Abstract
Clinical biomarkers can predict normalization of HbA1c after Roux-en-Y gastric bypass (RYGB) surgery, but it is unclear which are the most predictive.The aim of this study was to compare biomarkers for insulin sensitivity and other clinical parameters in the prediction of normalization of HbA1c after RYGB surgery. This study included 99 (23 men) obese subjects (BMI > 35 kg/m2) undergoing a laparoscopic RYGB. Clinical and biochemical examinations were performed pre-operatively and up to 2 years after surgery. Pre-operatively, normal fasting glucose levels were found in 25 individuals (NG), prediabetes in 46 and type 2 diabetes (T2DM) in 28. At baseline IGF-I (SD), IGFBP-1 and adiponectin levels were low while leptin was high. Weight loss was observed in all three groups, most in the prediabetes group. After 2 years HbA1c was decreased in prediabetes and T2DM. In all three groups insulin, HOMA-IR, lipids and blood pressure improved, IGFBP-1 and adiponectin increased and leptin decreased. IGF-I (SD) increased only in T2DM. In those with prediabetes or T2DM (n = 74), HbA1c at 2 years correlated to baseline BMI (r = -0.27, p = 0.028), age (r = 0.43, p < 0.001), HbA1c (r = 0.37, p = 0.001) and IGFBP-1 (r = 0.25, p = 0.038), and was normalized in 45/74 (61%) at 1 year and in 36 subjects (49%) at 2 years. These responders were younger, had higher BMI, larger waist circumference, lower HbA1c and lower IGFBP-1 levels at baseline. In a multiple regression model age (negative, p = 0.021) and waist circumference (positive, p = 0.047) were the only predictors for normalized HbA1c. RYGB normalized HbA1c in 49% at two years follow-up, which was predicted by low baseline IGFBP-1 level, a marker of hepatic insulin sensitivty and insulin secretion. However,. younger age and larger waist circumference were the only predictors of normalized HbA1c in multivariate analysis.
Similar content being viewed by others
Introduction
The incidence of obesity is increasing at an alarming rate worldwide1. Obesity is associated with premature mortality and risk of comorbidities such as insulin resistance (IR), type 2 diabetes mellitus (T2DM), hypertension, cardiovascular disease, musculoskeletal disorders and some types of cancer2. Lifestyle modification and pharmacological therapy result in insufficient long-term weight loss in most obese patients, leaving bariatric surgery as a possible method to achieve long-term substantial weight loss3,4,5. There are several, randomized studies which clearly demonstrate that bariatric surgery is superior to best medical practice in treatment of severe obesity related comorbidities. In addition, long-term, non-randomized studies have demonstrated significant benefits of bariatric surgery on comorbidities6,7. One main purpose of bariatric surgery is to normalize blood glucose and/or reduce IR and thus prevent future cardiovascular disease and T2DM8. Since there are some severe side effects as well as recurrence of T2DM after bariatric surgery9,10, it is important to find simple predictors to identify which subjects will benefit the most from bariatric surgery with normalization of HbA1c and/or insulin sensitivity. It has been shown that fasting insulin level can be a better predictor than BMI of the outcome11.
Serum biomarkers of insulin sensitivity and insulin secretionare are adiponectin, insulin-like growth factor (IGF) bindingprotein-1 (IGFBP-1), IGF-I and leptin12,13,14,15,16,17,18,19. Insulin regulates the synthesis of IGFBP-1, which is one of the binding proteins for IGF-I regulating its activity14. IGF-I, regulated by growth hormone, insulin and amino acid, is of importance for glucose homeostasis through enhancing glucose uptake in muscles and by increasing insulin sensitivity14. IGFBP-1, produced in the liver, has both IGF dependent and independent effects20, which may explain the finding that conditions characterized by hyperinsulinemia such as insulin resistance and metabolic syndrome are associated with decreased levels of IGFBP‐118,21,22. Fasting IGFBP-1 is a marker of hepatic insulin sensitivity23 and low levels of IGFBP-1 predict development of prediabetes and T2DM24,25,26,27. In T2DM the IGFBP-1 levels are increased due to beta-cell dysfunction with decreased portal insulin delivery to the liver. Adiponectin produced in the adipose tissue negatively regulated by inflammatory cytokines is also marker of insulin sensitivity and low levels predict T2DM12,16. Leptin produced in the adipose tissue and a marker of adiposity and insulin sensitivity4,15. High levels are associated with both leptin resistance and Insulin resistance4.
