Abstract
Low ocular perfusion pressure (OPP) has been proposed as an important risk factor for glaucoma development and progression, but controversy still exists between studies. Therefore, we conducted a systematic review and meta-analysis to analyze the association between OPP and open-angle glaucoma (OAG). Studies were identified by searching PubMed and EMBASE databases. The pooled absolute and standardised mean difference in OPP between OAG patients and controls were evaluated using the random-effects model. Meta-regression analysis was conducted to investigate the factors associated with OPP difference between OAG patients and controls. A total of 43 studies were identified including 3,009 OAG patients, 369 patients with ocular hypertension, and 29,502 controls. The pooled absolute mean difference in OPP between OAG patients and controls was −2.52 mmHg (95% CI −4.06 to −0.98), meaning significantly lower OPP in OAG patients (P = 0.001). Subgroup analyses showed that OAG patients with baseline IOP > 21 mmHg (P = 0.019) and ocular hypertension patients also had significantly lower OPP than controls (P < 0.001), but such difference in OPP was not significant between OAG patients with baseline IOP of ≤21 mmHg and controls (P = 0.996). In conclusion, although no causal relationship was proven in the present study, our findings suggest that in patients with high baseline IOP, who already have a higher risk of glaucoma, low OPP might be another risk factor.
Similar content being viewed by others
Introduction
Glaucoma, the second worldwide leading cause of blindness1, is a progressive and chronic disease characterized by the degeneration of retinal ganglion cell and its axon with corresponding visual field defect2. Although therapeutic risk factors for preventing development and progression of glaucoma have been under wide investigation, lowering intraocular pressure (IOP) currently is the only effective treatment3,4,5.
Ocular perfusion pressure (OPP), the pressure to drive blood throughout the intraocular vasculature, with the degree of perfusion being influenced by the flow resistance, represents the blood flow and oxygen supplying the optic nerve head (ONH)6,7. Thus, it has long been proposed that a decrease in OPP may increase the vulnerability of optic disc, leading to an increased risk of glaucoma development or progression8,9,10,11,12. However, the association between OPP level and the risk of glaucoma has been debatable between studies. Some studies reported a significant association between low OPP and an increased risk of glaucoma8,9,10,11,12. In contrast, others have reported the statistically non-significant or limited impact of OPP on the risk of glaucoma13,14.
Another hindrance when referring to OPP in clinic is that as various levels of OPP have been reported depending on the study design, glaucoma type, and patient characteristics (e.g. presence of hypertension, use of anti-hypertensive medication), this has led to controversy over the significant difference in the level of OPP between glaucoma patients and controls. In light of these, we performed a systematic review and meta-analysis to investigate the pooled difference of OPP between glaucoma patients and controls and the association between mean OPP (MOPP) level and the risk of open-angle glaucoma (OAG).
Materials and methods
Search strategy and study selection
This study adhered to the PRISMA statement to follow the appropriate guidelines for systematic review and meta-analysis15. The Ovid interface was used to search for the keywords in the databases PubMed, EMBASE, and the Cochrane Library. The keywords for disease were “open-angle glaucoma”, “primary open-angle glaucoma”, “high tension glaucoma”, “normal tension glaucoma”, and “ocular hypertension. The keywords for ocular perfusion pressure were “ocular perfusion pressure”, “mean ocular perfusion pressure”, “systemic ocular perfusion pressure”, and “diastolic ocular perfusion pressure”. The following search terms were used: (glaucoma, open-angle [Medical Subject Headings {MeSH}] OR open angle glaucoma OR open-angle glaucoma OR OAG OR primary open angle glaucoma OR primary open-angle glaucoma OR POAG OR high tension glaucoma OR low tension glaucoma [MeSH] OR glaucoma, low tension OR low tension glaucoma OR normal tension glaucoma OR glaucoma, normal tension OR normal-tension glaucoma OR NTG OR ocular hypertension OR OHT) AND (ocular perfusion pressure OR OPP OR mean ocular perfusion pressure OR MOPP OR systolic ocular perfusion pressure OR SOPP OR diastolic ocular perfusion pressure OR DOPP). The literature search was conducted according to MeSH and no language restrictions were applied during the search. Two investigators (KEK, SJA) performed the literature search and study selection in an independent and masked fashion. Studies published before May 31 2019 were included. After screening titles and abstracts, full-text articles of eligible studies following the inclusion and exclusion criteria were attained.
