Abstract
Smoking increases systemic inflammation and circulating endothelin-1 (ET-1), both of which contribute to an elevated risk of cardiovascular disease (CVD). The present study sought to test the hypothesis that a 12-week smoking cessation intervention would contribute to a long-term reduction in circulating ET-1, tumor necrosis factor-alpha (TNF-α), and interleukin-6 (IL-6). 30 individuals participated in a 12-week evidence-based smoking cessation program at Augusta University. Serum cotinine, plasma inflammatory cytokines, and plasma ET-1 were determined at baseline, immediately after the 12-week cessation program (end of treatment, EOT), and 12-months (12M) following the cessation program. Serum cotinine was significantly reduced (p < 0.001) at EOT and 12M following the smoking cessation program. Compared to BL (7.0 ± 1.6 pg/mL), TNF-α was significantly reduced at EOT (6.3 ± 1.5 pg/mL, p = 0.001) and 12M (5.2 ± 2.7 pg/mL, p < 0.001). ET-1 was significantly lower at EOT (1.9 ± 0.6 pg/mL, p = 0.013) and at 12M (2.0 ± 0.8 pg/mL, p = 0.091) following smoking cessation compared with BL (2.3 ± 0.6 pg/mL). BL concentrations of cotinine were significantly associated with basal ET-1 (r = 0.449, p = 0.013) and the change in cotinine at 12M following smoking cessation was significantly associated with the change in plasma ET-1 at 12M (r = 0.457, p = 0.011). Findings from the present pilot investigation demonstrate that a 12-week smoking cessation program reduces circulating concentrations of ET-1 and TNF-α for at least a year. The reduction in serum cotinine was associated with the decrease in circulating ET-1. The attenuation in ET-1 and inflammation may in part, contribute to the lower risk of CVD that is observed with smoking cessation.
Similar content being viewed by others
Introduction
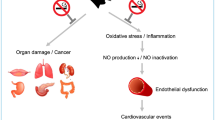
Nearly 500,000 smoking-related deaths occur each year, resulting in tobacco use as the leading preventable cause of death in the United States1. Smoking increases inflammation2 and oxidative stress3, both of which directly contribute to vascular endothelial dysfunction and subsequent increase in atherosclerosis and cardiovascular disease (CVD) risk4, 5. In fact, current smokers are at four times greater risk for developing CVD compared to those whom have never smoked or even those whom have recently quit6.
The American Heart Association has identified smoking status (i.e. smoking, never smoked, or quit smoking) as a major component of overall cardiovascular health7. If implemented early on, smoking cessation not only reduces excess health hazards by 90%, it can increase life expectancy by up to 10 years6. Regardless of smoking history, smoking cessation can favorably impact traditional CVD risk factors, including lowering blood pressure8 and reducing systemic inflammation5. The endothelin system plays a critical role in regulating vascular endothelial function and both smoking and secondhand smoke can transiently increase circulating concentrations of ET-19 and directly increase the expression of the vasoconstricting endothelin-A receptor10. On the other hand, cigarette smoke has multiple deleterious effects on the immune system that can cause immune deficiencies and augment the production of numerous pro-inflammatory cytokines11. Interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α) are arguably two of the most commonly studied pro-inflammatory cytokines12, 13, each of which has been reported to increase in both the lungs and circulation with smoking2, 14.
Although 70% of smokers express interest in quitting, most are discouraged by the high failure rates and only ~ 8% of smokers are successful at quitting without guidance1. Interestingly, the success of smoking cessation is more closely associated with the degree of nicotine dependence than the motivation to quit15. The success of a smoking cessation program is also directly related to the type of intervention (group or individual therapy), the number of intervention modalities, and the number of reinforcing sessions. Therefore, inclusion of the evidence-based components of both pharmacotherapy (i.e., to address the physical addiction) and behavioral skills counseling (i.e., to address the psychological addiction) are essential for smoking cessation success16, 17.
Smoke exposure increases ET-118 and augments systemic inflammation19; however, the effect of a short-term (12-week) smoking cessation program on ET-1 and markers of inflammation over time is unknown. Accordingly, the present pilot investigation sought to test the hypothesis that a 12-week smoking cessation program would contribute to a reduction in ET-1, TNF-α, and IL-6 over a 12-month post cessation period.