The aim of this study was to evaluate biomarkers of insulin sensitivity and other clinical parameters which can predict normalization of HbA1c as primary enpoint and improvement of insulin sensitivity as secondary endpoint after Roux-en-Y gastric bypass (RYGB) surgery in subjects with normal glucose levels (NG), prediabetes (preDM) or T2DM. Thus, we have studied fasting levels of IGF-I, IGFBP-1, adiponectin and leptin as well as HOMA-IR before and 1 year after surgery and anthropometric data and metabolic factors before, 1 and 2 years after surgery.
Results
Baseline characteristics
There were no differences between the three groups with regard to BMI, weight or waist circumference (Table 1). The T2DM and PreDM groups were significantly older (p < 0.001 for both groups), and had significantly higher fasting plasma (fP) glucose (p < 0.001 for both groups), HbA1c (p < 0.001 for both groups), fasting serum (fS) insulin and HOMA-IR compared to the NG group. T2DM had significantly higher glucose (p < 0.001) and HbA1c (p < 0.001) levels compared to the PreDM group.
Baseline levels of biomarkers of insulin sensitivity after 2 weeks of caloric restriction
There were no differences between the three groups with regards to mean IGF-I (SD), IGFBP-1, leptin or adiponectin at baseline (Table 2). Therefore, we analysed the mean levels of biomarkers at baseline for all 99 subjects together. In the whole group the mean IGF-I (SD) level, IGFBP-1 and adiponectin level were all low while the mean leptin level was high (Table 2). Four (4%) of the subjects had normal leptin levels (BMI 35.6 ± 2.6 kg/m2), 21 (21%) had normal adiponectin (BMI 41.4 ± 1.5 kg/m2) and 33 (33%) had normal IGFBP-1 levels after 2 weeks of caloric restriction.
Metabolic improvements 1 and 2 years after RYGB
Significant weight loss and decrease in BMI were observed in all three groups at 12 and 24 months after RYGB (Table 3), which was accompanied by significant decrease in waist circumference and improvement in fP-glucose and HbA1c at 12 months. The PreDM group had lost most weight compared to the NG and T2DM groups. After 24 months the mean HbA1c level in subject with normal glucose levels (NG) was at the baseline level, while the mean HbA1c levels in subject with PreDM and T2DM were lower compared to the pre-operative levels (p < 0.001 for both groups)(Table 3). Mean plasma levels of insulin were decreased 12 months after RYGB in all three groups. Mean values of insulin sensitivity determined as HOMA-IR improvd to normal values in all three groups. The lipid profiles were improved in all three groups at 12 months after surgery and remained improved after 24 months except for LDL in T2DM. The systolic and diastolic blood pressure was significantly lower at 12 and 24 months (Table 3).
Changes in biomarkers of insulin secretion and sensitivity (IGF-I, IGFBP-1, adiponectin and leptin) 1 year after RYGB surgery
IGF-I (SD) increased significantly after RYGB in T2DM but not in the NG and PreDM groups. Mean IGFBP-1 and adiponectin levels increased significantly to normal values in all three groups (p < 0.001 for all groups) and leptin decreased significantly in all three groups (p < 0.001 for all groups)(T able 4).
Correlations between clincal parameters including markers of insulin sensitivity/secretion at baseline-and HbA1c 2 years after RYGB
In those with abnormal glucose levels, the combined group of PreDM and T2DM (n = 74), the HbA1c levels 2 years after surgery correlated inversely to baseline BMI (r = − 0.27, p = 0.028) and positively to preoperative age (r = 0.43, p < 0.001), preoperative HbA1c (r = 0.37, p = 0.001) and to IGFBP-1 (r = 0.25, p = 0.038) but not to preoperative glucose, IGF-I (SD), lipids, adiponectin, insulin levels or HOMA-IR. There was a tendency to an inverse correlation between preoperative leptin levels and HbA1c 2 years after surgery (r = − 0.23, p = 0.060).
