level similar to that before anaesthesia (Table 1b).
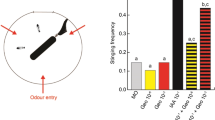
Response of slime mould Physarum polycephalum to trifluoroethane (Sigma Aldrich, UK). Electrical potential difference between two sites of 10 mm long protoplasmic tube was measured using aluminium electrodes, amplified and digitised with ADC-20 (Pico Technology, UK). (a) 5 \(\upmu \)L of trifluoroethane applied to a 5 mm \(\times \) 5 mm piece of filter paper placed in the Petri dish with slime mould. (b) 25 \(\upmu \)L applied. Arrows indicate moments when the piece of paper soaked in trifluoroethane was placed in a Petri dish with the slime mould.
The fungal responses to chloroform are similar to that recorded by us with slime mould Physarum polycephalum (unpublished results). A small concentration of anaesthetic leads to reduced frequency and amplitude of electrical potential oscillation spikes of the slime mould, and some irregularity of the electrical potential spikes (Fig. 6a). Large amounts of anaesthetic causes the electrical activity to cease completely and never recover (Fig. 6b). Similar electrical responses to anaesthetic again highlights the fact that slime mould exhibits same-degree affinities with fungi as with protozoa, and slime mould has been classified as fungi previously47,48,49.
Results presented in the paper contribute towards filling up the gaps in the taxonomy-related studies of anaesthesia, the living creatures from Protists to fungi to plants to insects to mammals are susceptible to anaesthetics. Effects of chloroform on fungi might also implicitly indicate presence of potassium channels, which are inhibited by anaesthetics50,51,52.
With regards to directions of future research, as far as we are aware, the present paper is the first in the field, and therefore it rather initiates the research than brings any closure or conclusions. We know that anaesthetics block electrical activity of fungi (as well as slime moulds) however we do not know exact biophysical mechanisms of these actions. The study of biophysics and molecular biology of fungi anaesthesia could be a scope for future research. Another direction of studies could be the analysis of the decision making abilities of fungi under the influence of anaesthetics. An experiment could be constructed when fungal hyphae are searching for an optimal path in a labyrinth when subjected to increasing doses of chloroform vapour. There may be an opportunity to make a mapping from concentrations of anaesthetic to geometry of the mycelium search path.
References
Sonner, J. M. A hypothesis on the origin and evolution of the response to inhaled anesthetics. Anesth. Analg. 107, 849 (2008).
Eckenhoff, R. G. Why can all of biology be anesthetized?. Anesth. Analg. 107, 859–861 (2008).
Grémiaux, A., Yokawa, K., Mancuso, S. & Baluška, F. Plant anesthesia supports similarities between animals and plants: Claude Bernard’s forgotten studies. Plant Signal. Behav. 9, e27886 (2014).
Bernard, C. et al. Lectures on the Phenomena of Life Common to Animals And Plants. Translation by Hebbel E. Hoff, Roger Guillemin [and] Lucienne Guillemin (1974).
Hiller, S. Action of narcotics on the ameba by means of microinjection and immersion. Proc. Soc. Exp. Biol. Med. 24, 427–428 (1927).
Oliver, A., Deamer, D. & Akeson, M. Sensitivity to anesthesia by pregnanolone appears late in evolution. Ann. N. Y. Acad. Sci. 625, 561–565 (1991).
Milne, A. & Beamish, T. Inhalational and local anesthetics reduce tactile and thermal responses in Mimosa pudica. Can. J. Anesth. 46, 287–289 (1999).
Nunn, J., Sturrock, J. E., Wills, E., Richmond, J. E. & McPherson, C. The effect of inhalational anaesthetics on the swimming velocity of Tetrahymena pyriformis. J. Cell Sci. 15, 537–554 (1974).
Verra, F. et al. Effects of local anaesthetics (lidocaine) on the structure and function of ciliated respiratory epithelial cells. Biol. Cell 69, 99–105 (1990).
