Abstract
Secoiridoid glycosides are anti-feeding deterrents of the Oleaceae family recently highlighted as potential biomarkers in Danish ash trees to differentiate between those tolerant and susceptible to the fungal disease ash dieback. With the knowledge that emerald ash borer has recently entered Europe from Russia, and that extensive selection trials are ongoing in Europe for ash dieback tolerant European ash (Fraxinus excelsior), we undertook comprehensive screening of secoiridoid glycosides in leaf extracts of trees tolerant and susceptible to ash dieback sampled from sites in the UK and Denmark. Here we report an unexpected diversity of secoiridoid glycosides in UK trees and higher levels of secoiridoid glycosides in the UK sample group. While it is unlikely that secoiridoid glycosides generally can serve as reliable markers for ash dieback susceptibility, there are differences between tolerant and susceptible groups for specific secoiridoids. We predict that the high levels—and structural diversity—of secoiridoids present in the UK group may provide a robust reservoir of anti-feeding deterrents to mitigate future herbivore threats such as the Emerald ash borer.
Similar content being viewed by others
Introduction
Iridoid glycosides are a large class of natural products prevalent in the plant kingdom. Derived from the monterpenoid iridotrial, they can be broadly divided into two subclasses: iridoids, containing an intact cyclopentene ring, and secoiridoids1,2,3. Predominantly, the iridoid glycosides identified in the Oleaceae family, which ash (Fraxinus) and olive (Olea) genera belong to, are secoiridoids. Secoiridoids are characterised by a 10-carbon core skeleton in which the bond between C-7 and C-8 in the cyclopentene ring is cleaved, generating carboxylic acid and olefin moieties (Fig. 1)1,2,4. Substitutions of the secoiridoid core typically occur on the two carboxylic acid groups located at C7 and C11, in addition to glycosylation (usually glucose) on C1 of the iridoid core. Further oxidations may occur at C-102. The secoiridoid glycoside biosynthetic pathways have been partially elucidated in the Madagascar periwinkle (Catharanthus roseus) and olive (Olea europaea)5,6. Many secoiridoid glycosides have previously been isolated from ash leaves (Fraxinus genus) including oleuropein, nuzhenide, excelsioside, GL3 and GL57,8.
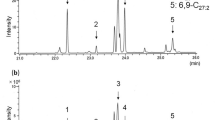
Summary of secoiridoid derived compounds previously identified from metabolite profiling. (a) General core structure of iridoid glycosides and secoiridoid glycosides (b) Fraxinus excelsior affected by ash dieback (mid-Devon, UK). (c) Extracted ion chromatograms m/z 589.1732 (1/P2/N2); 709.2315 (2/P5 and 3/P1/N4); 933.3007 (4/P7) representing [M+Na]+ adducts of compounds previously identified as markers of susceptibility to ADB in Danish ash27. (d) Positive ion mode mass spectra of 1–4.
Numerous biological activities for secoiridoid glycosides have been reported, including antioxidant activity associated with tyrosol and hydroxytyrosol groups9,10,11,12. Oleuropein, a major constituent of olive oil, has modest antibiotic activity and has been reported to stabilise α-synuclein, aggregation of which is a key step in the development of Parkinson’s disease13,14. There are numerous reports of aglycones and dialdehyde derivatives of oleuropein and ligustroside (ligstroside) acting as anti-feeding molecules15,16. Interestingly, secoiridoid glycoside biosynthetic genes are wound induced in common Centaury (Centaurium erythraea Rafn)17, and systemic responses to wounding and herbivory strongly overlap18.
European ash is currently under threat from ash dieback (ADB) caused by the fungus Hymenoscyphus fraxineus which has devastated ash across mainland Europe over the past 25 years19,20. ADB was first reported in the UK in 2012, though recent reports suggest H. fraxineus arrived as early as the late 1990s21. Meanwhile, across North America, the Emerald ash borer (EAB; Agrilus planipennis) has destroyed millions of trees of native ash species. EAB is currently found in Russia and Ukraine is likely to become a major pest in Europe22,23,24.
Whilst there has been extensive effort to identify robust DNA molecular markers for ADB tolerance or susceptibility, observation of disease states in the field is critical to provide the necessary germplasm to facilitate the development of robust genetic markers for ADB25. Recently, infrared spectroscopy has been shown to distinguish bark extracts of ash trees with low, intermediate and high susceptibility to ADB26. A complementary approach is to identify small molecule chemical markers. These may better reflect the susceptibility to ADB of a given genotype in the context of other biotic and abiotic factors in the location where the tree is growing and provide some mechanistic insight into disease and defence mechanisms of ADB. Untargeted metabolite screening of Danish ash leaf extracts identified five secoiridoid glycoside-like metabolites—P2/N2, P5, P1/N4, P7, N5, and N3, and the related metabolites P3/P4—that were more abundant in ADB susceptible ash trees27, leading to the hypothesis that if future breeding strategies selected ADB tolerant trees with reduced iridoid glycosides levels, then there may be inadvertent selection for trees with greater susceptibility to herbivore pests such as EAB.
To extend this study, we undertook a detailed investigation into the structural diversity of secoiridoid glycosides in UK F. excelsior leaf extracts from trees predicted to be tolerant and susceptible to ADB. Additionally, we compared secoiridoid glycoside profiles of UK and Danish ash to ascertain whether increased secoiridoid glycoside abundance is also observed in UK ash susceptible to ADB. We also profiled F. mandschurica secoiridoid glycosides, as both A. planipennis and H. fraxineus naturally co-exist on this species23,28,29.
Results
Identification of previously reported secoiridoid-like compounds in Danish ash
We first examined whether ADB discriminatory secoiridoid glycosides identified in the study of Danish ash were also (i) present in UK ash leaf samples, and (ii) more abundant in susceptible UK ash. We profiled UK samples from polytunnel grown grafts of trees identified in their natural environment, primarily Norfolk, as healthy trees amongst heavily infected and dying trees. Leaves were harvested in late July, similar to the previously sampled Danish trees. The original Danish and new UK leaf material was extracted in parallel (see Methods) and LC–MS/MS data acquired in positive and negative ion modes.
