Abstract
Reduced haemodynamic response in the frontotemporal cortices of patients with major depressive disorder (MDD) has been demonstrated using functional near-infrared spectroscopy (fNIRS). Most notably, changes in cortical oxy-haemoglobin during a Japanese phonetic fluency task can differentiate psychiatric patients from healthy controls (HC). However, this paradigm has not been validated in the English language. Therefore, the present work aimed to distinguish patients with MDD from HCs, using haemodynamic response measured during an English letter fluency task. One hundred and five HCs and 105 patients with MDD took part in this study. NIRS signals during the verbal fluency task (VFT) was acquired using a 52-channel system, and changes in oxy-haemoglobin in the frontal and temporal regions were quantified. Depression severity, psychosocial functioning, pharmacotherapy and psychiatric history were noted. Patients with MDD had smaller changes in oxy-haemoglobin in the frontal and temporal cortices than HCs. In both regions of interest, oxy-haemoglobin was not associated with any of the clinical variables studied. 75.2% and 76.5% of patients with MDD were correctly classified using frontal and temporal region oxy-haemoglobin, respectively. Haemodynamic response measured by fNIRS during an English letter fluency task is a promising biomarker for MDD.
Similar content being viewed by others
Introduction
Major depressive disorder (MDD) is a common global disorder that impairs daily living for sufferers, and causes significant societal and economic burden1,2. It is characterised by low mood and anhedonia for almost every day in a two-week period. These core symptoms are often accompanied by several other somatic, psychological and cognitive symptoms, that vary between individuals in terms of their occurrence and severity3. Patients are assessed during clinical interviews, but given the heterogenous nature of this disorder, subjective patient reports, and reliance on individual clinical judgement, there is poor inter-rater reliability in diagnosing MDD4. Despite the disease burden, and inaccuracies of clinical interviews, there are currently no quantitative biological tests for MDD. There is however, growing evidence for the utility of functional neuroimaging, particularly functional near-infrared spectroscopy (fNIRS), to aid in the diagnosis of MDD.
Light in the NIR spectrum has the unique property of penetrating tissues, and being preferentially absorbed by haemoglobin5. The absorbance spectra of haemoglobin is dependent on its binding with oxygen, which enables fNIRS devices to continuously monitor both oxy-haemoglobin and deoxy-haemoglobin in the cerebral cortex6. NIRS signals are a surrogate measure of the underlying neural activity, described by a phenomenon known as neurovascular coupling7. Regional neuron activity triggers an increase in blood flow and volume that is disproportionately higher than the metabolic demand. Therefore, cerebral haemodynamic response typically involves a nett increase in oxy-haemoglobin, and a simultaneous slight decrease in deoxy-haemoglobin8. Since the changes in oxy-haemoglobin are greater than deoxy-haemoglobin, the former is used as an indicator of cerebral activity9. Although NIR light cannot reach subcortical regions, the practical advantages of fNIRS make it a suitable tool for assessing psychiatric patients. Compared to conventional functional neuroimaging methods, such as functional magnetic resonance imaging (MRI) and positron emission tomography (PET), fNIRS is cost-effective and does not involve ionising radiation, restraints or loud noise10.
fNIRS studies have consistently reported reduced oxy-haemoglobin in the frontotemporal cortices during the verbal fluency test (VFT) in patients with MDD compared to healthy controls (HC)11. This cognitive task assesses language production and word retrieval controlled by the posterior inferior frontal gyrus, and semantic processing controlled by the anterior inferior temporal gyrus12. Thus, from a neuropsychological perspective, reduced frontotemporal haemodynamic response to cognitive stimuli may be associated with the cognitive symptoms of MDD. At the same time, reduced haemodynamic response may reflect neurophysiological changes associated with MDD. Although the biological mechanism of haemodynamic dysfunction is not clear, post-mortem and animal models suggest neurons and glial cells responsible for maintaining haemodynamic response lose their function in patients with MDD13.