Methods
Experimental design
The present investigation was comprised of three experimental visits: baseline (BL), 12-weeks (end of treatment, EOT), and 12-months (12M). The study timeline is presented in Fig. 1.
The three experimental visits occurred at various times throughout the day to accommodate participant’s schedules. The BL visit included an initial physical exam by a board certified nurse practitioner (NP) who was able to help assess the best pharmacotherapy in discussion with the participant. On each follow-up visit (EOT and 12M), demographics and vitals were collected and a venous blood sample was obtained. The NP was available at these two visits and seen by the participant only if there were concerns with the vital signs or any problems with the prescribed smoking cessation pharmacotherapy.
Participants
Thirty-five volunteers agreed to participate in this study. Participants were eligible if they met the following criteria: (1) were ≥ 18 years of age, (2) scored at least a 6 out of 10 on the Readiness to Quit Ladder20, which indicates “I definitely plan to quit smoking within the next 6 months,” and (3) were either apparently healthy or if with chronic illness(es), were well controlled on a treatment plan. Participants who were scheduled for a cessation clinic appointment and met the inclusion criteria were invited to enroll in the study. Research participants completed the written informed consent document (ICD) process after all questions were answered by the principal investigator or a study team member trained in the ICD process. This study was approved by the Institutional Review Board at Augusta University and all experiments were performed in accordance with relevant guidelines and regulations.
Smoking cessation program including pharmacotherapy
The smoking cessation program was 12-weeks long. For the first 8-weeks, participants were contacted by phone each week and attended weekly in-person counseling sessions with a cessation counselor/coach. In-person coaching sessions were conducted in groups of 6–10 with each session lasting 45–60 min. Topics that were addressed and covered during these sessions included the following: the effects of nicotine on the body, physical and psychological addiction of smoking, triggers to smoking, and the importance of being prepared when tempted and with cravings. The format was an open discussion with a cessation counselor who was experienced in both tobacco treatment and motivational interviewing, a technique shown to be effective with smoking cessation16, 17. Following the conclusion of the 12-week smoking cessation program, participants were contacted to monitor smoking cessation status every 2 weeks for 3-months and then monthly for the remaining 6-month period.
Participants started their respective pharmacotherapy within 3-days of the baseline visit. Each participant was prescribed an individual pharmacotherapy based on the recommendation of the NP on the initial visit. The pharmacotherapies included a daily oral dose of Varenicline (1 mg/day for the first week, then increased to 2 mg/day) or use of a nicotine replacement therapy (NRT), specifically the patch. Initial pharmacotherapy was prescribed for a month at the time and participants were instructed to take the medication minimally for 8 weeks and at maximum for 16-weeks. Any side effects experienced from the pharmacotherapy were reported to the counselor who contacted the NP immediately to address as needed. Compliance of the pharmacotherapy was measured at each experimental visit by self-report. Participants were asked if they were still using the therapy, if so, how often and if not, when they last used the therapy. This study was completed prior to any of the current concerns and holds on varenicline usage.
Blood analysis: cotinine, ET-1, TNF-a and IL-6
A venous blood sample was collected into serum separator or EDTA treated collection tubes (BD, United States) and serum or plasma, respectively, was separated out by centrifugation. Serum and plasma samples were flash frozen in liquid nitrogen and stored at − 80 °C until later analyzed.
Cotinine, the primary metabolite of nicotine21, is considered the “gold standard” for the assessment of smoking status, including verification of smoking cessation22. Serum cotinine was assessed at BL, EOT, and 12M using an established protocol of liquid chromatography and mass spectrometry by Salimetrics, LLC. Concentrations of plasma ET-1, TNF-α, and IL-6 were determined using a Simple Plex cartridge on the Ella Platform (Protein Simple, San Jose, CA) according to the manufacturer’s instructions. The limit of detection of human ET-1, TNF-α, and IL-6 are 0.250–1000 pg/mL, 1.14–290 pg/mL, and 0.41–3850 pg/mL, respectively.