Extracted ion chromatograms were generated corresponding to the compounds 1–4 [where 1(P2/N2), 2(P5), 3(P1/N4) and 4(P7) relate to previous nomenclature putatively assigned to be iridoid glycosides]27. 1–4 were confirmed in positive and negative ion modes (Fig. 1 and Supplementary Fig. S1–S6). Compound 5 (N5 in Sollars et al.) was only observed in negative ion mode and is an adduct of 1(N2) having lost a hexose moiety from the [2M-H]− dimer formed in the electrospray source, Supplementary Fig. S427,30. N3 from Sollars et al. was not observed in any of our ash leaf extracts. However, a compound 6, was observed at the expected retention time for N3 and its peak intensity followed the same trend as observed for N3 with greater abundance observed in susceptible than tolerant Danish leaf extracts (Supplementary Fig. S5). The calculated molecular formula of 6, C18H25O13 could correspond to [N3-hydroxytyrosol]. We did not observe m/z 247.06 (C11H12O5Na) corresponding to the secoiridoid-like metabolites P3 and P4. However, peaks with m/z 225.0762 corresponding to [M+H]+ adducts (C11H13O5) were observed co-eluting with 1 and 3 (Supplementary Fig. S6), suggesting P3 and P4 may arise from in-source fragmentation of 1 and 3, respectively. The presence of 1–4 and 6 were confirmed in UK ash (Fig. 1, Supplementary S1, S5).
Identification of characteristic features in mass spectra of secoiridoid glycoside standards
To identify iridoid related leaf metabolites we first analysed commercial standards of iridoid glycosides, monotropein 7 and loganic acid 8, and secoiridoid glycosides nuzhenide 9, oleuropein 10, and GL3 11 by LC-HRMS/MS (Fig. 2 and Supplementary Fig. S7–S15). The predominant fragment ions in positive ion mode for secoiridoids 9–11 represented losses of 162 Da (C6H10O5) corresponding to loss of a glucosyl moiety and 194 Da (C7H14O6), corresponding to loss of a glucosyl and a methoxy group (Supplementary Fig. S7). MS2 spectra of monotropein 7 and loganic acid 8 feature a predominant peak corresponding to loss of 162 Da (C6H10O5). However, instead of corresponding to loss of 194 Da, the predominant peak corresponds to loss of 180 Da (C6H12O6), indicative of a glucosyl group and H2O, implying a hydroxyl group. Minor peaks corresponding to [M–C6H12O6+H]+ were also observed in the MS2 spectra of the secoiridoids 9–11 (Fig. 2 and Supplementary Fig. S7).
Mass spectra of iridoid glycoside standards in positive ion mode (a) and negative ion mode (b). (a) The predominant peaks arise from [M+Na]+ and major fragment ions arise from losses of C6H10O5 (162 Da) and C7H14O6 (194 Da). (b) The predominant pseudomolecular ion peak corresponds to [M–H]− and major fragment ions arise from a C11H11O5 (m/z 223) fragment and neutral losses of C10H16O6 (232 Da) and C17H22O10 (386 Da).
Full scan MS1 spectra for each compound 7–11 revealed two discrete peaks with m/z difference of 202—the [M+Na]+ adduct of each molecule, and a second peak [M–C6H12O6+H]+ arising from the in-source fragmentation31 and GL3(11) showed an additional peak at 713.2427, corresponding to [M–2C6H12O6+H]+. 9 and 10 showed the same peak at m/z 225.0658 as noted for 1–4 (Supplementary Fig.S7). In addition, the MS2 spectrum of oleuropein(10) has a minor fragment ion m/z = 265.0682 (formula C11H14O6Na), probably representing the secoiridoid aglycone core fragment.
In negative ion mode, MS1 spectra of the standards exhibit one predominant peak corresponding to [M–H]−. Fragment ions corresponding to loss of 232 Da (C10H16O6 arising from loss of the glucosyl moiety and rearrangement of the pyran ring)32,33 were observed in the [M–H]− MS2 spectra of secoiridoids 9–11 but not the C-10 iridoids 7 and 8 (Fig. 2 and Supplementary Fig. S8–S13). MS2 spectra of 7 and 8 exhibit loss of 224—C7H12O8—representing glucosyl, CO2 and OH groups (Fig. S9-10)34. Additional peaks in the MS2 spectra of nuzhenide(9) and GL3(11) m/z = 299.1131 and m/z = 685.2350, respectively, correspond to neutral loss of a 386 Da oleoside-11-methyl ester moiety, C17H22O1032,35. m/z = 299.1131 corresponds to a salidroside fragment C14H19O7, also observed in the MS2 spectrum of GL3(11) (Fig. 2). Minor peaks in the MS2 spectra of nuzhenide(9) and oleuropein(10) (m/z = 421.1502 and 275.0929 respectively) correspond to loss of C10H16O8 (264 Da). Supplementary Figures S9–S13 show assignments of fragment ions from the standards analysed. Additionally, MS2 spectra of the secoiridoids 9–11 but not the C-10 iridoids 7 and 8 featured m/z 223.0610, (C11H11O5) corresponding to the core secoiridoid aglycone moiety (Fig. 2 and Supplementary Fig. S9–S13). In negative ion mode, loganic acid eluted much earlier due to the eluent used for negative ion mode analyses (Supplementary Fig. S14).
Confirmation of 1–4 as secoiridoid glycosides and identification of additional secoiridoid glycosides in ash leaves
Using the above criteria, constant neutral loss chromatograms were generated for 162 Da in positive ion mode and 232 Da and 386 Da losses in negative ion mode for both Danish and UK ash leaf extracts. MS and MS2 spectra for compounds 1–4 were compared to the MS2 spectra of the standards, confirming 1–4 to be secoiridoids and leading to identification of 22 additional putative secoiridoid glycosides 12–33 (examples shown in Fig. 3 and Supplementary Fig. S15).
These findings are summarised in Table 1 and Supplementary Tables S1–S3. Fragment ions and neutral losses from the [M+Na]+ and [M–H]− ions for each compound 1–33 are visualised in network diagrams (Supplementary Fig. S16–S18 and Supplementary Tables S4 and S5). 6 (related to N3 from Sollars et al.) does have some shared fragments with some of the compounds 12–33 assigned as secoiridoids (Supplementary Fig. S17 and Table S5) however it lacks any of the characteristic neutral losses of 232, 264 or 386 observed for secoiridoids.