While several variations of the VFT have been published in the fNIRS literature14,15,16,17, the VFT and NIRS signal processing protocol proposed by Takizawa et al.18 was designed specifically for neuropsychiatric assessment. Subsequent studies using this protocol have demonstrated that frontotemporal oxy-haemoglobin response magnitude could distinguish HCs and patients with common psychiatric disorders, namely MDD, schizophrenia and bipolar disorder19. Moreover, frontotemporal oxy-haemoglobin response magnitude is associated with depression severity20, social functioning21 and family psychiatric history22 in patients with common psychiatric disorders. Despite these promising results, this paradigm has not been modified for the English language. Hence, the objective of this study is to validate an fNIRS diagnostic test for MDD in English-speaking adults.
We hypothesise that low activation in the frontotemporal cortex is a salient trait of patients with MDD, and that frontotemporal oxy-haemoglobin response magnitude can differentiate patients with MDD from HCs with acceptable accuracy. Hence, the first aim of this study was to demonstrate lower frontotemporal oxy-haemoglobin response magnitude during an English version of the VFT in patients with MDD compared to HCs. The second aim was to investigate the relationship between cortical oxy-haemoglobin and several clinical factors such as depression severity, pharmacotherapy and clinical history. Lastly, we report the sensitivity and specificity of classifying patients with MDD and HCs with frontotemporal oxy-haemoglobin response magnitude.
Results
Demographic and clinical data
HCs and patients with MDD did not differ in age, gender, ethnicity, handedness and family psychiatric history (p > 0.05) (Table 1). Compared to controls, patients with MDD had fewer years of education [t = 5.28, df = 186.46, p ≤ 0.001, d = 0.73, 95% CI, (1.08 to 2.37)], generated fewer words [t = 4.2, df = 203.62, p ≤ 0.001, d = 0.63, 95% CI, (1.89 to 5.24)], had higher HAM-D scores [t = -15.9, df = 123.33, p ≤ 0.001, d = 2.19, 95% CI, (-13.79 to -10.73)] and lower GAF scores [t = 17, df = 176.21, p ≤ 0.001, d = 2.35, 95% CI, (21.72 to 27.43)]. Approximately 70% of patients had no prior admission to a psychiatric ward, and approximately 35% were medication naïve. Amongst patients with MDD receiving pharmacotherapy, majority were on antidepressants, and a fraction were on combination anxiolytics or antipsychotics (Supplementary Table 2).
Comparing haemodynamic response between controls and patients
HCs and patients with MDD did not differ in the number of available channels in the frontal (MDD, n = 101; HC, n = 101; p = 0.094) and temporal (MDD, n = 102; HC, n = 103; p = 0.952) regions. Patients had lower integral values than controls in both the frontal [t = 4.76, df = 200, p ≤ 0.001, d = 0.67, 95% CI, (39.24 to 94.81)] and temporal [t = 6.99, df = 199.42, p ≤ 0.001, d = 0.98, 95% CI, (68.16 to 139.65)] regions (Fig. 1), but centroid values did not differ between controls and patients (frontal region, p = 0.567; temporal region, p = 0.689).
Factors associated with haemodynamic response
The frontal region integral value was not associated with demographic factors, clinical factors and channel availability for both HCs and patients with MDD. Similarly, the temporal region integral value was not associated with demographic and clinical factors for HCs and patients with MDD (supplementary table 3 and 4). However, the temporal region integral value negatively correlated with the number of available channels amongst HCs (r = −0.222, p = 0.024), but not patients with MDD. Stepwise linear regression also revealed associations between temporal region integral value and number of available channels amongst HCs (β = −6.68, SE = 2.92, p = 0.024, adjusted R2 = 0.04). Despite this statistically significant association, the number of available channels only accounts for 4% variability in temporal integral value. Thus, both the frontal and temporal integral values were used in ROC analysis.
Differentiating controls and patients with major depression
The area under the ROC curve for the frontal and temporal region integral values, were 0.76 [95% CI, (0.7 to 0.83)] and 0.82 [95% CI, (0.76 to 0.88)] respectively (Fig. 2). Using an optimal threshold value of 51, the frontal region integral value correctly classified 75.2% of patients with MDD (proportion of patients/measurements: 76/101) and 74.3% of HCs [proportion of controls/measurements: 75/101; positive predictive value (PPV) = 0.75; negative predictive value (NPV) = 0.75]. Similarly, with an optimal threshold value of 81, the temporal region integral value correctly classified 76.5% of patients with MDD (78/102) and 76.7% of HCs (79/103; PPV = 0.77; NPV = 0.77; Table 2).
