Abstract
Pancreatic cystic neoplasms (PCNs) are a heterogeneous group with varying risks of malignancy. To explore the clinical utility of liquid biopsy in cyst type classification, we analyzed the GNAS/KRAS mutations in circulating cell-free DNA (cfDNA) obtained from 57 patients with histologically diagnosed PCNs, including 34 with intraductal papillary mucinous neoplasms (IPMNs) and compared the mutant allele prevalence and variant patterns with the paired resected specimens using next-generation sequencing. The positive prevalence of GNAS mutations in cfDNA of patients with IPMN (n = 11, 32%) was significantly higher than that in those with other PCNs (0%, P = 0.002). Conversely, KRAS mutations were detected in cfDNA of only 2 (6%) IPMN patients. The paired-sample comparison revealed highly concordance between the GNAS mutation status of cfDNA and resected IPMN specimens. Similar distributions of GNAS mutation positivity in cfDNA were observed across the different histological grades, whereas IPMNs with intestinal subtype showed a significantly higher prevalence of GNAS mutations than other subtypes (P = 0.030). GNAS mutation positivity in cfDNA was significantly associated with the acellular mucin pool of histological findings in primary IPMN lesions (P = 0.017). Detection of GNAS mutation in cfDNA can serve as a novel biomarker for cyst type classification and differentiation of intestinal subtype IPMN from the other PCNs.
Similar content being viewed by others
Introduction
The prevalence of pancreatic cystic neoplasms (PCNs) has been increased due to a consequence of the greater use of high-quality, cross-sectional abdominal imaging1. PCNs represent heterogeneous group of diseases with variable malignant potential and several clinical guidelines have been adopted to assist clinicians in determining the management in each cyst type2,3,4. Serous cystic neoplasms (SCNs) are usually benign and have low potential for malignancy, and when asymptomatic, can be managed through observation. However, resection at the time of diagnosis without any surveillance is recommended for mucinous cystic neoplasms (MCNs) and solid pseudopapillary neoplasms (SPNs), both of which have high malignancy potential. Intraductal papillary mucinous neoplasms (IPMNs) exhibit a wide spectrum of histological transformation ranging from low-grade dysplasia (LGD) to invasive carcinoma (INV). Only high-grade dysplasia (HGD) and INV are recommended to be resected and LGD should be managed by surveillance5,6,7. Taken together, the major challenges for the better practical management of PCNs is the accurate stratification of cyst type and their risk assessment for the presence of malignancy. Currently, a set of clinical and radiologic parameters based on the clinical guidelines are used to diagnose, risk-stratify, and manage PCNs3,5,8. Nevertheless, there is a need for better identification of cysts with malignant potential. Pancreatic resection is an invasive procedure and it is associated with a significant risk of morbidity and mortality, which may be unnecessary when the cyst has no malignant potential. Therefore, finding potential reliable predictors for cyst type classification and malignancy risk must be the focus for clinicians.
Previous reports have revealed potential biomarkers in predicting cyst type and malignancy risk9,10,11. The PCNs were well-characterized by the mutational profiles with high specificity in each cyst type12. For example, VHL alterations were unique to SCNs, CTNNB1 mutations were found in SPNs and GNAS mutations in IPMNs13. The identification of these PCN-associated genes from patients’ samples, such as cyst fluid and pancreatic juice samples, provides strong diagnostic information for the classification of cyst type14,15. As a less-invasive and simpler approach, detection of gene mutations in circulating cell-free DNA (cfDNA) from the patients with PCNs was investigated by Berger and colleagues, and they demonstrated that the GNAS mutant alleles were detectable specifically in IPMN patients with high positive prevalence (~ 70%), but not KRAS mutants16. However, no data were available regarding the differences in GNAS/KRAS mutant positivity in the cfDNA of patients with different histological grades of IPMNs. Furthermore, the reasons for the markedly higher prevalence of GNAS mu tations than KRAS in the cfDNA remain unclear.