Comparisons between those with and without normalized HbA1c at 1 and 2 years
The HbA1c levels were normalized (< 39 mmol/mol) in 45 out of 74 (61%) subjects with abnormal glucose levels 1-year post-op. The number decreased to 36 subjects (49%) 2 years post-op. The group with normalized HbA1c 2 years after RYGB were at baseline compared to to the group who did not normalize HbA1c significantly younger (mean age 46.8 ± 1.7 years vs 54.0 ± 1.6 years, p = 0.004), had significantly higher BMI (mean 44.0 ± 1.2 kg/m2 vs 40.6 ± 5.6 kg/m2, p = 0.040), larger waist circumference (mean 133.1 ± 2.7 cm vs 124.5 ± 2.5 cm, p = 0.028), lower HbA1c (mean 44.9 ± 2.1 mmol/mol compared to 49.4 ± 2.7 mmol/mol, p = 0.027) and lower IGFBP-1 levels (mean 18.0 ± 2.3 μg/L compared to 21.6 ± 2.7 μg/L, p = 0.050). All other biomarkers were at baseline not different between responders and non-responders.
In a stepwise multiple regression model with preoperative age, BMI, waist, glucose, HbA1c, HOMA-IR, IGFBP-1, leptin and adiponectin, the only significant predictors were age (unstandardized beta value -0.0672, p = 0.021) and waist (unstandardized beta value 0.0417, p = 0.047) of normalized HbA1c 2 years after surgery. IGFBP-1 levels was at baseline significantly correlated inversely to insulin (r = − 0.45, p < 0.001), BMI (r = − 0.26, p = 0.026), waist circumference (r = − 0.26, p = 0.012), and positively to age (r = 0.404, p < 0.001), adiponectin (r = 0.44, p < 0.001) and HDL-cholesterol (r = 0.36, p < 0.001) but not to glucose, HbA1c, triglycerides, LDL-cholesterol or blood pressure.
Seventeen out of the 25 subjects (68%) with normal glucose control (NG) had low IGFBP-1 before RYGB of whom 15 (92%) had increased their levels above 20 µg/L 1 year after RYGB, thus 2 (8%) subjects continue to have low levels. Eighteen out of 20 subjects (90%) had normalized adiponectin levels.
In the group with normal glucose the subgroup with low IGFBP-1 levels (≤ 20 µg/L) (n = 15) had preoperatively increased HOMA-IR and a tendency to increased weight, insulin levels, triglycerides and diastolic blood pressure compared to the subgroup with high IGFBP-1 (> 20 µg/L). Similarly, in the group with PreDM the subgroup with low IGFBP-1 levels (n = 32) at baseline had signs of the metabolic syndrome with decreased HDL and adiponectin as well as increased triglycerides, insulin, HOMA-IR and IGFSD compared to the subgroup with high IGFBP-1 (n = 13). Moreover, the subgroup with low IGFBP-1 at baseline had after 1 year, in spite of significant weight loss, lower adiponectin and IGFBP-1 levels. Thus, those with low IGFBP-1 at baseline had increased risk for future T2DM. Futhermore, patients with T2DM and low IGFBP1 levels (n = 17) had after 1 and 2 years still signs of the metabolic syndrome in contrast to the patients with T2DM and high IGFBP-1 (n = 11).
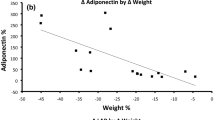
At baseline 4 subjects (4%) had low-normal levels of leptin in spite of obesity, which suggest relative leptin deficiency as a cause of obesity. The delta weight change after 1 and 2 years were negatively associated to baseline IGFBP-1 (1 year p = 0.006; 2 year p = 0.064), but not to leptin and adiponectin.
Discussion
The prevalence of prediabetes (46%) and T2DM (28%) were common in this cohort of obese subjects selected because of high BMI. The fasting glucose levels were high in spite of prior 2 weeks caloric restriction. Other studies of bariatric surgery have reported similar number of obese subjects to have prediabetes or T2DM and that bariatric surgery is particular effective in treating T2DM11,28,29.
In this study significant weight loss was observed in obese subject with normal glucose, preDM and T2DM at 12 and 24 months after RYGB. In parallel with weight loss significant improvement in the risk markers measured (fP-glucose, HbA1c, lipids and blood pressure) was observed including all markers of insulin resistance. These results are in line with previous studies30. IGFBP-1 was the only biomarker at baseline which predicted normalization of HbA1c. However, in a stepwise multiple regression model younger age and larger waist circumference at baseline were the only predictor for normalization of HbA1c when corrected for confounders. However, since baseline IGFBP-1 correlated to both age and waist circumference before surgery this may explain this finding.