Studies met the following criteria were included: (1) providing information on MOPP (2/3 [diastolic BP + 1/3 (systolic BP-diastolic BP)] – IOP) level represented as mean ± standard deviation (SD) in both controls and patients (2) IOP values represented as mean ± SD, measured by Goldmann applanation tonometry (3) open-angle glaucoma diagnosed with structural change (ONH, retinal nerve fiber layer) and corresponding functional changes, (4) ocular hypertension (OHT) diagnosed as IOP > 21 mmHg without any glaucomatous structural or functional damage. (5) studies approved by an institutional review board or ethics committee and followed the guidelines from the Declaration of Helsinki.
Exclusion criteria were: (1) studies reported an association between OPP and glaucoma in the form of correlation coefficients or odds ratio, (2) experimental studies involving non-human population, (3) angle-closure glaucoma or open-angle glaucoma with any secondary cause (e.g., uveitis, pseudoexfoliation syndrome), (4) glaucoma patients that underwent other ocular surgeries or received treatments other than topical IOP-lowering medication, (5) patients with uncontrolled hypertension, (6) papers not available in English, (7) studies involving patients less than 18 years of age, (8) abstracts or conference proceedings that were not published in peer-reviewed journals.
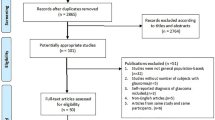
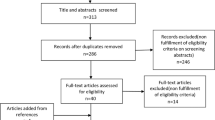
The study arms were divided into glaucoma/OHT and controls. The included studies had data on the following groups of glaucoma patients: (1) OAG (OAG without definite information on baseline IOP for inclusion criteria), (2) primary open-angle glaucoma (POAG; OAG patients with baseline IOP > 21 mmHg) and, (3) normal-tension glaucoma (NTG; OAG patients with baseline IOP ≤ 21 mmHg). The OAG arm in the present study combined OAG, POAG, and NTG patients. Additionally, we performed separate analyses for POAG, NTG, and OHT groups. Only controlled (stable) glaucoma patients were included. If more than one published article reported on the similar findings within the same study population by the same researcher group, the most recent publication or the publication with the higher level of evidence, or larger number of study subjects has been selected. If the IOP, BP, or OPP values were measured several times throughout the day, the first measurements were included in the analyses. The electronic search strategy and sequential exclusion are outlined in Fig. 1.
Data extraction and quality assessment
Two investigators (KEK and SUB) independently extracted data in a masked manner using a data extraction form. Discrepancies between the investigators were resolved by the third investigator (JWJ). Following data were extracted from the studies: (1) study characteristics, including the year of publication, name of the first author, country, study design, number of included eyes, patient demographics; (2) type of glaucoma, including OAG, POAG, NTG, and OHT; (3) outcome measurements, including OPP (MOPP, systolic OPP, diastolic OPP), IOP, and BP (mean arterial pressure [MAP], systolic BP [SBP], diastolic BP [DBP]); (4) patient characteristics, including proportion of patients under IOP-lowering medication, proportion of patients with hypertension or under hypertension medication.
The quality of included studies was assessed using the Risk of Bias Assessment Tool for Nonrandomized Studies (RoBANS 2.0) method by two independent investigators (KEK and SUB)16. The RoBANS tool consists of eight domains: comparability of participants, selection of participants, confounding variables, intervention (exposure) measurement, blinding of outcome assessment, outcome evaluation, incomplete outcome data, and selective outcome reporting. The risk of bias for each domain was categorized as low risk, high risk, and unclear risk.
Statistical methods
The pooled OPP difference, which is to say, the difference in OPP between the glaucoma and control groups from the meta-analysis of the included studies, was presented as the mean difference and the standardised mean difference with 95% confidence interval (CI). Heterogeneity in meta-analysis refers to the variation in study outcomes between or among studies. The I2 statistic, representing the percentage of variation across studies that is due to heterogeneity rather than to sampling error, was evaluated for the degree of unexplained variation in the OPP/glaucoma association17,18. The values can range from 0 to 100%, 0% indicating statistical homogeneity and 100% statistical heterogeneity. It has been suggested that the adjectives low, moderate, and high be ascribed to I2 values of 25, 50, and 75%, respectively19. Due to high levels of heterogeneity, differences in mean IOP, OPP, BP levels between glaucoma patients and control were analyzed using random-effects model, which assumes that the true underlying effect between studies varies20. Meta-regression analysis was used to find possible potential factors that could result in OPP difference between glaucoma patients and controls. Variables including age, gender (proportion of men), MAP, SBP, DBP and IOP were included in the analyses. For studies not having MAPs but only SBP and DBPs, MAPs were calculated as DBP + [1/3 (SBP – DBP)]. All statistical analyses used 95% CI and P-values with a cut-off point of 0.05. All statistical analyses were performed using the software package R version 3.6.221.