Statistical analyses
All statistical analyses were performed using SPSS version 24 (SPSS Inc, Chicago, IL). All data are presented as mean ± standard error of the mean unless otherwise noted. All data was assessed for normal distribution and sphericity. Mauchly’s test of sphericity was performed and if rejected, the Greenhouse–Geisser correction was applied. A repeated measures analysis of variance (ANOVA) was performed to determine changes in participant characteristics, serum cotinine, and plasma ET-1, IL-6, and TNF-α over the 12-month study period. When a significant main-effect was found, Tukey’s post-hoc analysis with Bonferroni correction was used. Pearson’s correlations were performed to evaluate the relationship between serum cotinine and circulating ET-1. Statistical significance was set a priori to an alpha level of ≤ 0.05.
Ethics approval and consent to participate
This study was approved by the Institutional Review Board at Augusta University (IRB Number: 0805252). Research participants completed the written informed consent document (ICD) process prior to participation, after all questions were answered by the principal investigator or a study team member trained in the ICD process.
Results
Participant characteristics
During the enrollment period, 35 participants agreed to participate in the pilot study. Of these 35 individuals, 31 (89%) completed all three experimental visits, and the cessation counseling sessions. Four participants (11%) were lost to follow-up: 2 chose to stop participating in the study prior to its conclusion, and 2 were non-responsive to all follow-up attempts to reach them. For the purpose of this study, one participant was excluded from the final analysis due to insufficient plasma for the ET-1 and inflammatory marker analysis. Participant characteristics are presented in Table 1.
Compared to BL, body weight was significantly (p = 0.001) higher at 12M. There was no change in any other anthropometric variables during the 12-week smoking cessation program or throughout the 12M study period. The pharmacotherapies, treatment compliance and the side-effects experienced while on the therapy are presented in Table 2.
Twenty-eight participants were prescribed an oral dose of Varenicline, one participant used NRT (patch), while one did not use any pharmacotherapy. Although pharmacotherapies were prescribed for a maximum of 16 weeks, participants could stop treatment prior to EOT if successful with quitting and restart the pharmacotherapy after EOT to help resist urges and/or if they had relapsed.
Biochemical analyses
Concentrations of serum cotinine at BL, EOT, and 12M are presented in Table 3. Compared to BL, a significant decrease in cotinine was observed at EOT (p < 0.001) and persisted through the 12M visit (p < 0.001).
Concentrations of circulating ET-1, TNF-α, and IL-6 at BL, EOT, and 12M are presented in Table 3. Compared to BL, concentrations of ET-1 were significantly reduced at EOT (p = 0.013) and this reduction was maintained at 12M (p = 0.091). Compared to BL, TNF-α was significantly (p = 0.001) lower at EOT. In addition, circulating concentrations of TNF-α continued to decrease out to 12M compared to both BL (p < 0.001) and EOT (p = 0.002). IL-6 data for all three time points were only available for 28/30 participants due to insufficient sample volume at 12M for two participants. Although not statistically significant, IL-6 tended (p = 0.114) to decrease from BL at EOT and the decrease was sustained at 12M.
Figure 2A illustrates the significant correlation between concentrations of cotinine and circulating ET-1 (r = 0.449, p = 0.013). In addition, the change in cotinine at 12M following smoking cessation was positively associated with the change in ET-1 at 12M (r = 0.457, p = 0.011) (Fig. 2B).
Discussion
Smoking can have a negative impact on circulating concentrations of ET-19 and systemic inflammation19, both of which are independent risk factors of CVD. Smoking cessation can significantly reduce the overall risk of CVD6; however, whether or not smoking cessation is associated with changes in ET-1 and inflammatory status had not as yet been determined. The present investigation sought to test the hypothesis that a 12-week, evidence-based smoking cessation intervention would contribute to a long-term reduction in circulating concentrations of ET-1, TNF-α, and IL-6. Findings of the present investigation demonstrate that a 12-week smoking cessation intervention is effective at not only reducing both circulating ET-1 and TNF-α, but also sustaining the reduction for at least 12-months. Perhaps most importantly, the reduction in circulating cotinine over time was associated with the reduction in plasma ET-1.