Investigating markers of insulin sensitivity (IGFBP-1, HOMA-IR, leptin and adiponectin) showed that baseline levels of IGFBP-1 and adiponectin were low in the three groups despite prior caloric restriction for 2 week. This suggests presence of hyperinsulinemia and insulin resistance in the majority of subjects. In a previous study strict caloric restriction for a shorter period reduced insulin secretion, and IGF-1 levels and increased IGFBP-1 both in overweight NG subjects and in T2DM31. It is known that 2 weeks hypocaloric diet improves hepatic insulin sensitivity greatly which is associated with the increase in IGFBP-123. This indicate that the subjects in the present study probably before the calorie restriction had even lower baseline fasting IGFBP-1 and higher insulin and IGF-I SD levels than the present data. Also adiponectin levels increase with hypocaloric diet32, thus our study goups most probably had lower levels before the 2 weeks of hypocaloric diet. Consequently , the majority of the NG and prediabetes subjects in our study had a high risk of future prediabetes or T2DM.
The majority (61%) of the subjects with abnormal glucose levels had normalized HbA1c after 1 year and somewhat less (49%) after two years, although the mean weight/BMI was not changed at 2 years. A sustained improvement of glucose and weight has been shown post-RYGB in patients with and without T2DM33. It is belived that both weight loss and improved beta-cell function contribute to the sustained improved glycemic control.
IGFBP-1 was the only biomarker at baseline which predicted normalization of HbA1c. Since IGFBP-1 levles is determined by insulin secretion our finding support previous report on fasting insulin as a better predictor than BMI for the beneficial effect of RYGB on metabolic control11. Interestingly, in addition, the lower baseline IGFBP-1, the higher weight loss after RYGB. Low IGFBP-1 is associated with hepatic insulin resistance and hyperinsulinemia. Our study suggests that only those with preoperatively beta-cells with remaining capacity to produce enough insulin when needed may normalize the metabolic control after improvement of hepatic insulin resistance. This is in line with the recent study by Jorgensen et al.33. It has been shown that improvement of hepatic insulin resistance is an important factor in combination with adequate postprandial insulin response explaining the metabolic effect of RYGB34,35. Weght loss, reduced insulin secretion and improved hepatic insulin sensitivity are associated with inceased IGFBP-1 levels29,36,37. Indeed, weight loss was accompanied by increased mean levels of both IGFBP-1 and adiponectin 1 year after the surgery.
In our cohort low adiponectin did not predict normalization of HbA1c after weight loss, which may be due to the fact that adiponectin is a marker of insulin resistance in adipose tissue, muscle and the whole body and not of beta-cell function38. In contrats to IGFBP-1, adiponectin nor leptin levels do not indicate beta-cell function per se.
The majority of the 71 subjects with abnormal glucose levels before surgery had low levels of IGFBP-1 (n = 49) and adiponectin (n = 56). It has previously been shown that low fasting IGFBP-1 and adiponectin levels predict future T2DM, especially in those with increased waist circumference25,26,39.
IGF/IGF-I (SD) levels were low in all groups before surgery, most probably due both to the caloric restriction and reduced GH secretion in obesity. However, the levels were not significantly changed in spite of weight loss after 1 year except for that in patients with T2DM who showed somewhat increased levels, compared to that before surgery. Thus, IGF-I was not responsible for improvement in HbA1c, which is in line with previous study by Brynskov et al.37.
In multivariate analysis only younger age and larger waist circumference were predictive parameters of HbA1c normalization at 2 years of follow-up. Younger age has been shown to be a predictor of weight loss in bariatric surgery in previous studies40. Intuitively, younger age should be a predictor since the glycaemic disturbances should not have been present as long as in elderly and the beta-cells more functional. Thus, HbA1c normalization would be easier with weight loss. Why a high waist circumference will predict a beneficial metabolic effect of RYGB is not fully explained by the present study. Unless the explaination is that the increased waist is a surrogate marker of liver steatosis, hepatic insulin resistance, and low IGFBP-1 levels23 which is corrected by RYGB reducing liver steatosis thus improving insulin sensitivity and glucose tolerance. In summary our study suggests that the metabolic control will be improved, even normalized, by RYGB particularly in subjects with low IGFBP-1 levels, which is a biomarker of liver steatosis, hepatic insulin resistance and preserved beta-cell function. However, both age and waist circumference are easier parameters to collect preoperatively compared to analysis of IGFBP-1 serum levels.
At baseline 4% had low-normal levels of leptin in spite of obesity, which suggest relative leptin deficiency as a cause of obesity. The delta weight change after 1 and 2 years were not associated to baseline leptin levels. These subjects may benefit more of treatment with the new synthetic leptin drug41.