Results
Characteristics of included studies
We identified 1,152 studies through database searches. After reviewing abstracts, we excluded 1,080 studies that were not relevant, leaving 72 studies for full-text evaluation (Fig. 1). Of these, 43 studies were finally included in the current systematic review and meta-analyses. Among them, 4 studies were population-based, cross-sectional studies8,22,23,24 and the others were hospital-based, clinical case-control studies14,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62. These 43 studies included 3,009 patients with OAG (1,294 OAG, 926 POAG, and 789 NTG), 369 OHT patients, and 29,502 controls from 19 countries and their characteristics are summarized in the Table 1. The quality of the evidence was generally good, but the risk of bias caused by confounding variables was high in 12 (27.9%) studies, and unclear in 3 (7.0%) studies.
Ocular perfusion pressure difference between glaucoma patients and control groups
The main outcome of the present study was the difference in OPP (measured in mmHg) between patients with and without OAG and its significance. The pooled average difference in OPP between patients with and without OAG was −2.52 mmHg (95% CI, −4.06 to −0.98, P = 0.001), with a high degree of heterogeneity (I2 = 92.3%), presented in Fig. 2A. The pooled standardised average difference in OPP between OAG patients and control was −0.38 (95% CI, −0.56 to −0.20, P < 0.001), also with high degree of heterogeneity (I2 = 90.1%, Fig. 2B). These showed that OAG patients had significantly lower OPP than controls.
Random-effects meta-analysis of ocular perfusion pressure (OPP) difference between open-angle glaucoma patients and controls. Pooled OPP difference was presented as (A) the mean difference (MD) and (B) the standardised mean difference (SMD) with 95% confidence interval (CI). SD = standard deviation.
Subgroup analyses showed that POAG patients had significantly lower pooled average OPP compared to controls (−4.20 mmHg, 95% CI −7.58 to −0.81, P = 0.019, Fig. 3A) and the similar trend was found in patients with OHT (−6.01 mmHg, 95% CI −8.61 to −3.42, P < 0.001, Fig. 3B). However, the pooled average difference in OPP between NTG patients and controls was not significant (−0.01 mmHg, 95% CI −2.14 to 2.12 mmHg, P = 0.996, Fig. 3C). The standardised mean difference in OPP also showed similar relationships between the subgroup of glaucoma patients and controls (Fig. 4A–C).
Random-effects meta-analysis of the mean ocular perfusion pressure (OPP) difference between (A) primary open-angle glaucoma (open-angle glaucoma [OAG] patients with baseline intraocular pressure [IOP] of>21 mmHg), (B) ocular hypertension, (C) normal-tension glaucoma (OAG patients with baseline IOP of ≤ 21 mmHg) and controls. SD = standard deviation; MD = mean difference; CI = confidence interval.
Random-effects meta-analysis of the standardised mean ocular perfusion pressure (OPP) difference between (A) primary open-angle glaucoma (open-angle glaucoma [OAG] patients with baseline intraocular pressure [IOP] of>21 mmHg), (B) ocular hypertension, (C) normal-tension glaucoma (OAG patients with baseline IOP of ≤21 mmHg) and controls. SD = standard deviation; SMD = standardised mean difference; CI = confidence interval.
Additional analyses
The study that contributed the most to the heterogeneity for the OPP difference between controls and OAG patients was Mroczkowska et al.36 Meta-regression analyses using random-effects model were performed to investigate the potential risk factors associated with the pooled standardised average difference in OPP between OAG patients and controls. Random effects meta-regression analyses showed that age, systolic BP, diastolic BP, mean arterial pressure, and study design were not significantly associated with the standardised mean difference in OPP. However, studies with larger proportion of men showed increasing trend of standardised mean difference in OPP (P = 0.040, Fig. 5A) and the OPP difference was larger in studies with lower mean OPP level (P = 0.029, Fig. 5B).
Random-effects meta-regression of standardised mean difference in ocular perfusion pressure (OPP) between patients with open-angle glaucoma (OAG) and controls according to (A) proportion of men and (B) mean OPP level. The line represents a line of best fit from meta-regression analysis. This suggests that the standardised mean difference in OPP levels between patients with OAG and controls was the largest in study population with large proportion of men (P = 0.040) and low mean OPP level (P = 0.029).