Efficacy of the smoking cessation program
A ‘former smoker’ is defined by the US Centers for Disease Control and Prevention as a non-smoking adult that has smoked at least 100 cigarettes in their lifetime23. It often takes several attempts for a smoker to successfully quit smoking and self-reported measures of smoking and smoking cessation are highly imprecise and inaccurate21. Cotinine, the major metabolite of nicotine, is regarded as the most objective biochemical measure of smoke exposure22. The concentration of cotinine is positively related to the amount of nicotine an individual is exposed to, with greater exposure eliciting higher concentrations of cotinine21. When an individual quits smoking, concentrations of cotinine begin to decline, and with prolonged cessation, cotinine concentrations decrease to undetectable levels21. The significant reduction in serum cotinine after 12-weeks of smoking cessation in the present investigation confirms the adherence and effectiveness of our evidence-based program. The average serum concentrations of cotinine at EOT was 47.0 ng/mL, indicative of a reduction in smoking but not 100% adherence. These results suggest a reduction in smoking frequency; however, this was not a primary focus of the present study. Importantly, compared to BL, serum cotinine remained lower at 12M and indicates that many participants continued to abstain from smoking for at least 9-months after the 12-week cessation program had ended.
Smoking cessation and endothelin-1
ET-1 is a potent vasoconstrictor peptide that has an important pathological role in the pathogenesis of hypertension24, 25 and overall CVD risk26. ET-1 provides opposing actions by acting through a vaso-constricting ETA-receptor (ETAR) and a vaso-dilating ETB-receptor (ETBR)27. Additionally, ET-1 increases renal vascular constriction and proteinuria in the kidney26, all of which contribute to the development of hypertension and subsequent CVD. The baseline concentrations of ET-1 measured in this study are consistent with previous investigations that have documented an increase in circulating concentrations of ET-1 with active smoking9. Findings of the present investigation are the first to demonstrate that (1) a short-term smoking cessation program elicits a significant reduction in circulating ET-1, and (2) the reduction in ET-1 is maintained for at least 12M with continued cessation. Perhaps most relevant, those who exhibited the greatest reductions in cotinine at 12M had the greatest reductions in circulating ET-1. The reduction in circulating ET-1 limits the number of ligands available to bind to the potent ETAR, thereby attenuating acute vasoconstriction and improving vascular tone25, 27. Taken together, these data suggest that the decline in cotinine with smoking cessation may contribute in part, to the decrease in circulating ET-1. Nonetheless, the evidence-based smoking cessation program at Augusta University resulted in a significant decrease in circulating ET-1, which likely contributes to an overall reduction in CVD risk.
Smoking and markers of inflammation
Chronic inflammation also plays a central role in the development of CVD and a reduction in inflammation offers cardio-protection28. In particular, there is great interest in targeting a decrease in TNF-α28 and IL-629, two hallmark pro-inflammatory cytokines. Elevated inflammatory cytokines have a direct impact on vascular resistance and endothelial cell apoptosis and both TNF-α and IL-6 can be stimulated by smoking19. As a vicious cycle, smoke exposure results in immediate tissue damage and this damage elicits an inflammatory immune response2 that continues to aid in repair. Chronic inflammation, such as that observed with long-term smoking, is associated with CVD14. Consistent with previous reports30, baseline concentrations of inflammation were elevated in the participants of the present investigation. With smoking cessation; however, a significant reduction in TNF-α was observed at EOT and persisted lower at 12M. In addition, IL-6 was reduced following the 12-week smoking cessation program, although it did not reach statistical significance. Previous studies have suggested that statistically significant reductions in IL-6 do not occur until 5–9 years following smoking cessation31, 32. Nonetheless, the reductions in circulating IL-6 and TNF-α observed in the present investigation may be clinically significant as the decrease in each individual cytokine may act synergistically to contribute to an overall greater reduction in CVD risk. In fact, targeting risk factors of CVD with a multifactorial approach has been proposed to be more effective in lowering CVD risk compared with modifying a single risk factor by itself33. Taken together, findings of the present investigation provide evidence that a short-term smoking cessation program results in an overall reduction in the systemic inflammatory profile.
Experimental considerations
In addition to elevated ET-1 at baseline, participants of the current investigation were classified as having stage 1 hypertension34, with an average blood pressure of 130/83 mm Hg. While not statistically significant, there was a modest, but clinically meaningful reduction in mean arterial pressure (MAP; 2 mm Hg) following cessation that may also have contributed to a reduction in CVD risk35. Interestingly, smoking cessation has been observed to coincide with an increase in blood pressure, particularly in those who had quit for over a year36. The modest change in blood pressure we observed in the current investigation could be due to (1) a less than 100% adherence in quitting and/or (2) we only tracked our participants out for 1 year. Reductions in ET-1 are associated with reductions in blood pressure37, which could, in part, explain the clinically significant reduction in MAP.