The increase in mean IGFBP-1 and adiponectin levels 1 year after RYGB to normal levels suggest reduced hepatic inuslin resistance, improved insulin sensitivity and protection against future prediabetes and/or T2DM in those with normal glucose levels and prediabetes12,42,43.
There are several clinical scoring systems to predict longterm T2DM remission after RYBG relying on basic clinical parameters such as age, BMI, HbA1c, and the use of insulin therapy and oral hypoglycemic agents, and sometimes C-peptid44. How less conventional biomarkers, which we have included in the present study (e.g., IGF-I, IGFBP-1, adiponectin and leptin) would perform if included in such scoring system are unclear but would be worth evaluating in future studies of larger cohorts.
This study had several limitations. Like all retrospective studies the design has its inherent disadvantages, particularly ascertainment bias and negative selection bias. The follow-up time was only 2 years with all hormone levels only available at 1 year. Moreover, the number of included individuals were limited, especially in the three subgroups. Another limitation is that we do not know the levels of the biomarkers as well as the weight and waist circumference before the 2 weeks of calorie restriction. These weeks of moderate calorie restriction should have improved insulin, insulin sensitivity, lipid profile and glucose levels. It would be of interest to see if the response to short-term calorie restriction can predict the response to RYGB. The strength of the study is the availability of many hormones preoperatively and at least at 1 year of follow-up.
In conclusion, this study suggests that the majority of subjects with low fasting IGFBP-1 and adiponectin levels benefit from bariatric surgery with improved insulin sensitivity and glucose tolerance as well as reduced risk factors for cardiovascular disease and future abnormal glucose tolerance. IGFBP-1 was the only biomarker that predicted normalization of HbA1c, supporting that preserved beta-cell function is of importance. However, in multivariate analysis corrected for confounders younger age and larger waist circumference were the only predictors of normalized HbA1c 2 years after RYGB.
Material and methods
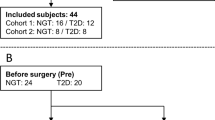
This is a retrospective clinical study of 99 consecutive (23 men) obese subjects referred for laparoscopic RYGB during 2 years. Inclusion criteria was a BMI greater than 35 and all were studied after an overnight fast.
In this cohort 25 subjects (3 men) had normal glucose levels (NG) (fasting plasma-glucose < 5.6 mmol/L and HbA1c < 39 mmol/mol), 46 subjects (14 men) had PreDM (fasting glucose 5.6–6.9 mmol/L and/or HbA1c 39–47 mmol/mol) and 28 subjects (6 men) had T2DM (fasting P-glucose ≥ 7 mmol/l and/or Hba1c ≥ 48)45. The 28 subjects with T2DM were treated with diet only (n = 10), metformin (n = 16), sulfonylurea (n = 1), thiazolidinedione (n = 2) and/or insulin (n = 5).
The subjects were studied prior to and 12 and 24 months after RYGB. They were asked to adhere to caloric restriction with 1,000 kcal/day during 2 weeks prior to the surgery, when a standard 100 cm laparoscopic RYGB was performed46. Clinical characteristic of the subjects at baseline before RYGB after 2 weeks of recommended caloric restriction are shown in Table 1.
Plasma glucose, serum lipid profile and HbA1c were analysed according to routine methods. HbA1c was determined using the MonoS method, Unimate (Roche Diagnostics, Basel, Switzerland). The values obtained with the MonoS method was recalculated to IFCC standard (mmol/mol): IFCC = (10.11*Mono-S) − 8.94. Normal HbA1c were 27–42 mmol/mol (< 50 years) and 31–46 mmol/mol (≥ 50 years), respectively. Serum insulin levels were determined using the ELISA technique (DakoCytomation). Serum IGF-I was determined by an in-house RIA after separation of IGFs from IGFBPs47. The detection level of the RIA was 3.0 µg/L. Cross-reactivity with IGFBP-2 and -3 was < 0.5 and < 0.05%, respectively. To minimize interference of remaining IGFBPs, des(1–3) IGF-I was used as radio-ligand. The intra- and inter-assay CV were 4% and 11%, respectively. Serum levels of IGF-I decrease with age, and are thus expressed as standard deviation (SD) score = [(10logIGF-I-observed + 0.00693 * age) − 2.581]/0.12048. Serum IGFBP-1 (μg/L) was analyzed with an in-house RIA49. The sensitivity of the RIA was 3 µg/L and the intra- and inter-assay CV were 3% and 10%, respectively. Leptin (μg/L) and adiponectin (mg/L) were analysed with double antibody RIA (Linco Research, St. Charles, MO). Homeostatic Model Assessment of Insulin Resistance (HOMA-IR), a measure of fasting whole body insulin sensitivity, was calculated according to Matthew et al.50 from fasting serum insulin and blood glucose levels using a computer-solved homeostasis model assessment: HOMA-IR = (fasting insulin x fasting glucose/22.5). Blood pressure was measured after 10 min of rest with patient sitting. Body mass index (BMI) was determined by body-weight (kg)/height (m2). Waist circumference was measured standing at the horizontal level two cm above umbilicus.