Discussion
Results from this study lend support for an fNIRS paradigm as a supplementary tool for MDD diagnosis. In addition to patients with MDD having significantly lower integral values than HCs, integral values were not associated with any of the demographic and clinical factors. In particular, the independence of integral values from illness duration and treatment with psychotropic medications, suggests that the proposed fNIRS paradigm may be used to assess patients at any stage of their disorder. A similar conclusion was made in a recent publication on patients at various stages of psychosis23. Additionally, the independence of integral values from the number of words generated during the VFT and years of education, suggests that the proposed test is suitable for most people with and without MDD.
Another significant finding in this study is that the integral values could distinguish HCs and patients with MDD with acceptable sensitivity and specificity. The classification rates reported in this study are similar to an earlier report on Japanese subjects19. Our finding provides a cross-cultural validation of fNIRS metrices, as a marker for common disorders like MDD24. However, the optimal cut-off integral values in our sample were lower than the Japanese study19. This variation may be due to differences in the design of the VFT. While we used English alphabets, the Japanese VFT utilises syllabary. Since lexical retrieval strategies differ between alphabetic and non-alphabetic languages25, it may have an effect on cortical activity. Likewise, amongst fNIRS studies done on Chinese-speaking patients with MDD, cortical activity in the temporal region differs between HCs and patients during the Chinese phonological VFT26, whereas differences in prefrontal cortex activity was observed during the Chinese categorical VFT27. Furthermore, variations of the Chinese letter fluency task have been proposed in fNIRS studies on patients with schizophrenia, because different populations use different syllable systems28. Specifically, the letter fluency task designed by Quan et al.29 for subjects in mainland China differed from that used by Chou et al.30 for subjects in Taiwan. Hence, language specific validation of this fNIRS paradigm is important.
In research and clinical settings alike, the presence and severity of depressive symptoms are typically assessed by instruments like the HAM-D and GAF. Although we did not observe an association between these scales and integral values, this association was previously reported. However, the methodological differences between studies, and limitations of these scales should be noted. For instance, Kawano et al.20 recruited patients with various psychiatric disorders, and found that frontal region integral values negatively correlated with the total score of the 21-item HAM-D. The HAM-D can differentiate symptomatic and remitted patients with excellent accuracy31, but its discriminatory power for mild, moderate and severe depression is lower32. Moreover, the recommended threshold values for depression severity categories vary between publications33. Hence, total HAM-D scores may not be a reliable measure of depression severity for a correlation analysis with integral values. Still, negative correlation between oxy-haemoglobin and individual HAM-D items have been reported, albeit in differ cortical region of interest. Items include depressed mood, work and activities34, insomnia early, psychomotor retardation35, and suicidal ideation36. Yet, the options for many HAM-D items do not vary as a function of depression severity. Thus, individual item scores may not be a reliable indicator for disorder severity37. The level of oxy-haemoglobin measured at individual channels of patients with MDD has also been linked to the GAF38. The GAF score is a single measurement of overall impairment, that takes psychological symptoms and social and occupational functioning into consideration. However, much like the HAM-D items, the GAF scale also lacks continuity between intervals39. Nevertheless, low frontotemporal cortical activity is associated with the syndromic criteria for MDD in the fifth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-5)11.
Low frontal and temporal region integral values may represent a decline in physiological function amongst MDD patients. Concerted evidence from other neuroimaging modalities and post-mortem studies indicate that pathophysiological changes occur in the prefrontal cortex. Compared to HCs, neuron size and glia cell density are reduced in patients with MDD40. MRI techniques reveal grey matter volume reduction41 and blood-oxygen-level-dependent response attenuation during the VFT42. Furthermore, a previous study using PET reported reduced blood flow and metabolism in the prefrontal cortex of patients with MDD43. Biomarker validation and pathophysiology elucidation for MDD is especially challenging, since there are no established biological gold standards. Regrettably, both psychological and somatic symptoms can only be evaluated by clinical interviews. Therefore, psychiatric nosology may dampen the classification accuracy of potential biomarkers44. On the other hand, low integral values may be particularly useful to evaluate the cortical function of psychiatric patients who may underreport their symptoms, remitted patients at risk of relapse45,46 and patients with poor prognosis26,47,48,49.