It is important to note that sex and race differences have been identified throughout the endothelin system38, 39. Although an equal number of men and women were recruited for this study, no sex differences in any outcomes were identified. In addition, the present study was not powered to test the effects of race as only 20% of our cohort identified as African Americans. In a similar line, this pilot study only included a small sample size of 30 individuals. Future studies are warranted to replicate the results of the current study in a larger cohort considering equal representation of sex and race.
Vascular dysfunction is not only a consequence of a heightened endothelin system27 but smoking as well40. While outside the scope of the current investigation, inclusion of other vascular measures, such as flow-mediated dilation, arterial stiffness or microvascular function could provide further insight into the CVD risk benefits of reducing ET-1 with smoking cessation. In a similar line, changes in nutrition41, alcohol intake42, or exercise43 have a strong interrelationship with smoking and smoking cessation. Smoking has appetite suppressive effects that often leads to weight loss44. Therefore, it is not surprising that we observed a significant increase in weight throughout the study. It is important to note; however, that we cannot rule out the impact of dietary or exercise changes that may have occurred during the treatment period, which can affect body weight. While not measured in the current study, future investigations that incorporate lifestyle and behavior and vascular health assessments with biomarkers are warranted.
In addition, further research is needed that examines the relationship between nicotine dependence of smokers [typically measured using the Fagerstrom Test for Nicotine Dependence (FTND)] and both ET-1 and inflammatory markers. Findings may provide important information on possible linkages between dependence and inflammatory markers pre and post-cessation as well as provide insight into the impact of the addiction level on the success of quitting. Although the pharmacotherapy prescribed was based on the number of cigarettes smoked per day, which is an acceptable measure of nicotine addiction and dependence, this study used the Readiness to Quit Ladder as inclusion criteria versus the FTND. Nonetheless, the FTND is an important test for addiction and future studies should include this factor due to the novel and important findings that may emerge.
Conclusions
The positive effects of smoking cessation on overall cardiovascular health are well established5. However, there are numerous factors that can contribute to the increased CVD risk with smoking, such as elevated ET-126 and inflammation19. Findings from the present investigation demonstrate that an evidence-based 12-week smoking cessation program is not only effective, it can also reduce circulating concentrations of ET-1, TNF-α, and IL-6, all of which can contribute to long-term reductions in CVD risk. Perhaps most important, the change in cotinine was associated with the change in ET-1, suggesting that reductions in CVD risk associated with smoking cessation may in part, be due to the decrease in circulating ET-1.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- ET-1:
-
Endothelin-1
- CVD:
-
Cardiovascular disease
- TNF-α:
-
Tumor necrosis factor-alpha
- IL-6:
-
Interleukin-6
- BL:
-
Baseline
- EOT:
-
End of treatment
- 12M:
-
12-Months
- NP:
-
Nurse practitioner
- ICD:
-
Informed consent document
- NRT:
-
Nicotine replacement therapy
- ANOVA:
-
Analysis of variance
- BMI:
-
Body mass index;
- SBP:
-
Systolic blood pressure
- DBP:
-
Diastolic blood pressure
- MAP:
-
Mean arterial pressure
- ETAR:
-
ETA-receptor
- ETBR:
-
ETB-receptor
References
Smoking & Tobacco Use: Fast Facts and Fact Sheet Centers for Disese Control and Prevention2019 Available from: https://www.cdc.gov/tobacco/data_statistics/fact_sheets/index.htm.
Goncalves, R. B. et al. Impact of smoking on inflammation: Overview of molecular mechanisms. Inflamm. Res. 60(5), 409–424 (2011).
Burke, A. & Fitzgerald, G. A. Oxidative stress and smoking-induced vascular injury. Prog. Cardiovasc. Dis. 46(1), 79–90 (2003).
Deanfield, J. E., Halcox, J. P. & Rabelink, T. J. Endothelial function and dysfunction: Testing and clinical relevance. Circulation 115(10), 1285–1295 (2007).
Ambrose, J. A. & Barua, R. S. The pathophysiology of cigarette smoking and cardiovascular disease: An update. J. Am. Coll. Cardiol. 43(10), 1731–1737 (2004).