Of the 26 compounds 1–4 and 12–33 identified in the F. excelsior leaf extracts, 24 were observed in positive ion mode and 25 in negative ion mode. In positive ion mode, 22 compounds share a pair of fragment ions corresponding to neutral losses of 162 (C6H10O5; red edges in Supplementary Fig. S16) and 194 (C7H14O6; black edges in Supplementary Fig. S16) characteristic of the secoiridoid glycoside standards. The predominant pair of fragment ions in the MS2 spectra of 1 and 28, correspond to [M–2xC6H10O5+Na]+ and [M–C6H10O5–C7H14O6+Na]+, implying the loss of two glucosyl moieties (Supplementary Fig. S15). As seen in Supplementary Fig. S16, 9 compounds share a fragment ion m/z 265.0682 (C11H14O6Na), that was also observed in the oleuropein standard(10). Of these, 8 also have the fragment ion m/z = 233.0417 (C10H10O5Na). MS2 spectra of [M+Na]+ adducts of 10 compounds also have a peak at m/z 165.0550 (C9H9O3), possibly representing a fragment derived from a rearranged secoiridoid (Supplementary Fig. S16)36.
(a–f) Extracted ion chromatograms showing relative abundance of [M+Na]+ adducts of several iridoid glycosides identified in UK and Danish ash leaf extracts. Insets show low intensity EICs only for clarity. Venn diagrams showing relative distribution (fc threshold 1.5) of each compound between tolerant and susceptible genotypes of (g) UK trees and (h) Danish trees. Compounds shown in white have p value < 0.05 and compounds in yellow have p values < 0.1. 18 and 2 were only observed in 5/24 UK leaf extracts so are omitted from panel (g) and 20 was only observed in 7/18 Danish leaf extracts so is omitted from panel (h). Compounds proposed to be novel are boxed. 1–4 are P2/N2, P5, P1/N4 and P7, respectively from Sollars et al.27.
In negative ion mode, 13 compounds displayed fragment ions arising from neutral loss of 232 Da i.e.[M–C10H16O6–H]−, indicative of loss of a glucose moiety and rearrangement of the pyran ring characteristic of secoiridoids standards 9–11. Eleven of these compounds also exhibited [M–C10H16O8–H]− fragments corresponding to loss of 264 Da, as in nuzhenide(9) and oleuropein(10), whereas two compounds, 17 and 21, instead lose 248 Da (C10H16O7). Crucially, thirteen compounds lose a neutral 386 Da (C17H22O10) fragment, as observed in the MS2 spectra of nuzhenide(9) and GL3(11), indicative of a terminal oleoside-methyl ester moiety35 and eighteen compounds share fragment ion m/z 223.0610 (C11H11O5), corresponding to the secoiridoid aglycone core (Supplementary Fig. S17–S18 and Table S5).
Supplementary Figure S16 demonstrates the high degree of similarity between the compounds identified as secoiridoid glycosides, with isomers clustering together, for example 1 and 15 (C23H34O16); 21 and 23 (C25H32O13); 24 and 30 (C25H32O12); 2, 3, 18 and 20 (C31H42O17); and 4, 29 and 31 (C42H54O22). The MS2 spectra of both 1 and 15 suggest loss of 2 glucosyl units therefore 1 or 15 could be methlyglucooleoside (7-β-1-D-glucopyranosyl-11-methyl oleoside), previously isolated from F. excelsior leaves, or an isomer thereof7,35,38. Thus, if one structure is methlyglucooleoside, the other is novel. In positive ion mode, compounds 21 and 23 (C25H32O13) share fragment ions m/z 401, 385, 383, 369 and compounds 24 and 30 (C25H32O12) share a set of fragments m/z 385, 369, 367, 353, and 311. Compound 4 (P7; C42H54O22) shares fragments m/z 577, 609 and 753 with its isomer 31 in addition to the ion pair m/z 771 and 739 that are also present in the MS2 spectrum of the isomer 29.
In negative ion mode (Supplementary Fig. S17–S18), different fragmentation patterns of these isomers allows some structural inference. For example, compounds 21 and 23 have the same molecular formula as oleuropein, C25H32O13. 23 elutes slightly later, but with identical retention time and fragmentation patterns in positive and negative mode as the oleuropein standard (10), thus is assigned as oleuropein. Conversely, the fragmentation patterns of [M+Na]+ and [M–H]− adducts of 21 imply a core secoiridoid aglycone with one extra oxygen atom, i.e. m/z = 281 (C11H14O7Na) in positive and m/z 239 (C11H11O6) in negative ion modes (Supplementary Figs. S16–S19). This could be 10-hydroxyligustroside, previously identified in F. excelsior leaves7,36. The peak at m/z = 281 (C11H14O7Na) in positive ion mode is also present in the MS2 spectra of 12 (C17H24O12Na; assigned as 10-hydroxyoleoside methyl ester) and 17 (C25H32O14; assigned as 10-hydroxyoleuropein)36,38, which also has a peak at m/z 239 (C11H11O6) in negative ion mode.
Of the two isomers 24 and 30, (C25H32O12) 30 elutes later and has a pair of fragment ions m/z 259.0986 (C15H15O4) and m/z 291.0886 (C15H15O6) in negative ion mode, consistent with a secoiridoid aglycone containing a tyrosol moiety as in ligustroside, rather than hydroxytyrosol as in oleuropein (Supplementary Fig. S17, S19), thus is assigned as ligustroside. Conversely, 24, eluting earlier, has a different fragmentation pattern (Supplementary Figs. S17, S19) and is predicted to be excelsioside, where the iridoid C-7 is linked to the hydroxytyrosol moiety via the phenol O rather than the ethanolic O atom35,36. Both have previously been identified in F. excelsior leaves7.
The m/z 259/291 fragment ion pair appears in MS2 spectra of five other compounds, including 4 (P7 from Sollars et al.; C42H54O22) and its isomer 31. These are absent from the MS2 spectrum of the other isomer 29. 29 has a number of neutral losses (524 Da, 686 Da, 730 Da, 748 Da) shared with other compounds, (Supplementary Figs. S18, S20). These could be GL5, previously been reported from F. excelsior27,35, or isomers jaspolyanoside, austrosomide and/or 6′-elenolylnicotiflorine41,48,49. The m/z 259/291 fragment ion pair is also observed in the MS2 spectra of the nuzhenide isomers 2, 18 and 20, and are absent from their isomer 3. Both 2 and 3 have an additional peak m/z 299.1139, suggesting a salidroside moiety, also present in the nuzhenide standard (Fig. 3 and Supplementary Fig. S21).