Discussion
Our systematic review and meta-analysis showed that patients with OAG had lower mean OPP compared to controls. The pooled mean absolute difference in OPP level between OAG patients and controls was −2.52 mmHg. Moreover, patients with POAG and OHT also showed significantly lower OPP than controls, with the pooled mean absolute difference of −4.20 mmHg and −6.01 mmHg, respectively. However, this trend of the relationship was not significant in NTG patients. We concluded that low OPP may be a significant risk factor for OAG patients with high baseline IOP, and thus, control of IOP leading to appropriate OPP may be important in terms of regulating vascular factors for glaucoma treatment.
Vascular factors have long been suspected of playing an important role in the glaucomatous process in addition to IOP. In this aspect, previous studies have reported a significant association between low OPP and glaucoma. Low OPP represents hypo-perfusion to ONH, ultimately leading to ONH deprived of nutrition and oxygenation. However, several studies have shown no association between them14. This may be due to the fact that IOP and BP, the major constitutes of OPP, were measured in different clinical settings (e.g. study population, types of glaucoma, use of topical drugs). Moreover, various BP- and OPP-related parameters (e.g., MOPP, systolic OPP, diastolic OPP, MAP, SBP, DBP) were used, which rendered interpretation of the effect of OPP on glaucoma more complex, in line with the study by Barbosa-Breda et al.63 Therefore, to address the gap in consideration of heterogeneity between studies, we conducted systematic review and meta-analysis and confirmed that OAG patients had significantly lower MOPP than controls.
Our results showed that POAG patients, whose baseline IOP of more than 21 mmHg, showed significantly lower OPP than controls. Previous studies have suggested that NTG patients may be more affected by ischemic injury associated with vascular factors than mechanical injury by elevated IOP44,64,65,66,67,68,69. In this aspect, one may expect significantly lower OPP in NTG patients than in controls. However, this was not proven in the present study. Following reasons may explain our findings. First, OPP itself is not the only vascular factor causing ischemic injury to the optic nerve head. Moreover, several studies reported that OPP fluctuation or degree of its variability, rather than one single OPP value may be more important in development and progression of NTG65,70,71. Second, several studies have suggested that NTG patients may have vascular dysregulation or weak vascular regulating system to defense against provocative stimulation. Thus, their vulnerability to vascular insults may not be revealed under normal (resting) condition14,72. All of the included studies measured OPP at resting and sitting position, and thus, our results could not reveal the OPP results in NTG patients under provocative stimulation. Third, the present study only included studies with definite MOPP values presented as mean ± SD. Thus, several large, population-based studies including Baltimore Eye Survey73, Egna-Neumarkt Study12, Los Angeles Latino Eye Study74 which reported the association between low OPP and increased risk of glaucoma in terms of odds ratio were excluded. Since these studies also had a large proportion of patients with baseline IOP less than 21 mmHg, these could have affected the present meta-analysis.
The actual OPP should be determined by the difference between arterial pressure at the entrance to the eye and the venous pressure at the exit of the eye. However, currently available methods cannot directly measure such pressures. Therefore, OPP has been estimated by the difference between arterial pressure measured in the arm and IOP, which may not reflect actual measures. Based on current equation, either decrease in BP or increase in IOP may influence the decrease in OPP. However, these parameters cannot be evaluated separately for the association with glaucoma, since they are all included in the same equation. We conducted direct comparison of OPP, IOP, and BP between POAG and NTG subgroups in an attempt to explain the effects of these parameters on OPP difference. However, it was not possible, since only 18.6% (8/43) of studies had both POAG and NTG groups. Also, some studies presented “untreated” IOP values, while others presented “treated” IOP values for the subjects’ baseline characteristics. Thus, we indirectly calculated the pooled average OPP, IOP, and BP differences between POAG and NTG groups using network meta-analysis (Supplemental Table S1)75. Despite the high heterogeneity and the limited number of studies, we confirmed that the POAG group showed lower OPP and higher IOP compared with the NTG group. By contrast, there was no significant difference in pooled average BP difference between POAG and NTG patients by network meta-analysis. These additional findings seem to imply that high IOP might be the basis for low OPP in patients with POAG.
Several studies reported that diastolic BP is more important in determining OPP than other BP parameters. As the degree of BP is larger than that of IOP, OPP may be more sensitive to changes in BP than those in IOP. We used meta-regression analyses to examine the potential evidence as to which BP parameter would be associated with OPP difference. However, none of the BP parameters showed any association with OPP difference. This could be attributable to the fact that all of the studies included patients with no hypertension or hypertension under controlled BP with or without medication. Another possible cause is the fact that not all of the studies had available BP values. To overcome the limitation that only 32.6% (14/43) studies had mean arterial pressures, we even calculated them additionally based on systolic BP and diastolic BP provided in 65.1% (28/43) of studies, but the association was insignificant. As only mean OPP was used in the analyses, the future investigation is needed on whether BP parameters could have effect on other OPP parameters (e.g. diastolic, systolic).