Herein, we aimed to clarify the utility of GNAS/KRAS genotyping using cfDNA to identify the IPMNs differentiating from other PCNs and to segregate high-risk lesions. Furthermore, to explore the pathogenesis of the GNAS mutant allele presence in their systemic circulation, we also evaluated the clinical and histological features of primary IPMN lesions in the cases representing the GNAS mutant alleles in cfDNA.
Results
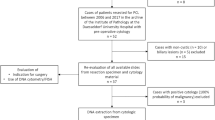
A total of 57 enrolled patients underwent pancreatic resection and all PCNs were histologically proven in terms of cyst type classification and grade of dysplasia. Of the 57 PCNs, 34 patients had IPMN, 9 had MCN, 10 had SPN, and 4 had SCNs (Table 1). Clinical features of the age, sex, and tumor location in each cyst type were similar to previous evidence2. The PCNs with HGD/INV were found in 27 of 34 IPMN patients and only one of 9 MCN patients (Table 1).
Figure 1 shows the overall GNAS/KRAS mutations detected in cfDNA and paired resected specimens of surgically aspirated cyst fluids or tissues from the patients with PCNs. Only one case (#51), with low-quality and unavailable tissue sample, was excluded from further genetic analysis. Next-generation sequencing revealed GNAS and KRAS mutations were found in 23 (70%) and 25 (76%) of the 33 IPMN resected specimens, respectively (Fig. 1). The median yields of cfDNA in plasma was 7.5 ng (range 3.0–24.4) per mL of plasma. The GNAS mutations in cfDNA were detected in 11 (32%) of the all 34 IPMN patients and 10 (43%) of the 23 IPMN patients harboring GNAS mutation in their primary lesions (Fig. 1). The representative cases harboring GNAS mutation in cfDNA are shown in Fig. 2. Paired comparison of mutant allele frequencies (MAFs) of GNAS between cfDNA and resected specimen revealed the much higher MAF in resected specimen than cfDNA; however, not all cfDNA positive cases showed the highly abundance of GNAS mutant alleles in their primary lesion (Supplementary Figure S1). No GNAS mutations were identified in cfDNA from the patients with non-IPMN PCNs of MCN, SPN, and SCN. The KRAS mutations in cfDNA were detected in only 2 (6%) of the 34 IPMN patients and both of two also had KRAS mutation in their IPMN lesions (Fig. 1). No KRAS mutations were detected in cfDNA of patients with non-IPMN PCNs. One case (#64) with MCN with LGD harbored the 7% of KRAS mutant alleles in the surgically aspirated cyst fluid.
Illustration of overall mutational status in cell-free DNA and resected specimens for all enrolled patients with pancreatic cystic neoplasms. IPMN, intraductal papillary mucinous neoplasm; MCN, mucinous cystic neoplasm; SPN, solid-pseudopapillary neoplasm; SCN, serous cystic neoplasm; LGD, low-grade dysplasia; HGD, high-grade dysplasia; INV, invasive carcinoma; GAS, gastric subtype; INT, intestinal subtype; PB, pancreatobiliary subtype; ONC, oncocytic subtype; N/A, not applicable; CF, surgically aspirated cyst fluid sample; T, tussue sample. cfDNA, circulating cell-free DNA; WT, wild-type; Mut, mutant positive.
Representative cases harboring GNAS mutation of R201H (A) and R201C (B) in both cfDNA and resected specimen. In the upper side of 2-D plot, pink lines showed the thresholds of fluorescent value of FAM (blue droplets, mutant type) and HEX (green droplets, wild-type). Lower illustration in each case is a representation of the reads aligned to the reference genome, as provided by the Integrative Genomics Viewer (IGV) software for the hotspot mutations in the GNAS codon 201.
We then compared the prevalence of GNAS mutant positivity in cfDNA with that in paired resected specimen. All IPMN cases positive for GNAS mutations in cfDNA harbored identical mutation patterns in their resected specimens. All IPMN cases without GNAS mutations in their resected specimens had only GNAS wild-type alleles in their cfDNA. Of the 11 cases with GNAS mutation positive in their cfDNA, heterogeneous mutations were detected in 3 cases. Among these 3 cases, 1 (#59) had apparent multicentric branch-duct IPMNs with dilatation of main pancreatic duct, suggesting the possibility of polyclonality across the multicentric IPMNs (Supplementary Figure S2).