Carlile, M. J., Watkinson, S. C. & Gooday, G. W. The Fungi (Gulf Professional Publishing, 2001).
Smith, M. L., Bruhn, J. N. & Anderson, J. B. The fungus Armillaria bulbosa is among the largest and oldest living organisms. Nature 356, 428 (1992).
Dai, Y.-C. & Cui, B.-K. Fomitiporia ellipsoidea has the largest fruiting body among the fungi. Fungal Biol. 115, 813–814 (2011).
Hanson, K. L. et al. Fungi use efficient algorithms for the exploration of microfluidic networks. Small 2, 1212–1220 (2006).
Held, M., Edwards, C. & Nicolau, D. V. Examining the behaviour of fungal cells in microconfined mazelike structures. In Imaging, Manipulation, and Analysis of Biomolecules, Cells, and Tissues VI, vol. 6859, 68590U (International Society for Optics and Photonics, 2008).
Held, M., Edwards, C. & Nicolau, D. V. Fungal intelligence; or on the behaviour of microorganisms in confined micro-environments. J. Phys. Conf. Ser. 178, 012005 (2009).
Held, M., Lee, A. P., Edwards, C. & Nicolau, D. V. Microfluidics structures for probing the dynamic behaviour of filamentous fungi. Microelectron. Eng. 87, 786–789 (2010).
Held, M., Edwards, C. & Nicolau, D. V. Probing the growth dynamics of Neurospora crassa with microfluidic structures. Fungal Biol. 115, 493–505 (2011).
Boddy, L., Hynes, J., Bebber, D. P. & Fricker, M. D. Saprotrophic cord systems: dispersal mechanisms in space and time. Mycoscience 50, 9–19 (2009).
Adamatzky, A. Developing proximity graphs by Physarum polycephalum: Does the plasmodium follow the toussaint hierarchy?. Parallel Process. Lett. 19, 105–127 (2009).
Adamatzky, A. (ed.) Bioevaluation of World Transport Networks (World Scientific, 2012).
Adamatzky, A. (ed.) Advances in Physarum Machines: Sensing and Computing with Slime Mould (Springer, 2016).
Nakagaki, T., Yamada, H. & Tóth, Á. Intelligence: Maze-solving by an amoeboid organism. Nature 407, 470 (2000).
Nakagaki, T. Smart behavior of true slime mold in a labyrinth. Res. Microbiol. 152, 767–770 (2001).
Nakagaki, T., Yamada, H. & Toth, A. Path finding by tube morphogenesis in an amoeboid organism. Biophys. Chem. 92, 47–52 (2001).
Nakagaki, T. et al. Minimum-risk path finding by an adaptive amoebal network. Phys. Rev. Lett. 99, 068104 (2007).
Tero, A. et al. Rules for biologically inspired adaptive network design. Science 327, 439–442 (2010).
Shirakawa, T., Adamatzky, A., Gunji, Y.-P. & Miyake, Y. On simultaneous construction of Voronoi diagram and delaunay triangulation by Physarum polycephalum. Int. J. Bifurc. Chaos 19, 3109–3117 (2009).
Jones, J. & Adamatzky, A. Computation of the travelling salesman problem by a shrinking blob. Nat. Comput. 13, 1–16 (2014).
Slayman, C. L., Long, W. S. & Gradmann, D. “Action potentials’’ in Neurospora crassa, a mycelial fungus. Biochim. Biophys. Acta Biomembr. 426, 732–744 (1976).
Olsson, S. & Hansson, B. Action potential-like activity found in fungal mycelia is sensitive to stimulation. Naturwissenschaften 82, 30–31 (1995).
Adamatzky, A. On spiking behaviour of oyster fungi Pleurotus djamor. Sci. Rep. 8, 1–7 (2018).
Iwamura, T. Correlations between protoplasmic streaming and bioelectric potential of a slime mold, Physarum polycephalum. Shokubutsugaku Zasshi 62, 126–131 (1949).