For middle age healthy NG females normal serum levels were for adiponectin 13–15 mg/L, for leptin 13–20 µg/L, and for IGFBP-1 40–46 µg/L15,49. For middle age healthy NG males normal serum levels were for adiponectin 8–9 mg/L, for leptin 7–9 µg/L and for IGFBP-1 27–31 µg/L15,42.
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The study was approved by the Stockholm Ethical Committee and all subjects gave written informed consent to participate.
Statistics
Statistical analyses were carried out using STATISTICA software, version 13 (StatSoft, Tulsa, OH, USA). P-values < 0.05 were considered statistically significant. Variables are presented as mean ± SEM. Normality of variables was tested using the Kolmogorov–Smirnov and Lilliefors tests. Differences between 2 dependent values were tested using Wilcoxon Matched Pair test. Differences between 2 groups were tested using Mann-Whiney test. Differences between the three groups’ variables were tested using Kruskal–Wallis ANOVA and Median test. Differences within each group over the 2 years were tested using Friedman ANOVA. Correlations were tested using Spearman Rank Order Correlation. Logistic regressions were preformed to test which parameters affected primary outcome.
References
Ng, M. et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 384, 766–781 (2014).
Picot, J. et al. The clinical effectiveness and cost-effectiveness of bariatric (weight loss) surgery for obesity: A systematic review and economic evaluation. Health Technol. Assess 13(1–190), 215–357 (2009).
Gloy, V. L. et al. Bariatric surgery versus non-surgical treatment for obesity: A systematic review and meta-analysis of randomised controlled trials. BMJ 347, f5934 (2013).
Schauer, P. R. et al. Bariatric surgery versus intensive medical therapy for diabetes–3-year outcomes. N. Engl. J. Med. 370, 2002–2013 (2014).
Sjostrom, L. Review of the key results from the Swedish Obese Subjects (SOS) trial - A prospective controlled intervention study of bariatric surgery. J. Intern. Med. 273, 219–234 (2013).
Schauer, P. R. et al. Bariatric surgery versus intensive medical therapy for diabetes - 5-year outcomes. N. Engl. J. Med. 376, 641–651 (2017).
Schiavon, C. A. et al. Effects of bariatric surgery in obese patients with hypertension: The GATEWAY randomized trial (gastric bypass to treat obese patients with steady hypertension). Circulation 137, 1132–1142 (2018).
Aminian, A. et al. Association of metabolic surgery with major adverse cardiovascular outcomes in patients with type 2 diabetes and obesity. JAMA 322, 1271–1282 (2019).
Feng, X., Andalib, A., Brethauer, S. A., Schauer, P. R. & Aminian, A. How safe is bariatric surgery in patients with class I obesity (body mass index 30–35 kg/m(2))?. Surg. Obes. Relat. Dis. 15, 253–260 (2019).
Souteiro, P. et al. Long-term diabetes outcomes after bariatric surgery-managing medication withdrawl. Int. J. Obes. (Lond.) 43, 2217–2224 (2019).
Sjostrom, L. et al. Bariatric surgery and long-term cardiovascular events. JAMA 307, 56–65 (2012).
Li, S., Shin, H. J., Ding, E. L. & van Dam, R. M. Adiponectin levels and risk of type 2 diabetes: A systematic review and meta-analysis. JAMA 302, 179–188 (2009).
Friedman, J. The long road to leptin. J. Clin. Invest. 126, 4727–4734 (2016).
Clemmons, D. R. Modifying IGF1 activity: An approach to treat endocrine disorders, atherosclerosis and cancer. Nat. Rev. Drug Discov. 6, 821–833 (2007).