Several limitations should be considered for the interpretation of our results. First, our meta-analyses are based on cross-sectional studies. Thus, despite a significant association between low OPP and glaucoma, our results cannot provide evidence for a causal relationship between them. The Barbados Eye Study, a prospective, longitudinal study reported that low MOPP, systolic OPP, and diastolic OPP were all associated with a higher risk of developing glaucoma at 4 and 9 years of follow-up10. Despite this, further longitudinal studies providing sufficient clinical evidence are needed to address this causal relationship. Second, the heterogeneity of pooled studies was substantial. This may result from differences in study population, study design, and participant characteristics. We used random-effects analyses to overcome such limitation and no publication bias was detected by Egger’s test. Finally, the present study showed the absolute mean difference of OPP as approximately 2 mmHg between OAG patients and controls, but these were based on single measurements. Thus, further investigation is required to prove the clinical relevance of OPP fluctuation or dynamic range of OPP on glaucoma.
In conclusion, our systematic review and meta-analysis point to evidence for low OPP in OAG patients, which is in line with the current notion that decreased vascular supply to the ONH may increase its vulnerability to glaucomatous structural damage. Additionally, among OAG patients, those with high baseline IOP particularly, rather than those with low baseline IOP, showed significantly lower OPP than the controls. Although further investigation might be needed, our results imply that in patients with high baseline IOP, who already have a higher risk of glaucoma, low OPP might be another risk factor.
References
Congdon, N. G., Friedman, D. S. & Lietman, T. Important causes of visual impairment in the world today. JAMA 290, 2057–2060 (2003).
Jonas, J. B. et al. Glaucoma. Lancet 390, 2183–2193 (2017).
Group, C. Comparison of glaucomatous progression between untreated patients with normal-tension glaucoma and patients with therapeutically reduced intraocular pressures. Collaborative Normal-Tension Glaucoma Study Group. Am J Ophthalmol 126, 487–497 (1998).
Kass, M. A. et al. The Ocular Hypertension Treatment Study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch Ophthalmol 120, 701–713; discussion 829–730 (2002).
Leske, M. C. et al. Factors for glaucoma progression and the effect of treatment: the early manifest glaucoma trial. Arch Ophthalmol 121, 48–56 (2003).
Caprioli, J. & Coleman, A. L., Blood Flow in Glaucoma, D. Blood pressure, perfusion pressure, and glaucoma. Am J Ophthalmol 149, 704–712 (2010).
Costa, V. P. et al. Ocular perfusion pressure in glaucoma. Acta Ophthalmol 92, e252–266 (2014).
Zheng, Y. et al. Distribution of ocular perfusion pressure and its relationship with open-angle glaucoma: the singapore malay eye study. Invest Ophthalmol Vis Sci 51, 3399–3404 (2010).
Memarzadeh, F. et al. Blood pressure, perfusion pressure, and open-angle glaucoma: the Los Angeles Latino Eye Study. Invest Ophthalmol Vis Sci 51, 2872–2877 (2010).
Leske, M. C. et al. Risk factors for incident open-angle glaucoma: the Barbados Eye Studies. Ophthalmology 115, 85–93 (2008).
Leske, M. C. et al. Predictors of long-term progression in the early manifest glaucoma trial. Ophthalmology 114, 1965–1972 (2007).
Bonomi, L. et al. Vascular risk factors for primary open angle glaucoma: the Egna-Neumarkt Study. Ophthalmology 107, 1287–1293 (2000).
Xu, L., Wang, Y. X. & Jonas, J. B. Ocular perfusion pressure and glaucoma: the Beijing Eye Study. Eye (Lond) 23, 734–736 (2009).
Samsudin, A. et al. Ocular perfusion pressure and ophthalmic artery flow in patients with normal tension glaucoma. BMC Ophthalmol 16, 39 (2016).
Moher, D., Liberati, A., Tetzlaff, J., Altman, D. G. & Group, P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 339, b2535 (2009).
Kim, S. Y. et al. Testing a tool for assessing the risk of bias for nonrandomized studies showed moderate reliability and promising validity. J Clin Epidemiol 66, 408–414 (2013).
Hardy, R. J. & Thompson, S. G. Detecting and describing heterogeneity in meta-analysis. Stat Med 17, 841–856 (1998).