Three secoiridoids (13, 26 and 32) eluted earlier in negative mode compared to positive ion mode, as did loganic acid(8), probably due to the presence of free carboxylic acid moieties in these compounds. Free COOH groups for 26 (C27H38O15) are consistent with frameroside/epi-frameroside isolated previously from F. americana44. 13 and 26 both have fragment ions with m/z differences of 44 Da (CO2) in their MS2 spectra and 32 loses 44 Da in negative ion mode. 32 also has a peak at m/z 667.2617 [M–H]− in its MS2 spectrum corresponding to neutral loss of 276 Da (C10H16O6 + CO2; Supplementary Figs. S17–S18, S22), as do 14 and 16 (assigned as demethyloleuropein and demethylligustroside, respectively). As the only known iridoid glycoside with the same molecular formula C43H60O23 as 32 is the secoiridoid pulosarioside, isolated from Alyxia reinwardtii46, which lacks a terminal carboxylic acid group, we predict 32 is a novel compound.
Of the remaining compounds, 28 and 33 have not previously been reported from F. excelsior and are assigned as jaspofoliamosides A and E, respectively, or isomers thereof41,47. 22 has the same formula as secoiridoids oleoacetoside and isooleoacetoside (C46H58O25)40,42, though only oleoacetoside has previously been reported from F. excelsior35 therefore we assign 22 as oleoacetoside. Several others (25, 27, 32) probably represent new compounds. The only known iridoid glycosides with the same molecular formula as 25 (C33H50O17) are jashemslosides C and D, from Jasminum hemsleyi though these are not secoiridoids43. However, characteristic neutral losses of 232 Da, 264 Da and 386 Da in the [M–H]− MS2 spectrum of 25 and losses of 162 Da and 194 Da in its [M+Na]+ MS2 spectrum all suggest 25 is a secoiridoid (Supplementary Figs. S16–S18, S23, Table 1). The molecular formula of 27 (C36H56O17) is consistent with an unnamed C-10 iridoid isolated from Rehmannia glutinosa45, although our data clearly indicate neutral losses of 162 Da and 194 Da in positive ion mode and neutral losses of 232 Da and 264 Da in negative ion mode, (Table 1, Supplementary Figs. S16–S18, S24) indicative of a secoiridoid. Therefore we predict that 27 is another new secoiridoid.
Distribution of secoiridoid glycoside metabolites in UK and Danish tolerant and susceptible F. excelsior leaf extracts and Manchurian ash
The relative distributions of each compound between tolerant and susceptible UK and Danish ash samples were assessed by overlaying their extracted ion chromatograms and by fold change and t test on normalised peak areas, as illustrated in Figs. 4 and 5 and Supplementary Table S6–S8. Compound 1 was more abundant in susceptible UK ash leaf extracts compared to tolerant leaf extracts (t = − 1.8511, p 0.0776, d.f = 22), (Fig. 4a), though the different abundance of 1 between the Danish susceptible and Danish tolerant groups were more pronounced (t = − 3.7532, p 0.00174, d.f = 16). 28 discriminates susceptible from tolerant UK leaf extracts sampled (t = − 2.1913, p 0.0393, d.f = 22) and Danish leaves sampled (t = − 1.7874, p 0.0928, d.f = 16). 24 and 30 (excelsioside and ligustroside, respectively; Fig. 4c) are more abundant in the UK compared to Danish leaves, with 24 being slightly enriched in the UK tolerant subgroup. Conversely, 12 may discriminate tolerance in Danish leaf extracts (Fig. 4e) whereas 32 discriminates tolerant from susceptible UK leaves sampled but not tolerant Danish leaves sampled (Fig. 4b). 3 (an isomer of nuzhenide; Fig. 4f) is more abundant in the Danish group and enriched in the highly susceptible subgroup, whereas 25 both discriminates susceptibility in UK leaf extracts, and is much more abundant in the UK leaf extracts compared to Danish leaf extracts (Fig. 4d).
(a) Heat map showing relative abundance of 1–34 in UK and Danish F. excelsior (top row) and presence/absence of 1–34 in F. mandschurica. Green and purple shading indicate the difference between peak areas of each compound in UK and Danish samples that have p value < 0.05. *p value < 0.1. Cells that are split indicate that there was a different outcome of the t-test for [M+Na]+ and [M–H]− adducts of these compounds. (b) Extracted ion chromatograms representing [M+Na]+ adducts of 1–33 observed in F. mandschurica.
The distribution of secoiridoids in susceptible and tolerant groups of UK and Danish trees analysed are illustrated in Fig. 4g and h. These data reveal a dramatic difference in the relative distribution of secoiridoids between the tolerant and susceptible UK and Danish tolerant and susceptible groups. Compounds 1 and 28 appear to discriminate susceptibility in both UK and Danish groups. Fold change analysis showed ten additional compounds 2, 3, 4 (original biomarkers of ADB), 13, 14, 18, 26, 27, 29 and 31 are more abundant in susceptible than tolerant Danish leaves sampled. However three of those compounds (26, 29 and 31) are more abundant in UK tolerant compared to susceptible trees sampled and the difference in abundance of 31 were not significant (p > 0.1). Two compounds (2 and 18) were only detected in 5/24 UK leaf extracts and 13 shows no difference between UK tolerant and susceptible trees sampled (Supplementary Figs. S25–S26). Conversely, of the remaining four compounds that distinguish the UK susceptible group (15, 22, 25 and 28), low abundance of 15 and 25 in Danish trees precluded discrimination between tolerant and susceptible groups and the greater level of 22 in the UK susceptible group was not statistically significant (p > 0.1) The other two compounds higher in abundance in the UK tolerant group, 24 and 32, do not clearly discriminate tolerance in the Danish group. Conversely, fold change analysis showed 12 was more abundant in Danish tolerant leaf extracts (Fig. 4e), although this difference was not statistically significant (p = 0.1122).
The differences in distribution of 1–4 and 12–33 between the UK and Danish populations are summarised in Fig. 4g and h and Fig. 5. In total, 13/26 secoiridoid glycosides were more abundant in UK compared to Danish leaf extracts (fold change > 1.5 and p value < 0.1), including one molecule (20) only detected in 7/18 of the Danish samples (Supplementary Fig. S1). In general, the secoiridoids more abundant in UK extracts tended to be larger and more structurally complex, with the exception of 15 (methlyglucooleoside or isomer) and 19 (oleoside dimethyl ester). By contrast, 6/26 secoiridoid glycosides were present in Danish leaf extracts in greater amounts (fold change > 1.5 and p value < 0.1). Two of these compounds, 2 and 18 were only observed in 5/24 of the UK samples, and of the remaining four, one is a smaller secoiridoid 12 (10-hydroxyoleoside 11-methyl ester).