Despite the strong concordance of GNAS mutational patterns between cfDNA and tissue specimens of the same individual, not all IPMN cases harboring GNAS mutations in their primary lesion presented GNAS mutation positive in cfDNA. We then evaluated the specific features of IPMNs harboring GNAS mutations in cfDNA. No significant differences of clinical findings of primary IPMN lesions were seen between the cases with GNAS mutant positive in cfDNA and negative (Supplementary Table S1). The positive prevalence of GNAS mutation in cfDNA showed similar distribution across the different histological grades (Fig. 3A). With respect to histological phenotype of mucin-hypersecretion, GNAS mutation detection in cfDNA had a significantly higher positive prevalence in cases with intestinal subtype than in cases with other subtypes (P = 0.030, Fig. 3B). Although their prevalence was low (only two positive cases), KRAS mutant alleles were detected in cfDNA of only cases with HGV/INV (Fig. 3C). Moreover, the IPMN cases with intestinal subtype showed a trend of higher values of GNAS mutant allele frequencies in their cfDNA than those with other subtypes (P = 0.069, Fig. 4). In contrast to the GNAS, no IPMN cases with intestinal subtypes showed KRAS mutant alleles in cfDNA (Fig. 3D).
Positive prevalence of GNAS mutations in cell-free DNA and resected specimens according to the histological grade (A) and histological subtype (B). Likewise, KRAS mutant positivity in cfDNA stratified by the histological grade (C) and subtype (D). LGD, low-grade dysplasia; HGD, high-grade dysplasia; INV, invasive carcinoma; PB, pancreatobiliary subtype; ONC, oncocytic subtype; GAS, gastric subtype; INT, intestinal subtype. cfDNA, circulating cell-free DNA.
The GNAS mutant allele frequency in cfDNA from the IPMN cases with or without intestinal subtypes. The longer horizontal bar represents the median value. Dotted line represents the 0.2%, approximately correspondence to 2 positive droplets. cfDNA; circulating-ceil free DNA; INT, intestinal subtype. cfDNA, circulating cell-free DNA.
To test whether the GNAS mutant alleles detected in cfDNA were derived from the primary IPMN lesions, we compared the positive prevalence and allele frequency of the GNAS mutant between pre- and post-operative pairs of blood samples. Among the 10 evaluable cases, only 3 showed the GNAS mutation in cfDNA before pancreatic resection. After pancreatic resection, no GNAS mutations were found in any of the 3 cases (Table 2).
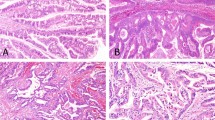
Based on the highly prevalent GNAS mutation in cases of IPMN with intestinal subtype, we evaluated the association between GNAS mutant positivity in cfDNA and local histological findings. The presence of acellular mucin pool lacking neoplastic epithelium was more frequently found in primary lesions from the IPMN cases with GNAS mutant positive cfDNA than those with negative (P = 0.017, Fig. 5A,C). Rupture of pancreatic ducts was also evaluated, and no significant difference in frequency was observed between the IPMN cases with GNAS mutant positive and negative in cfDNA (P = 0.363, Fig. 5B,D).
Association of GNAS mutant positivity in cfDNA and histological findings of primary IPMN lesions. The presence of acellular mucin pool (A) and rupture of ducts (B) in cases with or without GNAS mutation in their cfDNA. Representative histological images of acellular mucin pool (C) and rupture of ducts (D).
Discussion
Appropriate management of patients with PCNs depends on the accurate classification of the cysts. Because of the increasing detection of asymptomatic PCNs, a novel non-invasive, repeatable, and reliable diagnostic test are urgently needed. Despite the insufficiently accurate for predicting the histological grades of IPMN, GNAS mutation detection in plasma cfDNA from patients with PCNs can be an evolving tool distinguishing IPMN from other types of PCNs such as MCN, SCN, and SPN. Furthermore, to the best of our knowledge, this is the first study to report following the two notable findings: first, mutant GNAS in cfDNA was derived from the primary IPMN lesion based on the genetic analysis using the resected specimens and pre- and post-operative blood samples. Second, GNAS mutant positivity in cfDNA is attributable to the histological disruption and mucin-hypersecretion at the primary IPMNs, especially known as intestinal subtype.