Higgins, J. P. & Thompson, S. G. Quantifying heterogeneity in a meta-analysis. Stat Med 21, 1539–1558 (2002).
Higgins, J. P., Thompson, S. G., Deeks, J. J. & Altman, D. G. Measuring inconsistency in meta-analyses. BMJ 327, 557–560 (2003).
Ades, A. E., Lu, G. & Higgins, J. P. The interpretation of random-effects meta-analysis in decision models. Med Decis Making 25, 646–654 (2005).
R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria, http://www.r-project.org (2019).
Cantor, E., Mendez, F., Rivera, C., Castillo, A. & Martinez-Blanco, A. Blood pressure, ocular perfusion pressure and open-angle glaucoma in patients with systemic hypertension. Clin Ophthalmol 12, 1511–1517 (2018).
Tham, Y. C. et al. Inter-relationship between ocular perfusion pressure, blood pressure, intraocular pressure profiles and primary open-angle glaucoma: the Singapore Epidemiology of Eye Diseases study. Br J Ophthalmol 102, 1402–1406 (2018).
Jonas, J. B., Wang, N. & Nangia, V. Ocular Perfusion Pressure vs Estimated Trans-Lamina Cribrosa Pressure Difference in Glaucoma: The Central India Eye and Medical Study (An American Ophthalmological Society Thesis). Trans Am Ophthalmol Soc 113, T6 (2015).
Mursch-Edlmayr, A. S. et al. Differences in Optic Nerve Head Blood Flow Regulation in Normal Tension Glaucoma Patients and Healthy Controls as Assessed With Laser Speckle Flowgraphy During the Water Drinking Test. J Glaucoma 28, 649–654 (2019).
Hidalgo-Aguirre, M., Costantino, S. & Lesk, M. R. Pilot study of the pulsatile neuro-peripapillary retinal deformation in glaucoma and its relationship with glaucoma risk factors. Curr Eye Res 42, 1620–1627 (2017).
Gao, Y., Wan, B., Li, P., Zhang, Y. & Tang, X. Short-term reproducibility of intraocular pressure and ocular perfusion pressure measurements in Chinese volunteers and glaucoma patients. BMC Ophthalmol 16, 145 (2016).
Abegao Pinto, L. et al. Ocular blood flow in glaucoma - the Leuven Eye Study. Acta Ophthalmol 94, 592–598 (2016).
Modrzejewska, M., Grzesiak, W., Zaborski, D. & Modrzejewska, A. The role of lipid dysregulation and vascular risk factors in glaucomatous retrobulbar circulation. Bosn J Basic Med Sci 15, 50–56 (2015).
Goharian, I. et al. Reproducibility of retinal oxygen saturation in normal and treated glaucomatous eyes. Br J Ophthalmol 99, 318–322 (2015).
Willekens, K., Abegao Pinto, L., Vandewalle, E., Marques-Neves, C. & Stalmans, I. Higher optic nerve sheath diameters are associated with lower ocular blood flow velocities in glaucoma patients. Graefes Arch Clin Exp Ophthalmol 252, 477–483 (2014).
Sehi, M. et al. Retinal blood flow in glaucomatous eyes with single-hemifield damage. Ophthalmology 121, 750–758 (2014).
Abegao Pinto, L., Vandewalle, E., Willekens, K., Marques-Neves, C. & Stalmans, I. Ocular pulse amplitude and Doppler waveform analysis in glaucoma patients. Acta Ophthalmol 92, e280–285 (2014).
Wang, J. et al. Estimation of ocular rigidity in glaucoma using ocular pulse amplitude and pulsatile choroidal blood flow. Invest Ophthalmol Vis Sci 54, 1706–1711 (2013).
Ramli, N., Nurull, B. S., Hairi, N. N. & Mimiwati, Z. Low nocturnal ocular perfusion pressure as a risk factor for normal tension glaucoma. Prev Med 57(Suppl), S47–49 (2013).
Mroczkowska, S. et al. Primary open-angle glaucoma vs normal-tension glaucoma: the vascular perspective. JAMA Ophthalmol 131, 36–43 (2013).
Gugleta, K., Turksever, C., Polunina, A. & Orgul, S. Effect of ageing on the retinal vascular responsiveness to flicker light in glaucoma patients and in ocular hypertension. Br J Ophthalmol 97, 848–851 (2013).
Gherghel, D., Mroczkowska, S. & Qin, L. Reduction in blood glutathione levels occurs similarly in patients with primary-open angle or normal tension glaucoma. Invest Ophthalmol Vis Sci 54, 3333–3339 (2013).