We additionally profiled Fraxinus mandschurica leaf extracts by (i) generating extracted ion chromatograms for [M+Na]+ and [M–H]− for all secoiridoid glycosides 1–33 and (ii) constant neutral loss chromatograms for 162 Da and 194 Da in positive ion mode and 232 Da and 386 Da in negative ion mode. This revealed that 20 out of 26 of secoiridoid glycosides 1–33 were observed in F. mandschurica, including 12 compounds more abundant in UK than Danish F. excelsior extracts and 4 compounds that were more abundant in Danish F. excelsior extracts (Fig. 5). We identified an additional secoiridoid glycoside, 34, that has the same molecular formula as jaspolyoside and its isomers isojaspolyoside A-C50. Reanalysing UK and Danish spectra, 34 was detected in UK extracts and lower amounts detected in Danish extracts (Fig. 5 and Supplementary Fig. S27).
Discussion
This study, for the first time, comprehensively profiled secoiridoid glycosides in a panel of UK ash leaves and compared them to validated ADB susceptible and tolerant Danish leaf extracts. We significantly expand our original identification of four secoiridoid glycosides 1–4 enriched in susceptible Danish genotypes of F. excelsior to 27 F. excelsior secoiridoid glycosides. Seven of these had not been previously reported and at least four (25, 27, 32 and 1 and/or 15) are predicted to represent new structures2, 35,39. Our data reinforce our previous finding that compound 1 is a discriminatory metabolite of ADB susceptibility in both UK and Danish ash trees sampled. Notably, of the new secoiridoid glycosides identified; 15 and 25 are much more abundant in the UK group and, interestingly, discriminate susceptibility in the UK but not the Danish group. For compounds 27 and 32, there was no difference in abundance between samples when grouped by geographical origin. However, within the Danish sample group, 27 was more abundant in susceptible compared to tolerant leaf extracts, whereas within the UK sample group, 32 was more abundant in the tolerant compared to susceptible leaf extracts. Furthermore, we undertook the most comprehensive profiling of secoiridoids in F. mandschurica leaf extracts to date, substantially increasing the number of secoiridoids detected in this species to 2151,52,53.
A key finding is that secoiridoid abundance is generally not correlated with ADB tolerance or susceptibility. Rather, our comprehensive profiling enabled us to identify specific secoiridoid glycosides that may serve as potential biomarkers of ADB tolerance or susceptibility. Of the secoiridoids initially reported as markers of ADB susceptibility in Danish leaf extracts27, we confirmed 1 was also present in higher levels in leaf extracts from susceptible compared to tolerant UK trees sampled (Fig. 4), thus representing a possible robust biomarker of ADB susceptibility in European ash. Particularly interesting was the geographical specificity in distribution and abundance of secoiridoid glycosides. For example, compounds 26 and 29 were more abundant in susceptible Danish, yet more abundant in tolerant UK leaf extracts analysed, highlighting heterogeneity in distribution and raising the question of what selective advantage such heterogeneity confers. While a core subset of seven compounds (16, 17, 19, 21, 23, 30, 33) did not appear to distinguish ADB tolerance from susceptibility within either the UK or Danish leaves sampled, five of these compounds are more abundant in the UK compared to Danish leaf extracts, reflecting a geographic influence on these secoiridoid levels. Whether these have evolved due to biotic pressures in their respective environments needs further investigation. In summary, our results suggest there is greater abundance of secoiridoid glycosides in UK than Danish F. excelsior leaf extracts (Fig. 5), and notably, these are generally more structurally complex. This could arise from altered gene expression, or activity of enzymes utilising different secoiridoid biosynthetic intermediates in UK ash. Given the associated metabolic cost, the reason for this diversity in structure and abundance is of fundamental interest. It will be important to determine whether these differences discussed here are predictive of ADB tolerance and susceptibility in the wider UK population; larger scale sampling is planned to address this.
It is particularly striking that, whilst our study involved a relatively small number of genotypes and grafts of each genotype, both 15 (potentially the same structure as methlyglucooleoside) and 1 (N2) correlated to susceptibility to ADB in our study. This observation is in agreement with a recent study of bark extracts from a larger panel of F. excelsior54. Other commonalities included a molecule with the same HRMS m/z (943.3353) as 32 (although no MS2 data is available to validate this) that was enriched in tolerant ADB genotypes. Thus, 1, 15 and 32 may be robust markers of relative ADB susceptibility in bark and leaf tissues54. As observed in our Danish leaf extracts, 26 (frameroside/epi-frameroside) and one compound m/z = 909.2920 also had a weak association with ADB susceptibility. While it is tempting to speculate that this molecule has the same structure as 31 or 4, further chemical interrogation is needed to validate this prediction.
In its native environment, F. mandschurica co-exists with both EAB and ADB28,29,55, so it is interesting that our panel of European ash leaves showed greater diversity of secoiridoids than F. mandschurica with only 21 out of 27 F. excelsior secoiridoids identified in F. mandschurica. Of the six F. excelsior specific secoiridoids, two of them (2 and 18) were much more abundant in the Danish leaf extracts. Notably, 28 was enriched in susceptible populations of both UK (p = 0.0393) and Danish trees (p = 0.0928). Interestingly, 1, 3 and 4, enriched in susceptible Danish F. excelsior genotypes, were all observed in F. mandschurica leaf extracts.
In a previous study of Swedish F. excelsior seedlings treated with the H. fraxineus toxin viridiol, oleuropein 23(10) levels were lower in an ADB tolerant genotype, whereas demethylligustroside 16 and demethyloleuropein 14 accumulated more in another tolerant genotype. However the overall differences in secoiridoid profile between tolerant and susceptible populations were much more ambiguous39. In our study of older, unchallenged trees, 16 and 23 did not discriminate tolerant from susceptible genotypes, although 14 was slightly enriched in Danish susceptible trees (Fig. 4; p = 0.0846).