Pirie, K. et al. The 21st century hazards of smoking and benefits of stopping: A prospective study of one million women in the UK. Lancet 381(9861), 133–141 (2013).
Benjamin, E. J. et al. Heart disease and stroke statistics-2018 update: A report from the American heart association. Circulation 137(12), e67–e492 (2018).
Verdecchia, P. et al. Cigarette smoking, ambulatory blood pressure and cardiac hypertrophy in essential hypertension. J. Hypertens. 13(10), 1209–1215 (1995).
Haak, T., Jungmann, E., Raab, C. & Usadel, K. H. Elevated endothelin-1 levels after cigarette smoking. Metabolism 43(3), 267–269 (1994).
Tanus-Santos, J. E., Sampaio, R. C., Hyslop, S., Franchini, K. G. & Moreno, H. Jr. Endothelin ET(A) receptor antagonism attenuates the pressor effects of nicotine in rats. Eur. J. Pharmacol. 396(1), 33–37 (2000).
Arnson, Y., Shoenfeld, Y. & Amital, H. Effects of tobacco smoke on immunity, inflammation and autoimmunity. J. Autoimmun. 34(3), J258–J265 (2010).
Papanicolaou, D. A. & Vgontzas, A. N. Interleukin-6: The endocrine cytokine. J. Clin. Endocrinol. Metab. 85(3), 1331–1333 (2000).
Levine, B., Kalman, J., Mayer, L., Fillit, H. M. & Packer, M. Elevated circulating levels of tumor necrosis factor in severe chronic heart failure. N. Engl. J. Med. 323(4), 236–241 (1990).
Yanbaeva, D. G., Dentener, M. A., Creutzberg, E. C., Wesseling, G. & Wouters, E. F. Systemic effects of smoking. Chest 131(5), 1557–1566 (2007).
Ussher, M., Kakar, G., Hajek, P. & West, R. Dependence and motivation to stop smoking as predictors of success of a quit attempt among smokers seeking help to quit. Addict. Behav. 53, 175–180 (2016).
Kottke, T. E., Battista, R. N., DeFriese, G. H. & Brekke, M. L. Attributes of successful smoking cessation interventions in medical practice. A meta-analysis of 39 controlled trials. JAMA 259(19), 2883–2889 (1988).
Fiore, M. C. et al. Treating Tobacco Use and Dependence: 2008 Update. Clinical Practive Guideline (US Department of Health and Human Services, Public Health Service, 2008).
Kowalczyk, A., Kleniewska, P., Kolodziejczyk, M., Skibska, B. & Goraca, A. The role of endothelin-1 and endothelin receptor antagonists in inflammatory response and sepsis. Arch. Immunol. Ther. Exp. (Warsz) 63(1), 41–52 (2015).
Johannsen, A., Susin, C. & Gustafsson, A. Smoking and inflammation: Evidence for a synergistic role in chronic disease. Periodontol 2000 64(1), 111–126 (2014).
Abrams, D. B. The Tobacco Dependence Treatment Handbook: A Guide to Best Practices xviii, 365 (Guilford Press, 2003).
Benowitz, N. L. Cotinine as a biomarker of environmental tobacco smoke exposure. Epidemiol. Rev. 18(2), 188–204 (1996).
Hukkanen, J., Jacob, P. 3rd. & Benowitz, N. L. Metabolism and disposition kinetics of nicotine. Pharmacol. Rev. 57(1), 79–115 (2005).
CDC. National Health Interview Survey (2017).
Spratt, J. C. et al. Systemic ETA receptor antagonism with BQ-123 blocks ET-1 induced forearm vasoconstriction and decreases peripheral vascular resistance in healthy men. Br. J. Pharmacol. 134(3), 648–654 (2001).
Schiffrin, E. L. Role of endothelin-1 in hypertension and vascular disease. Am. J. Hypertens. 14(6 Pt 2), 83S-S89 (2001).
Haynes, W. G. & Webb, D. J. Endothelin as a regulator of cardiovascular function in health and disease. J. Hypertens. 16(8), 1081–1098 (1998).
Davenport, A. P. et al. Endothelin. Pharmacol. Rev. 68(2), 357–418 (2016).
Golia, E. et al. Inflammation and cardiovascular disease: From pathogenesis to therapeutic target. Curr. Atheroscler. Rep. 16(9), 435 (2014).