Despite the lower prevalence, the GNAS mutation pattern of missense substitution in cfDNA was highly concordant with the resected specimens. This result suggests that not all IPMNs harboring GNAS mutation are detectable by liquid-biopsy; however, GNAS mutation detection in cfDNA is a 100% positive predictive value for the presence of IPMN since there was no IPMN case in which GNAS mutation was positive in the cfDNA but negative in tissues. Furthermore, no GNAS mutations were detected from available postoperative blood samples. Taken together, GNAS mutant in cfDNA, definitively derived from the primary lesions harboring the identical mutation, can serve as a specific predictor of IPMN differentiating from other PCNs.
A few cases showed mutational heterogeneity of GNAS in cfDNA even though homogeneous mutation patterns were found in the primary IPMN lesions. As shown in Supplementary Figure S2, only one patient (#59) with multiple IPMNs underwent pancreatectomy for only high-risk lesions in body and tail of the pancreas. This patient harbored the low-risk branch-duct IPMN in the remnant pancreas head. Regrettably, a postoperative blood sample was unavailable and, therefore, it was difficult to further investigate whether the mutational heterogeneity of GNAS in cfDNA is mainly attributable to the difference in the mutation pattern between the resected and remained IPMNs. One more possible explanation for the heterogeneous mutations in cfDNA is presence of minute and invisible IPMN, the so-called incipient IPMN, in the remnant pancreas. Another possible reason is intratumoral heterogeneity with polyclonal nature during the unifocal IPMN development.
Our study is notable for the significant difference in the positive prevalence between the KRAS and GNAS mutations in cfDNA. Despite the similar positive prevalence between the KRAS and GNAS mutations (70–80%) in the resected IPMN specimens, a much lower prevalence of KRAS mutation than that of GNAS was detected in cfDNA. Recent studies have revealed that circulating pancreatic epithelial cells were found in peripheral blood samples collected from the patients of IPMN without invasion; however, the GNAS/KRAS mutational status for those circulating cells is still unelucidated17,18,19. Definitive reasons remain unclear; however, quite different prevalence of mutant positivity between KRAS and GNAS in cfDNA can be explained by a difference in biological signatures according to the histological subtypes.
Intestinal subtype IPMN, highly prevalent GNAS mutations, has been recognized to represent a quite different biological behavior compared to other histological subtypes20,21,22. A representative feature of intestinal subtype is mucin-hypersection, associated with a dilated papilla with mucin extrusion23. Intestinal subtype also tended to be observed in main-duct type with high-grade dysplasia21. Furthermore, once IPMN with intestinal subtype invade to stroma, invasive component act as a mucinous carcinoma, better prognosis than tubular carcinoma derived from IPMN with other histological subtypes24. On the other hand, pancreatobiliary subtype IPMNs are known to have aggressive biological behaviors with inconspicuous mucin secretion and highly prevalent KRAS mutation21,24,25, explaining why two IPMN cases with HGD/INV (case #90 and #111) harboring the KRAS mutant in cfDNA showed wild-type GNAS genotype in the primary lesions. Taken together, as shown in Figs. 3 and 4, we classified the histological subtypes in terms of the mucin-hypersecretion and compared intestinal with non-intestinal subtypes. Notably, the present study supports one possible mechanism that the mucin-hypersecreting IPMNs induce local tissue disruption with acellular mucin pool containing high abundance GNAS mutant alleles, then involve the local microvessels, and finally enter the bloodstream. Further investigation is required to explore whether the “mechanical” tissue disruption, characterized by GNAS mutation, is more prone to induce bloodstream entry than the “invasive” tissue disruption with KRAS mutation.