Whilst EAB larvae feed in the phloem, adults feed on ash leaves. If secoiridoids do contribute to EAB tolerance as feeding deterrents, UK trees may exhibit greater tolerance as higher levels of the secoiridoids identified were observed in the UK leaves sampled. While detailed feeding studies are needed to assess this, biological activity may be further complicated by subsequent chemical modification upon herbivory of this diverse repertoire of secoiridoids16. Interestingly, the diversity of secoiridoids in F. excelsior identified here and in bark extracts is greater than that reported in green and white ash species native to North America2,44,52,54. F. excelsior may be more tolerant to EAB than Fraxinus species native to North America as recently reported56, consistent with complementary studies showing that adult A. planipennis has a much greater feeding preference for leaves of green, white and black ash compared to Manchurian and European ash57. It remains to be determined whether F. excelsior in European forests is more or less tolerant to A. planipennis than F. pennsylvanica24. Clearly, establishing any biological function for the diversity of the secoiridoids identified in leaf (this study) and bark extracts of F. excelsior on tolerance or susceptibility to EAB requires further analytical dissection. Our study, along with the recent analysis of F. excelsior bark secoiridoids54 provides the necessary analytical framework to further pursue such studies and represents a complementary approach to the FT-IR based identification of trees tolerant and susceptible to ADB using bark extracts26. A future objective will be to increase sampling density and geographic distribution of F. mandschurica and F. excelsior leaf extracts for secoiridoid profiling.
In conclusion we found an unexpected diversity of secoiridoids in European F. excelsior leaf extracts. While secoiridoid glycosides collectively are not suitable biomarkers to distinguish ADB susceptible from tolerant UK ash trees, we identify several specific secoiridoid glycosides enriched in trees sampled from different locations that could be potential markers of ADB susceptibility (3 and 27 in Danish ash and 25 in UK ash) and tolerance (32 in UK ash), with 1 and 28 emerging as robust markers of susceptibility in both sample groups. Encouragingly, the diversity and geographic variability of secoiridoid glycosides uncovered in these European F. excelsior samples may prove valuable reservoirs of antifeeding deterrents in mitigating the future threat of EAB. We currently lack knowledge of differences in the overall metabolic landscape of ADB tolerant and susceptible genotypes in a comprehensive, representative sample set. To address this, untargeted analysis of UK ash leaf extracts is underway to find further discriminatory metabolites that could be used as biomarkers for ash dieback susceptibility and tolerance among the wider UK population.
Methods
UK F. excelsior samples were grafted trees taken from woodlands that were deemed to be tolerant (four genotypes—BERTIE, CHRISTINA, RABBIT, VITALSTATISTIX), having survived in locales with high levels of disease and are surrounded by trees that are dead or dying from ADB. These individuals were monitored for leaf and bark damage at least twice per year from 2015. As of December 2019, these trees are still alive in their native environments. UK trees classified as susceptible (four genotypes—Fex203014, Fex203015, Fex303003, Fex405001) have succumbed to the disease in their native woodland. Three ramets (clones) of each genotype were sampled. Trees were grafted in February and March 2016 and transferred to a polytunnel. Leaves were harvested from three separate grafts of each genotype in July 2017, snap frozen in liquid nitrogen and lyophilised. Danish leaf samples were prepared from same leaf material used in Sollars et al.27,30. Leaf material from 18 month old grafts of three each of the tolerant (HGH-A, HGH-C, HGH-D) and susceptible (UGH-F, UGH-G, UGH-H) genotypes were selected. Manchurian ash samples were prepared from leaf material harvested from grafted F. mandschurica trees in 2014 (three samples). Lyophilised leaf material was ground to powder and 10 mg aliquots were extracted on ice in 400 µL 80% methanol containing 2.5 µg/mL d5-IAA then once more in 400 µL 80% methanol and supernatants combined, as reported in Sollars et al.27.
Samples were submitted to UPLC-HRMS analysis using a Dionex UltiMate 3000 UHPLC system and Agilent Eclipse Plus C18 UPLC column (2.1 × 150 mm, 1.8 µm particle size) with outflow routed to a Bruker MaXis II Q-TOF with an electrospray source. Samples were run in positive ion mode and then in negative ion mode. Solvents A and B were water and acetonitrile, respectively. For positive ion mode analyses, 0.1% formic acid was added to the mobile phase and for negative ion mode analyses, 0.1% ammonia was added. Compounds were eluted using the following gradient: isocratic elution with 95% solvent A and 5% solvent B for 5 min, followed by a gradient elution to 100% solvent B over min 28.7 min. 5 µL sodium formate (10 mM) was loop-injected as an internal standard. Samples were also analysed using the same elution profile in auto MS/MS mode with the three most intense peaks selected for MS2 data acquisition after each full scan. Standards of oleuropein, nuzhenide, GL3 and loganic acid were purchased from Extrasynthese (France) and monotropein was purchased from PhytoLab (Germany). Indole-2,4,5,6,7-d5-3-acetic acid (d5-IAA) was purchased from Sigma Aldrich (UK). A mixture of 10 µg/mL each of the iridoid glycoside standards was prepared in 80% methanol and submitted to UPLC-MS/MS analysis.
Peak areas for ions of interest were extracted using XCMS58 and checked by manually picking peaks in Bruker Compass Data Analysis. Misshaped peaks (i.e. for ions with closely eluting isomers or poorly defined peak shapes) were integrated manually using the peak-picking tool in Compass Data Analysis. Statistical analyses were performed using MetaboAnalyst v4.0 ( https://www.metaboanalyst.ca/ ).59 Peak areas were normalised to the Na(NaCOOH)3 adduct (m/z = 226.9515) of the sodium formate internal standard (positive ion mode) and the HCOO(NaCOOH)4 adduct (m/z = 316.9479) in negative ion mode. Missing values were replaced with 1/5 intensity of the minimum observed intensity for that compound. For the data in Fig. 4, samples were grouped into ‘tolerant’ and ‘susceptible’ groups and analysed separately based on location (UK and Denmark) and samples were grouped by geographical location for Fig. 5. Peak areas for each compound were submitted to fold change analysis (threshold 1.5) and t-test. Supplementary Tables S6–S8 show fc, t and p values used for generating Fig. 4 and 5.
Data availability
The datasets generated during and analysed during the current study are available from the corresponding author on reasonable request.
References
Dinda, B. Pharmacology and Applications of Naturally Occurring Iridoids (Springer, Berlin, 2019). https://doi.org/10.1007/978-3-030-05575-2.
Huang, Y. L. et al. The Oleaceae family: a source of secoiridoids with multiple biological activities. Fitoterapia 136, 104155 (2019).
Wang, C. et al. Iridoids: research advances in their phytochemistry, biological activities, and pharmacokinetics. Molecules 25, 287 (2020).
Jensen, S. R., Franzyk, H. & Wallander, E. Chemotaxonomy of the oleaceae: iridoids as taxonomic markers. Phytochemistry 60, 213–231 (2002).