Figueiredo, B. P., Cronemberger, S. & Kanadani, F. N. Correlation between ocular perfusion pressure and ocular pulse amplitude in glaucoma, ocular hypertension, and normal eyes. Clin Ophthalmol 7, 1615–1621 (2013).
Abegao Pinto, L., Vandewalle, E., De Clerck, E., Marques-Neves, C. & Stalmans, I. Lack of spontaneous venous pulsation: possible risk indicator in normal tension glaucoma? Acta Ophthalmol 91, 514–520 (2013).
Plange, N., Bienert, M., Remky, A. & Arend, K. O. Optic disc fluorescein leakage and intraocular pressure in primary open-angle glaucoma. Curr Eye Res 37, 508–512 (2012).
Sehi, M., Flanagan, J. G., Zeng, L., Cook, R. J. & Trope, G. E. The association between diurnal variation of optic nerve head topography and intraocular pressure and ocular perfusion pressure in untreated primary open-angle glaucoma. J Glaucoma 20, 44–50 (2011).
Portmann, N. et al. Choroidal blood flow response to isometric exercise in glaucoma patients and patients with ocular hypertension. Invest Ophthalmol Vis Sci 52, 7068–7073 (2011).
Galassi, F., Giambene, B. & Varriale, R. Systemic vascular dysregulation and retrobulbar hemodynamics in normal-tension glaucoma. Invest Ophthalmol Vis Sci 52, 4467–4471 (2011).
Kim, Y. K., Oh, W. H., Park, K. H., Kim, J. M. & Kim, D. M. Circadian blood pressure and intraocular pressure patterns in normal tension glaucoma patients with undisturbed sleep. Korean J Ophthalmol 24, 23–28 (2010).
Garhofer, G. et al. Retrobulbar blood flow velocities in open angle glaucoma and their association with mean arterial blood pressure. Invest Ophthalmol Vis Sci 51, 6652–6657 (2010).
Resch, H. et al. Effect of dual endothelin receptor blockade on ocular blood flow in patients with glaucoma and healthy subjects. Invest Ophthalmol Vis Sci 50, 358–363 (2009).
Pemp, B. et al. Diurnal fluctuation of ocular blood flow parameters in patients with primary open-angle glaucoma and healthy subjects. Br J Ophthalmol 93, 486–491 (2009).
Deokule, S. et al. Correlation among choroidal, parapapillary, and retrobulbar vascular parameters in glaucoma. Am J Ophthalmol 147, 736–743 e732 (2009).
Plange, N., Kaup, M., Remky, A. & Arend, K. O. Prolonged retinal arteriovenous passage time is correlated to ocular perfusion pressure in normal tension glaucoma. Graefes Arch Clin Exp Ophthalmol 246, 1147–1152 (2008).
Januleviciene, I., Sliesoraityte, I., Siesky, B. & Harris, A. Diagnostic compatibility of structural and haemodynamic parameters in open-angle glaucoma patients. Acta Ophthalmol 86, 552–557 (2008).
Galassi, F., Giambene, B. & Menchini, U. Ocular perfusion pressure and retrobulbar haemodynamics in pseudoexfoliative glaucoma. Graefes Arch Clin Exp Ophthalmol 246, 411–416 (2008).
Feke, G. T. & Pasquale, L. R. Retinal blood flow response to posture change in glaucoma patients compared with healthy subjects. Ophthalmology 115, 246–252 (2008).
Riva, C. E. et al. Flicker-evoked response measured at the optic disc rim is reduced in ocular hypertension and early glaucoma. Invest Ophthalmol Vis Sci 45, 3662–3668 (2004).
Okuno, T., Sugiyama, T., Kojima, S., Nakajima, M. & Ikeda, T. Diurnal variation in microcirculation of ocular fundus and visual field change in normal-tension glaucoma. Eye (Lond) 18, 697–702 (2004).
Hosking, S. L. et al. Ocular haemodynamic responses to induced hypercapnia and hyperoxia in glaucoma. Br J Ophthalmol 88, 406–411 (2004).
Gherghel, D., Hosking, S. L. & Cunliffe, I. A. Abnormal systemic and ocular vascular response to temperature provocation in primary open-angle glaucoma patients: a case for autonomic failure? Invest Ophthalmol Vis Sci 45, 3546–3554 (2004).
Galassi, F. et al. Nitric oxide proxies and ocular perfusion pressure in primary open angle glaucoma. Br J Ophthalmol 88, 757–760 (2004).