Andreasson, A. N., Unden, A. L., Elofsson, S. & Brismar, K. Leptin and adiponectin: Distribution and associations with cardiovascular risk factors in men and women of the general population. Am. J. Hum. Biol. 24, 595–601 (2012).
Weyer, C. et al. Hypoadiponectinemia in obesity and type 2 diabetes: Close association with insulin resistance and hyperinsulinemia. J. Clin. Endocrinol. Metab. 86, 1930–1935 (2001).
Iliodromiti, S. et al. Accuracy of circulating adiponectin for predicting gestational diabetes: A systematic review and meta-analysis. Diabetologia 59, 692–699 (2016).
Unden, A. L., Elofsson, S. & Brismar, K. Gender differences in the relation of insulin-like growth factor binding protein-1 to cardiovascular risk factors: A population-based study. Clin. Endocrinol. (Oxf) 63, 94–102 (2005).
Brismar, K., Grill, V., Efendic, S. & Hall, K. The insulin-like growth factor binding protein-1 in low and high insulin responders before and during dexamethasone treatment. Metabolism 40, 728–732 (1991).
Wheatcroft, S. B. & Kearney, M. T. IGF-dependent and IGF-independent actions of IGF-binding protein-1 and -2: Implications for metabolic homeostasis. Trends Endocrinol. Metab. 20, 153–162 (2009).
Lemne, C. & Brismar, K. Insulin-like growth factor binding protein-1 as a marker of the metabolic syndrome–A study in borderline hypertension. Blood Press 7, 89–95 (1998).
Halldin, M. et al. The metabolic syndrome and ECG detected left ventricular hypertrophy–influences from IGF-1 and IGF-binding protein-1. PLoS ONE 9, e108872 (2014).
Kotronen, A., Lewitt, M., Hall, K., Brismar, K. & Yki-Jarvinen, H. Insulin-like growth factor binding protein 1 as a novel specific marker of hepatic insulin sensitivity. J. Clin. Endocrinol. Metab. 93, 4867–4872 (2008).
Sandhu, M. S. et al. Circulating concentrations of insulin-like growth factor-I and development of glucose intolerance: a prospective observational study. Lancet 359, 1740–1745 (2002).
Lewitt, M. S. et al. IGF-binding protein 1 and abdominal obesity in the development of type 2 diabetes in women. Eur. J. Endocrinol. 163, 233–242 (2010).
Petersson, U., Ostgren, C. J., Brudin, L., Brismar, K. & Nilsson, P. M. Low levels of insulin-like growth-factor-binding protein-1 (IGFBP-1) are prospectively associated with the incidence of type 2 diabetes and impaired glucose tolerance (IGT): The Soderakra Cardiovascular Risk Factor Study. Diabetes Metab. 35, 198–205 (2009).
Rajpathak, S. N. et al. Insulin-like growth factor axis and risk of type 2 diabetes in women. Diabetes 61, 2248–2254 (2012).
English, W. J. & Williams, D. B. Metabolic and bariatric surgery: An effective treatment option for obesity and cardiovascular disease. Prog. Cardiovasc. Dis. 61, 253–269 (2018).
Song, Z. et al. Increased serum IGFBP-1 and reduced insulin resistance after Roux-En-Y gastric bypass in Chinese patients with type 2 diabetes: a 6-month follow-up. Obes. Surg. 28, 3165–3171 (2018).
Wolfe, B. M., Kvach, E. & Eckel, R. H. Treatment of obesity: Weight loss and bariatric surgery. Circ. Res. 118, 1844–1855 (2016).
Bang, P., Brismar, K., Rosenfeld, R. G. & Hall, K. Fasting affects serum insulin-like growth factors (IGFs) and IGF-binding proteins differently in patients with noninsulin-dependent diabetes mellitus versus healthy nonobese and obese subjects. J. Clin. Endocrinol. Metab. 78, 960–967 (1994).
Salehi-Abargouei, A., Izadi, V. & Azadbakht, L. The effect of low calorie diet on adiponectin concentration: A systematic review and meta-analysis. Horm. Metab. Res. 47, 549–555 (2015).
Jorgensen, N. B. et al. Sustained improvements in glucose metabolism late after Roux-En-Y gastric bypass surgery in patients with and without preoperative diabetes. Sci. Rep. 9, 15154 (2019).
Bojsen-Moller, K. N. et al. Early enhancements of hepatic and later of peripheral insulin sensitivity combined with increased postprandial insulin secretion contribute to improved glycemic control after Roux-en-Y gastric bypass. Diabetes 63, 1725–1737 (2014).