Miettinen, K. et al. The seco-iridoid pathway from Catharanthus roseus. Nat. Commun. 5, 3606 (2014).
Alagna, F. et al. Identification and characterization of the iridoid synthase involved in oleuropein biosynthesis in olive (Olea europaea) fruits. J. Biol. Chem. 291, 5542–5554 (2016).
Damtoft, S., Franzyk, H. & Jensen, S. R. Excelsioside, a secoiridoid glucoside from Fraxinus excelsior. Phytochemistry 31, 4197–4201 (1992).
Egan, P. et al. GI 5, a dimer of oleoside, from Fraxinus excelsior (Oleaceae). Biochem. Syst. Ecol. 32, 1069–1071 (2004).
De Marco, E., Savarese, M., Paduano, A. & Sacchi, R. Characterization and fractionation of phenolic compounds extracted from olive oil mill wastewaters. Food Chem. 104, 858–867 (2007).
Cardoso, S. M., Falcão, S. I., Peres, A. M. & Domingues, M. R. M. Oleuropein/ligstroside isomers and their derivatives in Portuguese olive mill wastewaters. Food Chem. 129, 291–296 (2011).
Schaffer, S. et al. Hydroxytyrosol-rich olive mill wastewater extract protects brain cells in vitro and ex vivo. J. Agric. Food Chem. 55, 5043–5049 (2007).
Medina, E., Brenes, M., Romero, C., García, A. & De Castro, A. Main antimicrobial compounds in table olives. J. Agric. Food Chem. 55, 9817–9823 (2007).
Palazzi, L. et al. Oleuropein aglycone stabilizes the monomeric α-synuclein and favours the growth of non-toxic aggregates. Sci. Rep. 8, 1–17 (2018).
Mohammad-Beigi, H. et al. Oleuropein derivatives from olive fruit extracts reduce—Synuclein fibrillation and oligomer toxicity. J. Biol. Chem. 294, 4215–4232 (2019).
Konno, K., Hirayama, C., Yasui, H. & Nakamura, M. Enzymatic activation of oleuropein: a protein crosslinker used as a chemical defense in the privet tree. Proc. Natl. Acad. Sci. USA 96, 9159–9164 (1999).
Volk, J. et al. Two novel methylesterases from Olea europaea contribute to the catabolism of oleoside-type secoiridoid esters. Planta 250, 2083–2097 (2019).
Božunović, J. et al. Secoiridoids metabolism response to wounding in common centaury (Centaurium erythraea rafn) leaves. Plants 8, 1–18 (2019).
Toyota, M. et al. Glutamate triggers long-distance, calcium-based plant defense signaling. Science 80(361), 1112–1115 (2018).
Semizer-Cuming, D., Krutovsky, K. V., Baranchikov, Y. N., Kjӕr, E. D. & Williams, C. G. Saving the world’s ash forests calls for international cooperation now. Nat. Ecol. Evol. 3, 141–144 (2019).
Hill, L. et al. The £15 billion cost of ash dieback in Britain. Curr. Biol. 29, R315–R316 (2019).
Wylder, B., Biddle, M., King, K., Baden, R. & Webber, J. Evidence from mortality dating of Fraxinus excelsior indicates ash dieback (Hymenoscyphus fraxineus) was active in England in 2004–2005. Forestry 91, 434–443 (2018).
Abella, S. R. et al. Fourteen years of swamp forest change from the onset, during, and after invasion of emerald ash borer. Biol. Invasions 21, 3685–3696 (2019).
Valenta, V., Moser, D., Kapeller, S. & Essl, F. A new forest pest in Europe: a review of Emerald ash borer (Agrilus planipennis) invasion. J. Appl. Entomol. 141, 507–526 (2017).
Orlova-Bienkowskaja, M. J. et al. Current range of Agrilus planipennis Fairmaire, an alien pest of ash trees, in European Russia and Ukraine. Ann. For. Sci. https://doi.org/10.1007/s13595-020-0930-z (2020).
Menkis, A. et al. Identifying Fraxinus excelsior tolerant to ash dieback: Visual field monitoring versus a molecular marker. For. Pathol. 50, 5–8 (2020).
Villari, C. et al. Advanced spectroscopy-based phenotyping offers a potential solution to the ash dieback epidemic. Sci. Rep. 8, 1–9 (2018).
Sollars, E. S. A. et al. Genome sequence and genetic diversity of European ash trees. Nature 541, 212–216 (2017).
Cleary, M. et al. Friend or foe? Biological and ecological traits of the European ash dieback pathogen Hymenoscyphus fraxineus in its native environment. Sci. Rep. 6, 1–11 (2016).
Inoue, T. et al. The life cycle of Hymenoscyphus fraxineus on Manchurian ash, Fraxinus mandshurica, in Japan. Mycoscience 60, 89–94 (2019).
Sambles, C. M. et al. Ash leaf metabolomes reveal differences between trees tolerant and susceptible to ash dieback disease. Sci. Data 4, 170190 (2017).
Kostova, M. B., Konaklieva, M. I., Alipieva, K. I., Popov, S. S. & Handjieva, N. V. ESIMS study of some C 10 iridoid glycosides. Instrum. Sci. Technol. 33, 691–702 (2005).
Michel, T. et al. UHPLC-DAD-FLD and UHPLC-HRMS/MS based metabolic profiling and characterization of different Olea europaea organs of Koroneiki and Chetoui varieties. Phytochem. Lett. 11, 424–439 (2015).
Zhong, X. et al. Metabolomics approach based on ultra-high-performance liquid chromatography coupled with quadrupole-time-of-flight mass spectrometry to identify the chemical constituents of the Traditional Chinese Er-Zhi-Pill. J. Sep. Sci. 40, 2713–2721 (2017).
Kucharska, A. Z. & Fecka, I. Identification of iridoids in edible honeysuckle berries (lonicera caerulea L. var. kamtschatica Sevast) by UPLC-ESI-qTOF-MS/MS. Molecules 21, 1157 (2016).
Kiss, A. K., Michalak, B., Patyra, A. & Majdan, M. UHPLC-DAD-ESI-MS/MS and HPTLC profiling of ash leaf samples from different commercial and natural sources and their in vitro effects on mediators of inflammation. Phytochem. Anal. 31, 57–67 (2020).
Song, J., Zhao, L., Rui, W., Guo, J. & Feng, Y. Identification and fragmentation pattern analysis of iridoid glycosides from Fructus ligustri lucidi by UPLC/ESI-QTOF-MS. J. Liq. Chromatogr. Relat. Technol. 37, 1763–1770 (2014).