Stenvinkel, P. et al. IL-10, IL-6, and TNF-alpha: Central factors in the altered cytokine network of uremia–the good, the bad, and the ugly. Kidney Int. 67(4), 1216–1233 (2005).
Petrescu, F., Voican, S. C. & Silosi, I. Tumor necrosis factor-alpha serum levels in healthy smokers and nonsmokers. Int. J. Chronic Obstr. Pulm. Dis. 5, 217–222 (2010).
Wannamethee, S. G. et al. Associations between cigarette smoking, pipe/cigar smoking, and smoking cessation, and haemostatic and inflammatory markers for cardiovascular disease. Eur. Heart J. 26(17), 1765–1773 (2005).
Reichert, V. et al. A pilot study to examine the effects of smoking cessation on serum markers of inflammation in women at risk for cardiovascular disease. Chest 136(1), 212–219 (2009).
Lorber, D. Importance of cardiovascular disease risk management in patients with type 2 diabetes mellitus. Diabetes Metab. Syndr. Obes. 7, 169–183 (2014).
Whelton, P. K. et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of High blood pressure in adults: A report of the American college of cardiology/American heart association task force on clinical practice guidelines. Hypertension 71(6), e13–e115 (2018).
Hardy, S. T. et al. Reducing the blood pressure-related burden of cardiovascular disease: Impact of achievable improvements in blood pressure prevention and control. J. Am. Heart Assoc. 4(10), e002276 (2015).
Lee, D. H., Ha, M. H., Kim, J. R. & Jacobs, D. R. Jr. Effects of smoking cessation on changes in blood pressure and incidence of hypertension: A 4-year follow-up study. Hypertension 37(2), 194–198 (2001).
Dhaun, N. et al. Role of endothelin-1 in clinical hypertension: 20 years on. Hypertension 52(3), 452–459 (2008).
Kellogg, D. L. Jr., Liu, Y. & Pergola, P. E. Selected contribution: Gender differences in the endothelin-B receptor contribution to basal cutaneous vascular tone in humans. J. Appl. Physiol. 91(5), 2407–2411 (2001) (discussion 389–90).
Ergul, S., Parish, D. C., Puett, D. & Ergul, A. Racial differences in plasma endothelin-1 concentrations in individuals with essential hypertension. Hypertension 28(4), 652–655 (1996).
Powell, J. T. Vascular damage from smoking: Disease mechanisms at the arterial wall. Vasc. Med. 3(1), 21–28 (1998).
Bakhru, A. & Erlinger, T. P. Smoking cessation and cardiovascular disease risk factors: results from the third national health and nutrition examination survey. PLoS Med. 2(6), e160 (2005).
Batel, P., Pessione, F., Maitre, C. & Rueff, B. Relationship between alcohol and tobacco dependencies among alcoholics who smoke. Addiction 90(7), 977–980 (1995).
Marcus, B. H. et al. The efficacy of exercise as an aid for smoking cessation in women: A randomized controlled trial. Arch. Intern. Med. 159(11), 1229–1234 (1999).
Filozof, C., Fernandez Pinilla, M. C. & Fernandez-Cruz, A. Smoking cessation and weight gain. Obes. Rev. 5(2), 95–103 (2004).
Acknowledgements
The authors would like to thank all the participants for their commitment to this research investigation.
Funding
This project was support in part by the Georgia Cancer Center, Augusta University to M. S. Tingen, PhD as PI.
Author information
Authors and Affiliations
Contributions
C.C.D. and S.J.S. performed the data analyses and drafted the manuscript. C.C.D. submitted the manuscript. M.S.T. was PI of the parent study including original design of the smoking cessation program. J.T. performed the blood analysis, primarily the measurements of ET-1 and inflammation. R.A.H., M.S.T., and M.A.T. provided conceptualization of the link between smoking cessation and ET-1. C.C.D., R.A.H., assisted in writing the manuscript methods, and the data collection. C.C.D., S.J.S., M.S.T., A.B., and R.A.H. assisted in editing the manuscript and creating figures. R.A.H. and M.S.T. provided overall supervision and final approval of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Derella, C.C., Tingen, M.S., Blanks, A. et al. Smoking cessation reduces systemic inflammation and circulating endothelin-1. Sci Rep 11, 24122 (2021). https://doi.org/10.1038/s41598-021-03476-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-03476-5
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.