Madsbad, S., Dirksen, C. & Holst, J. J. Mechanisms of changes in glucose metabolism and bodyweight after bariatric surgery. Lancet Diabetes Endocrinol. 2, 152–164 (2014).
Hellenius, M. L., Brismar, K. E., Berglund, B. H. & de Faire, U. H. Effects on glucose tolerance, insulin secretion, insulin-like growth factor 1 and its binding protein, IGFBP-1, in a randomized controlled diet and exercise study in healthy, middle-aged men. J. Intern. Med. 238 (1995).
Brynskov, T., Laugesen, C. S., Floyd, A. K., Frystyk, J. & Sorensen, T. L. The IGF-axis and diabetic retinopathy before and after gastric bypass surgery. Obes. Surg. 27, 408–415 (2017).
Wang, Y. et al. Plasma adiponectin levels and type 2 diabetes risk: A nested case-control study in a Chinese population and an updated meta-analysis. Sci. Rep. 8, 406 (2018).
Lewitt, M. S. et al. Insulin-like growth factor-binding protein-1 in the prediction and development of type 2 diabetes in middle-aged Swedish men. Diabetologia 51, 1135–1145 (2008).
Nickel, F. et al. Predictors of risk and success of obesity surgery. Obes. Facts 12, 427–439 (2019).
Dallner, O. S. et al. Dysregulation of a long noncoding RNA reduces leptin leading to a leptin-responsive form of obesity. Nat. Med. 25, 507–516 (2019).
Lu, J. et al. IGFBP1 increases beta-cell regeneration by promoting alpha- to beta-cell transdifferentiation. EMBO J. 35, 2026–2044 (2016).
Haywood, N. J., Slater, T. A., Matthews, C. J. & Wheatcroft, S. B. The insulin like growth factor and binding protein family: Novel therapeutic targets in obesity & diabetes. Mol. Metab. 19, 86–96 (2019).
Dicker, D. et al. Prediction of long-term diabetes remission after rygb, sleeve gastrectomy, and adjustable gastric banding using diarem and advanced-DiaRem scores. Obes. Surg. 29, 796–804 (2019).
American Diabetes, A. 2. Classification and diagnosis of diabetes: Standards of medical care in diabetes-2018. Diabetes Care 41, S13–S27 (2018).
Lonroth, H., Dalenback, J., Haglind, E. & Lundell, L. Laparoscopic gastric bypass. Another option in bariatric surgery. Surg. Endosc. 10, 636–638 (1996).
Bang, P., Eriksson, U., Sara, V., Wivall, I. L. & Hall, K. Comparison of acid ethanol extraction and acid gel filtration prior to IGF-I and IGF-II radioimmunoassays: improvement of determinations in acid ethanol extracts by the use of truncated IGF-I as radioligand. Acta Endocrinol. (Copenh) 124, 620–629 (1991).
Hilding, A. et al. Serum levels of insulin-like growth factor I in 152 patients with growth hormone deficiency, aged 19–82 years, in relation to those in healthy subjects. J. Clin. Endocrinol. Metab. 84, 2013–2019 (1999).
Soderberg, S. et al. Circulating IGF binding protein-1 is inversely associated with leptin in non-obese men and obese postmenopausal women. Eur. J. Endocrinol. 144, 283–290 (2001).
Matthews, D. R. et al. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28, 412–419 (1985).
Funding
This project was generously supported by the Family-Erling-Persson Foundation, Berth von Kantzow Foundation, Baltzar von Platen Foundation, Stockholm County Council, Strategic Research program in diabetes at Karolinska Institutet and Karolinska Institutet Foundations. Open Access funding provided by Karolinska Institute.
Author information
Authors and Affiliations
Contributions
E.N. and K.B. planned the study. E.N. included the participants. N.R.E. and H.F. processed and analysed the data. All coauthors contributed to study design, optimization and interpretation of results. N.R.E., H.F. and K.B. characterized the patients. N.R.E. and H.F. wrote the manuscript with contributions from all coauthors. All authors reviewed and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ekberg, N.R., Falhammar, H., Näslund, E. et al. Predictors of normalized HbA1c after gastric bypass surgery in subjects with abnormal glucose levels, a 2-year follow-up study. Sci Rep 10, 15127 (2020). https://doi.org/10.1038/s41598-020-72141-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-72141-0
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.