Shen, Y., Lin, C. & Chen, C. Secoiridoid glycosides from Jasminum multiflorum. Phytochemistry 29, 2905–2912 (1990).
Kiss, A. K., Mańk, M. & Melzig, M. F. Dual inhibition of metallopeptidases ACE and NEP by extracts, and iridoids from Ligustrum vulgare L. J. Ethnopharmacol. 120, 220–225 (2008).
Cleary, M. R. et al. Genotypes of Fraxinus excelsior with different susceptibility to the ash dieback pathogen Hymenoscyphus pseudoalbidus and their response to the phytotoxin viridiol—a metabolomic and microscopic study. Phytochemistry 102, 115–125 (2014).
Takenaka, Y., Tanahashi, T., Taguchi, H., Nagakura, N. & Nishi, T. Nine new secoiridoid glucosides from Jasminum nudiflorum. Chem. Pharm. Bull. 50, 384–389 (2002).
Tanahashi, T. et al. Six secoirioid glucosides from Jasminum polyanthum. Chem. Pharm. Bull. 45, 367–372 (1997).
Shen, Y. C., Lin, S. L. & Chein, C. C. Jaspolyside, a secoiridoid glycoside from Jasminum polyanthum. Phytochemistry 42, 1629–1631 (1996).
Tanahashi, T. et al. Structure elucidation of six acylated iridoid glucosides from Jasminum hemsleyi. Chem. Pharm. Bull. 43, 729–733 (1995).
Takenaka, Y. et al. Secoiridoid glucosides from Fraxinus americana. Phytochemistry 55, 275–284 (2000).
Liu, Y. F. et al. Hepatoprotective iridoid glycosides from the roots of Rehmannia glutinosa. J. Nat. Prod. 75, 1625–1631 (2012).
Kitagawa, I. et al. Pulosarioside, a new bitter trimeric-iridoid diglucoside, from an Indonesian jamu, the bark of Alyxia reinwardtii bl. (Apocynaceae). Chem. Pharm. Bull. 36, 4232–4235 (1988).
Takenaka, Y., Tanahashi, T. & Nagakura, N. Eight minor secoiridoid glucosides with a linear monoterpene unit from Jasminum polyanthum. Chem. Pharm. Bull. 46, 1776–1780 (1998).
Benkrief, R. et al. Monoterpene alkaloids, iridoids and phenylpropanoid glycosides from Osmanthus austrocaledonica. Phytochemistry 47, 825–832 (1998).
Liu, X. et al. Chemical constituents from the fruits of Ligustrum lucidum. Chem. Nat. Compd. 46, 701–703 (2010).
Tanahashi, T., Takenaka, Y. & Nagakura, N. Two dimeric secoiridoid glucosides from Jasminum polyanthum. Phytochemistry 41, 1341–1345 (1996).
Qazi, S. S., Lombardo, D. A. & Abou-Zaid, M. M. A metabolomic and HPLC-MS/MS analysis of the foliar phenolics, flavonoids and coumarins of the Fraxinus species resistant and susceptible to emerald ash borer. Molecules 23, 1–19 (2018).
Eyles, A. et al. Comparative phloem chemistry of manchurian (Fraxinus mandshurica) and two North American ash species (Fraxinus americana and Fraxinus pennsylvanica). J. Chem. Ecol. 33, 1430–1448 (2007).
Whitehill, J. G. A. et al. Interspecific comparison of constitutive ash phloem phenolic chemistry reveals compounds unique to manchurian ash, a species resistant to emerald ash borer. J. Chem. Ecol. 38, 499–511 (2012).
Nemesio-Gorriz, M. et al. Candidate metabolites for ash dieback tolerance in Fraxinus excelsior. J. Exp. Bot. 71, 6074–6083 (2020).
Villari, C., Herms, D. A., Whitehill, J. G. A., Cipollini, D. & Bonello, P. Progress and gaps in understanding mechanisms of ash tree resistance to emerald ash borer, a model for wood-boring insects that kill angiosperms. New Phytol. 209, 63–79 (2016).
Showalter, D. N. et al. Resistance of European ash (Fraxinus excelsior) saplings to larval feeding by the emerald ash borer (Agrilus planipennis). Plants People Planet 2, 41–46 (2020).
Pureswaran, D. S. & Poland, T. M. Host Selection and Feeding Preference of Agrilus planipennis (Coleoptera: Buprestidae) on Ash (Fraxinus spp.). Environ. Entomol. 38, 757–765 (2009).
Smith, C. A., Want, E. J., O'Maille, G., Abagyan, R. & Siuzdak, G. XCMS: Processing mass spectrometry data for metabolite profiling using nonlinear peak alignment, matching, and identification. Anal. Chem. 78, 779–787 (2006).
Chong, J. et al. MetaboAnalyst 4.0: towards more transparent and integrative metabolomics analysis. Nucleic Acids Res. 46, W486–W494 (2018).
Acknowledgements
This work was supported by BBSRC UKRI grants BB/N021452/1 (M.G & D.J.S), BB/R018944/1 (M.G) and the Elizabeth Creak Charitable Trust (M.G & J.D.S). J.L.P was funded by the NERC Research Experience Placement scheme. The authors wish to thank Martha Clarke and Prof. James Brown (John Innes Centre) and Dr. Robert Saville (East Malling Research) for providing the UK and Manchurian ash samples, and Prof. Erik Kjær and Dr. Lea McKinney (University of Copenhagen) for Danish ash leaf material.
Author information
Authors and Affiliations
Contributions
J.D.S, L.S and M.G designed experiments. D.J.S. and C.S provided preliminary data and analyses. J.D.S, J.L.P and M.G prepared samples and L.S ran the mass spectrometer. J.D.S, L.S and J.L.P analysed data. J.D.S, L.S and M.G wrote the manuscript. M.G. provided the photograph in Fig. 1. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sidda, J.D., Song, L., Parker, J.L. et al. Diversity of secoiridoid glycosides in leaves of UK and Danish ash provide new insight for ash dieback management. Sci Rep 10, 19566 (2020). https://doi.org/10.1038/s41598-020-76140-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-76140-z
This article is cited by
-
Anti-Herbivore Activity of Oregonin, a Diarylheptanoid Found in Leaves and Bark of Red Alder (Alnus rubra)
Journal of Chemical Ecology (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.