Based on the strong evidence that the recurrent GNAS mutation is specific for IPMN26,27, recent studies investigated GNAS mutation detection in other biological specimens such as cyst fluid and duodenal and pancreatic juice samples of patients with PCNs15,28,29,30,31. These studies revealed that a higher positive prevalence of GNAS mutation than reported in the present study. In fact, mutation detection sensitivity depends on the anatomical distance from the primary lesion and the lower positive prevalence in peripheral blood samples than in the cyst fluid and juice samples might be inevitable. Nevertheless, blood sample is easier to harvest and is less invasive and can be more applicable to pancreatic screening. Theoretically, combination of blood-based screening with cyst-based detailed examination appeared to be the best diagnostic approach. To overcome low sensitivity, a combination of GNAS mutation positivity with novel biomarkers can be explored in future studies.
This study is based on prospectively collected samples harvested from the consecutive patients, leading to minimal selection biases. The most critical limitation of this study is the small sample size, particularly non-IPMN samples such as MCN, SPN, and SCN. The second major limitation of our study is the lack of patients undergoing surveillance. This study include only patient undergoing pancreatic resection and therefore, the use of a surgical case series leads to an enriched study population for IPMN with HGD/INV (27 of 34 cases, 79%). The detection of GNAS mutations in cfDNA before the emergence of a visible IPMN highlights the potential of liquid-biopsy to help in the risk stratification of patients undergoing pancreatic screening and surveillance.
In conclusion, we revealed that the detection of GNAS mutations in cfDNA from peripheral blood of patients with pancreatic cysts is a highly specific indicator for IPMNs, specifically the intestinal subtype. In the future, the prospective large-scale validation including patients with PCNs being considered for resection or surveillance is needed.
Materials and methods
Patients and specimens
From November 2017 to September 2019, 57 consecutive patients with PCNs who underwent surgical resection in Tohoku University Hospital were prospectively enrolled in our observational study and biological specimens such as peripheral blood, cyst fluid, and tissue samples were collected. This study was approved by the Ethical Committee for genetic studies of the Tohoku University Graduate School of Medicine (institutional review board approval number is 2019-1-119). All eligible patients during the course of the study gave written informed consent prior to participation. All research was performed in accordance with relevant guidelines and regulations.
Patients’ blood samples were obtained in the week before surgery using PAX gene ccfDNA tube (PreAnalytiX, Hombrechtikon, Switzerland). The postoperative blood samples were collected just before the discharge. Regarding the resected specimens, we basically used the surgically aspirated cyst fluid samples; however, not all IPMN cases had surgically aspirated cyst fluid samples due to the technical difficulty of sampling or insufficient DNA yields. Only for cases with unavailable cyst fluid samples, we evaluated the mutations using the resected tissue samples. All cyst fluid samples were aspirated from the resected specimens in the operating room immediately after the pancreatic resection using a fine needle sterile syringe and were transferred on ice to the laboratory generally within one hour, where it was aliquoted and stored at − 80 °C until further use. Blood samples were immediately processed to isolate plasma by centrifugation at 1900g for 15 min at room temperature and plasma samples were aliquoted and stored. All of cyst fluid, blood, and tissue samples were subjected to linkable anonymization. All experiments were conducted in a blinded fashion, without any prior knowledge of pathological diagnosis.
Clinical and histological data assessment
Demographic, pathological, and clinical data were collected from a prospectively maintained database and medical records. All resected specimens were histologically evaluated by two of the authors (Y.O. and T.F.) specializing in pancreas pathology and graded as either LGD, HGD or INV based on criteria defined by a recent consensus32. Histological subtypes based on the morphological findings of intraductal proliferation and mucin expression were classified as described previously20. As a semi-quantitative histological evaluation, we defined the histology score as the number of slide sections with presence of specific findings, such as acellular mucin pool and rupture of pancreatic ducts.