Kamiya, N. & Abe, S. Bioelectric phenomena in the myxomycete plasmodium and their relation to protoplasmic flow. J. Colloid Sci. 5, 149–163 (1950).
Kishimoto, U. Rhythmicity in the protoplasmic streaming of a slime mold, Physarum polycephalum. I. A statistical analysis of the electric potential rhythm. J. Gen. Physiol. 41, 1205–1222 (1958).
Meyer, R. & Stockem, W. Studies on microplasmodia of Physarum polycephalum V: Electrical activity of different types of microplasmodia and macroplasmodia. Cell Biol. Int. Rep. 3, 321–330 (1979).
Trebacz, K., Dziubinska, H. & Krol, E. Electrical signals in long-distance communication in plants. In Communication in plants, 277–290 (Springer, 2006).
Fromm, J. & Lautner, S. Electrical signals and their physiological significance in plants. Plant Cell Environ. 30, 249–257 (2007).
Zimmermann, M. R. & Mithöfer, A. Electrical long-distance signaling in plants. In Long-Distance Systemic Signaling and Communication in Plants, 291–308 (Springer, 2013).
Simons, P. The role of electricity in plant movements. New Phytol. 87, 11–37 (1981).
Fromm, J. Control of phloem unloading by action potentials in mimosa. Physiol. Plant. 83, 529–533 (1991).
Sibaoka, T. Rapid plant movements triggered by action potentials. Bot. Mag. = Shokubutsu-gaku-zasshi 104, 73–95 (1991).
Volkov, A. G. et al. Mimosa pudica: Electrical and mechanical stimulation of plant movements. Plant Cell Environ. 33, 163–173 (2010).
Minorsky, P. Temperature sensing by plants: A review and hypothesis. Plant Cell Environ. 12, 119–135 (1989).
Volkov, A. G. Green plants: electrochemical interfaces. J. Electroanal. Chem. 483, 150–156 (2000).
Roblin, G. Analysis of the variation potential induced by wounding in plants. Plant Cell Physiol. 26, 455–461 (1985).
Pickard, B. G. Action potentials in higher plants. Bot. Rev. 39, 172–201 (1973).
Whittaker, R. H. On the broad classification of organisms. Q. Rev. Biol. 34, 210–226 (1959).
Talbot, P. Fungi with a plasmodial thallus division myxomycota: Slime-moulds. In Principles of Fungal Taxonomy, 99–108 (Springer, 1971).
Bruns, T. D., White, T. J. & Taylor, J. W. Fungal molecular systematics. Annu. Rev. Ecol. Syst. 22, 525–564 (1991).
Jan, L. Y. & Jan, Y. N. How might the diversity of potassium channels be generated?. Trends Neurosci. 13, 415–419 (1990).
Kindler, C. H., Yost, S. C. & Gray, A. T. Local anesthetic inhibition of baseline potassium channels with two pore domains in tandem. J. Am. Soc. Anesthesiol. 90, 1092–1102 (1999).
Steinberg, E., Wafford, K., Brickley, S., Franks, N. & Wisden, W. The role of k 2p channels in anaesthesia and sleep. Pflügers Archiv-Eur. J. Physiol. 467, 907–916 (2015).
Acknowledgements
This project has received funding from the European Union’s Horizon 2020 research and innovation programme FET OPEN “Challenging current thinking” under grant agreement No 858132. The authors would like to acknowledge the collaboration of Mogu S.r.l. providing the living materials used in the experiments. At the time of experiments AG was affiliated with Mogu S.r.l., Inarzo, Italy. All the experiments have been conducted in the Unconventional Computing Lab, UWE, Bristol.
Author information
Authors and Affiliations
Contributions
A.A. and A.G. conducted and analysed experiments. A.A. and A.G. wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Adamatzky, A., Gandia, A. Fungi anaesthesia. Sci Rep 12, 340 (2022). https://doi.org/10.1038/s41598-021-04172-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-04172-0
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.