Fuchsjager-Mayrl, G. et al. Ocular blood flow and systemic blood pressure in patients with primary open-angle glaucoma and ocular hypertension. Invest Ophthalmol Vis Sci 45, 834–839 (2004).
Kerr, J., Nelson, P. & O’Brien, C. Pulsatile ocular blood flow in primary open-angle glaucoma and ocular hypertension. Am J Ophthalmol 136, 1106–1113 (2003).
Hafez, A. S., Bizzarro, R. L. & Lesk, M. R. Evaluation of optic nerve head and peripapillary retinal blood flow in glaucoma patients, ocular hypertensives, and normal subjects. Am J Ophthalmol 136, 1022–1031 (2003).
Duijm, H. F., van den Berg, T. J. & Greve, E. L. Choroidal haemodynamics in glaucoma. Br J Ophthalmol 81, 735–742 (1997).
Barbosa-Breda, J. et al. Heterogeneity in arterial hypertension and ocular perfusion pressure definitions: Towards a consensus on blood pressure-related parameters for glaucoma studies. Acta Ophthalmol 97, e487–e492 (2019).
Meyer, J. H., Brandi-Dohrn, J. & Funk, J. Twenty four hour blood pressure monitoring in normal tension glaucoma. Br J Ophthalmol 80, 864–867 (1996).
Choi, J. et al. Circadian fluctuation of mean ocular perfusion pressure is a consistent risk factor for normal-tension glaucoma. Invest Ophthalmol Vis Sci 48, 104–111 (2007).
Choi, J., Jeong, J., Cho, H. S. & Kook, M. S. Effect of nocturnal blood pressure reduction on circadian fluctuation of mean ocular perfusion pressure: a risk factor for normal tension glaucoma. Invest Ophthalmol Vis Sci 47, 831–836 (2006).
Okumura, Y., Yuki, K. & Tsubota, K. Low diastolic blood pressure is associated with the progression of normal-tension glaucoma. Ophthalmologica 228, 36–41 (2012).
Flammer, J. & Mozaffarieh, M. What is the present pathogenetic concept of glaucomatous optic neuropathy? Surv Ophthalmol 52(Suppl 2), S162–173 (2007).
Joe, S. G., Choi, J., Sung, K. R., Park, S. B. & Kook, M. S. Twenty-four hour blood pressure pattern in patients with normal tension glaucoma in the habitual position. Korean J Ophthalmol 23, 32–39 (2009).
Sung, K. R. et al. Twenty-four hour ocular perfusion pressure fluctuation and risk of normal-tension glaucoma progression. Invest Ophthalmol Vis Sci 50, 5266–5274 (2009).
Lee, J., Choi, J., Jeong, D., Kim, S. & Kook, M. S. Relationship between daytime variability of blood pressure or ocular perfusion pressure and glaucomatous visual field progression. Am J Ophthalmol 160, 522–537 e521 (2015).
Bossuyt, J. et al. Vascular dysregulation in normal-tension glaucoma is not affected by structure and function of the microcirculation or macrocirculation at rest: a case-control study. Medicine (Baltimore) 94, e425 (2015).
Sommer, A. et al. Relationship between intraocular pressure and primary open angle glaucoma among white and black Americans. The Baltimore Eye Survey. Arch Ophthalmol 109, 1090–1095 (1991).
Francis, B. A. et al. Population and high-risk group screening for glaucoma: the Los Angeles Latino Eye Study. Invest Ophthalmol Vis Sci 52, 6257–6264 (2011).
Chaimani, A., Caldwell, D., Li, T., Higgins, J. & Salanti, G. Chapter 11: Undertaking network meta-analyses. (ed. Higgins, JPT.) Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019) (Cochrane (2019).
Acknowledgements
Supported by Grant Number 23-2015-0100 (2015-0291) from the SNUH Research Fund.
Author information
Authors and Affiliations
Contributions
Design of the study (K.E.K., J.W.J.); Conduct of the study (K.E.K., J.W.J.); Collection and management of data (K.E.K., S.U.B., S.J.A.); Analysis and interpretation of data (K.E.K., S.O., K.H.P., J.W.J.); Preparation of manuscript (K.E.K., J.W.J., S.O.); Review or approval of manuscript (J.W.J., K.H.P.).
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kim, K.E., Oh, S., Baek, S.U. et al. Ocular Perfusion Pressure and the Risk of Open-Angle Glaucoma: Systematic Review and Meta-analysis. Sci Rep 10, 10056 (2020). https://doi.org/10.1038/s41598-020-66914-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-66914-w
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.