DNA extraction
Before DNA extraction, second centrifugation for further plasma clearance was performed at 16,000g for 10 min at 4 °C to remove cellular debris. The cfDNA was extracted from 4 mL of plasma using the QIAamp Circulating Nucleic Acid Kit (QIAGEN, Hilden, Germany) according to the manufacturer’s instructions. Genomic DNA from cyst fluid (DNeasy blood and tissue kit, QIAGEN) and the formalin-fixed paraffin-embedded tissue (QIAamp DNA FFPE Tissue Kit, QIAGEN) were also extracted. Extracted DNA was quantified by a SYBR Green real-time PCR based method (LINE1-assay) in order to assess the amount of amplifiable DNA as described elsewhere33. Standard calibration curve was valid for fivefold serial dilution of human genomic DNA (Promega, Madison, WI).
Droplet digital PCR
KRAS mutation in cfDNA were examined using droplet digital PCR (ddPCR) system and the ddPCR KRAS multiscreening kit (Bio-Rad, Hercules, CA), which covers seven types of mutations in KRAS codon 12/13 (G12A, G12C, G12D, G12R, G12S, G12V and G13D). Mutation of GNAS p.R201C and p.R201H were also examined using respective PrimePCR™ ddPCR™ Mutation Assay (Bio-rad). Because the input cfDNA amount affects the detection sensitivity, we adjusted the loading cfDNA amount with dilution and applied ~ 10 ng into ddPCR as a template. In some cfDNA samples with relatively low quantity, maximum volume of 8.0 μL was applied. Pre-mix preparation, droplet generation, and thermal cycling were performed according to the manufacturer’s instructions. The fluorescence intensity in droplets was detected by a QX200 Droplet Reader (Bio-Rad). For all assays, no template controls were run to determine lack of contamination. We also used the DNA from PANC-1 and BxPC-3 cells as positive controls of KRAS mutant and wild, respectively (Supplementary Figure S3). Positive controls of GNAS wild type and mutant (R201C and R201H) were obtained as plasmids after the T/A cloning of PCR products from primary resected IPMN tissue samples. QuantaSoft version 1.7.4 analysis software (Bio-Rad) was used for data acquisition and analysis. Only tests providing more than 10,000 droplets were used for analysis. The threshold for distinguishing positive from negative droplets was manually determined. Specific amplification of wild type and each mutant of GNAS were validated using multiple positive and negative control samples (Supplementary Figure S3). To ensure the specific amplification and detection, all assays with ≥ 2 mutant droplets were considered positive for the KRAS and GNAS mutation.
Next-generation sequencing
The targeted sequencing was performed using Ion AmpliSeq technology (Thermo Fisher Scientific Inc., Waltham, MA, USA). and Ion AmpliSeq Cancer Hotspot Panel v.2 (Thermo Fisher Scientific), which contains 207 primer pairs and targets approximately 2800 hotspot mutations for 50 cancer-related genes. Sequencing libraries were prepared using the 5 ng of DNA and Ion AmpliSeq Library Kit Plus with Ion Xpress™ Library Barcode Adaptors (Thermo Fisher Scientific). Amplified libraries were cleaned up by Agencourt AMPure XP Reagent (Beckman Coulter Life Sciences, Brea, CA, USA). Emulsion PCR was performed on Ion OneTouch™ 2 System, followed by the template-positive Ion Sphere Particles enrichment on the Ion OneTouch™ ES Instrument (Thermo Fisher Scientific) according to the manufacturer's instructions. Sequencing was performed on the Ion Torrent Personal Genome Machine (PGM; Thermo Fisher Scientific) using 316v2 chips. Post-sequencing data processing, including alignment to the hg19 human reference genome and variant calling, were conducted by Torrent Suite version 5.0 software and Torrent Variant Caller (Thermo Fisher Scientific). Alignments and putative mutations were visually verified using the Integrative Genomics Viewer (IGV, v2.3; Broad Institute, Cambridge, MA, USA).
Statistical analysis
All statistical analysis was performed using the JMP Pro 15.0.0 statistical software (SAS Institute Inc., Cary, NC, USA) and GraphPad Prism Version 8.4.3 (GraphPad Software, San Diego, CA, USA). Continuous and categorical variables were reported as medians and as whole numbers and percentages. We used the non-parametric Mann–Whitney U test to compare continuous variables. Categorical variables were analyzed using the Fisher's exact test. P values < 0.05 was considered statistically significant.
References
van Huijgevoort, N. C. M., Del Chiaro, M., Wolfgang, C. L., van Hooft, J. E. & Besselink, M. G. Diagnosis and management of pancreatic cystic neoplasms: current evidence and guidelines. Nat. Rev. Gastroenterol. Hepatol. 16, 676–689 (2019).
Tanaka, M. et al. International consensus guidelines 2012 for the management of IPMN and MCN of the pancreas. Pancreatology 12, 183–197 (2012).
Vege, S. S. et al. American gastroenterological association institute guideline on the diagnosis and management of asymptomatic neoplastic pancreatic cysts. Gastroenterology 148, 819–822; quize812–813 (2015).
Del Chiaro, M. et al. European experts consensus statement on cystic tumours of the pancreas. Dig. Liver Dis. 45, 703–711 (2013).
Tanaka, M. et al. Revisions of international consensus Fukuoka guidelines for the management of IPMN of the pancreas. Pancreatology 17, 738–753 (2017).
Seo, N. et al. Validation of the 2012 international consensus guidelines using computed tomography and magnetic resonance imaging: branch duct and main duct intraductal papillary mucinous neoplasms of the pancreas. Ann. Surg 263, 557–564 (2016).
Hirono, S. & Yamaue, H. Surgical strategy for intraductal papillary mucinous neoplasms of the pancreas. Surg. Today 50, 50–55 (2020).
European Study Group on Cystic Tumours of the, P. European evidence-based guidelines on pancreatic cystic neoplasms. Gut 67, 789–804 (2018).
Raman, A. & Lennon, A. M. Cyst fluid biomarkers - diagnosis and prediction of malignancy for cystic lesions of the pancreas. Visc. Med. 34, 178–181 (2018).
Hata, T. et al. Diagnostic and prognostic impact of neutrophil-to-lymphocyte ratio for intraductal papillary mucinous neoplasms of the pancreas with high-grade dysplasia and associated invasive carcinoma. Pancreas 48, 99–106 (2019).
Tulla, K. A. & Maker, A. V. Can we better predict the biologic behavior of incidental IPMN? A comprehensive analysis of molecular diagnostics and biomarkers in intraductal papillary mucinous neoplasms of the pancreas. Langenbecks Arch. Surg. 403, 151–194 (2018).
Maker, A. V. et al. Cyst fluid biomarkers for intraductal papillary mucinous neoplasms of the pancreas: a critical review from the international expert meeting on pancreatic branch-duct-intraductal papillary mucinous neoplasms. J. Am. Coll. Surg. 220, 243–253 (2015).
Ohtsuka, T. et al. Clinical assessment of the GNAS mutation status in patients with intraductal papillary mucinous neoplasm of the pancreas. Surg. Today 49, 887–893 (2019).
Singhi, A. D. et al. Preoperative next-generation sequencing of pancreatic cyst fluid is highly accurate in cyst classification and detection of advanced neoplasia. Gut 67, 2131–2141 (2018).
Springer, S. et al. A combination of molecular markers and clinical features improve the classification of pancreatic cysts. Gastroenterology 149, 1501–1510 (2015).
Berger, A. W. et al. Detection of hot-spot mutations in circulating cell-free DNA From patients with intraductal papillary mucinous neoplasms of the pancreas. Gastroenterology 151, 267–270 (2016).
Rhim, A. D. et al. Detection of circulating pancreas epithelial cells in patients with pancreatic cystic lesions. Gastroenterology 146, 647–651 (2014).
Franses, J. W. et al. Improved detection of circulating epithelial cells in patients with intraductal papillary mucinous neoplasms. Oncologist 23, 121–127 (2018).
Poruk, K. E. et al. Circulating epithelial cells in intraductal papillary mucinous neoplasms and cystic pancreatic lesions. Pancreas 46, 943–947 (2017).
Furukawa, T. et al. Classification of types of intraductal papillary-mucinous neoplasm of the pancreas: a consensus study. Virchows Arch. 447, 794–799 (2005).
Furukawa, T. et al. Prognostic relevance of morphological types of intraductal papillary mucinous neoplasms of the pancreas. Gut 60, 509–516 (2011).
Hosoda, W. et al. GNAS mutation is a frequent event in pancreatic intraductal papillary mucinous neoplasms and associated adenocarcinomas. Virchows Arch. 466, 665–674 (2015).
Hata, T. et al. Dilated papilla with mucin extrusion is a potential predictor of acute pancreatitis associated with intraductal papillary mucinous neoplasms of pancreas. Pancreatology 13, 615–620 (2013).
Adsay, N. V. et al. Pathologically and biologically distinct types of epithelium in intraductal papillary mucinous neoplasms: delineation of an “intestinal” pathway of carcinogenesis in the pancreas. Am. J. Surg. Pathol. 28, 839–848 (2004).
Tan, M. C. et al. GNAS and KRAS mutations define separate progression pathways in intraductal papillary mucinous neoplasm-associated carcinoma. J. Am. Coll. Surg. 220, 845-854 e841 (2015).
Wu, J. et al. Recurrent GNAS mutations define an unexpected pathway for pancreatic cyst development. Sc.i Transl. Med. 3, 92ra66 (2011).
Furukawa, T. et al. Whole-exome sequencing uncovers frequent GNAS mutations in intraductal papillary mucinous neoplasms of the pancreas. Sci. Rep. 1, 161 (2011).
Hata, T. et al. Predicting the grade of dysplasia of pancreatic cystic neoplasms using cyst fluid DNA methylation markers. Clin. Cancer Res. 23, 3935–3944 (2017).
Kanda, M. et al. Mutant GNAS detected in duodenal collections of secretin-stimulated pancreatic juice indicates the presence or emergence of pancreatic cysts. Gut 62, 1024–1033 (2013).
Takano, S. et al. Deep sequencing of cancer-related genes revealed GNAS mutations to be associated with intraductal papillary mucinous neoplasms and its main pancreatic duct dilation. PLoS ONE 9, e98718 (2014).
Mateos, R. N. et al. Genomic analysis of pancreatic juice DNA assesses malignant risk of intraductal papillary mucinous neoplasm of pancreas. Cancer Med. 8, 4565–4573 (2019).
Basturk, O. et al. A revised classification system and recommendations from the Baltimore Consensus Meeting for Neoplastic Precursor Lesions in the Pancreas. Am. J. Surg. Pathol. 39, 1730–1741 (2015).
Diehl, F. et al. Analysis of mutations in DNA isolated from plasma and stool of colorectal cancer patients. Gastroenterology 135, 489–498 (2008).
Acknowledgements
We thank Biomedical Research Unit of Tohoku University Hospital for technical support. This work was supported by Japan Society for the Promotion of Science (JSPS) KAKENHI Grant number 18K16338.
Author information
Authors and Affiliations
Contributions
T.H. designed the study. T.H., M.M., F.M., Y.O., M.I., K.N., H.H., M.M. and T.F. collected and assessed the data. T.K., T.F., and M.U. contributed to writing the discussion. All authors have approved the final version of the article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hata, T., Mizuma, M., Motoi, F. et al. GNAS mutation detection in circulating cell-free DNA is a specific predictor for intraductal papillary mucinous neoplasms of the pancreas, especially for intestinal subtype. Sci Rep 10, 17761 (2020). https://doi.org/10.1038/s41598-020-74868-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-74868-2
This article is cited by
-
Pancreatic Cancer Biomarkers: Oncogenic Mutations, Tissue and Liquid Biopsies, and Radiomics—A Review
Digestive Diseases and Sciences (2023)
-
Circulating tumour DNA: a challenging innovation to develop “precision onco-surgery” in pancreatic adenocarcinoma
British Journal of Cancer (2022)
-
A case of ectopic pancreas of the stomach accompanied by intraductal papillary mucinous neoplasm with GNAS mutation
World Journal of Surgical Oncology (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.