This study has several limitations. Firstly, this cross-sectional study could not establish a causal relationship between low frontotemporal activity and onset of MDD. In contrast, previous longitudinal studies reported concurrent oxy-haemoglobin increase and MDD severity decrease50,51. However, these changes were only seen in 1 or 2 channels located at the temporal cortex. Therefore, integral value for the frontal and temporal regions may not vary over time. Longitudinal studies focussing on the same regions of interest are needed to verify this hypothesis. Moreover, consistency of frontal and temporal region integral values at different time points would demonstrate internal validity. Secondly, MDD subtypes were not compared in this study, even though it is a heterogenous disorder52. This may explain why clinical outcomes were not associated with integral values in this study. Future fNIRS investigations on other clinical outcomes, such as treatment response, may identify differences between MDD subtypes. Thirdly, this study was unable to determine the effect of individual psychotropic medications on haemodynamic response. Contrary to the present study, Takamiya et al.53 observed an association between oxy-haemoglobin at 5 channels across the frontotemporal region and daily defined dose of antidepressants. As antidepressants can improve depressive symptoms54 and improve cognitive function55, future fNIRS studies comparing antidepressants may provide insights into their mechanism of action. Lastly, majority of patients included in this study suffered from mild to moderate depression. Therefore, replication studies in severely depressed patients are needed to further support the fNIRS diagnostic paradigm. Despite these limitations, this is the first study to validate a single trial fNIRS VFT paradigm in the English language for MDD. Furthermore, the moderate sample size allows findings from this study to be generalised to other adult populations.
In conclusion, cortical oxy-haemoglobin changes measured by fNIRS provides a safe, non-invasive and direct measure of cerebral physiological function. Accordingly, diminished haemodynamic response in the frontal and temporal cortices of patients with MDD provides further evidence for the biological basis of this disorder. Threshold integral values determined in this study enable this fNIRS paradigm to be used as an objective and quantitative assessment of psychiatric patients who present with depressive symptoms.
Materials and methods
Participants
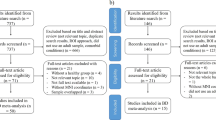
One hundred and five HCs and 105 age- and gender-matched patients with MDD participated in this study. All participants spoke English and were between 21–65 years old. Depressive symptoms and psychosocial functioning for each participant were evaluated on the day of participation using the 17-item Hamilton rating scale for depression (HAM-D)56 and global assessment of functioning (GAF)57, respectively.
HCs recruited from the community were assessed by a psychiatrist, and those included in this study were certified as being normal. HCs did not have a history of any psychiatric illnesses, including alcohol/substance abuse or addiction. HCs were excluded if they had conditions that could affect the central nervous system, including neurological illnesses such as epilepsy, traumatic brain injury, cerebrovascular diseases, respiratory diseases, hepatic diseases, kidney diseases, cancer or intellectual disability. Additionally, HCs who received psychotherapy in the past, had a HAM-D score of 8 or higher on the day of participation32, or reported drowsiness on the day of participation, were excluded.
Patients with MDD were recruited from the outpatient psychiatric clinic at the National University Hospital, Singapore. They were diagnosed by a psychiatrist, according to the DSM-5 criteria58. Patients with MDD were excluded if they had any neurological illnesses, traumatic brain injury, cerebrovascular diseases, respiratory diseases, hepatic diseases, kidney diseases, cancer, intellectual disability or alcohol/substance abuse or addiction. In addition, patients with MDD who received psychotherapy in the past, and those who reported drowsiness on the day of participation, were excluded.
Study details were fully explained to participants, and their written informed consent was obtained. This study was performed according to the Declaration of Helsinki, and the ethical principles in the Belmont Report. It was approved by the Domain Specific Review Board of the National Healthcare Group, Singapore (protocol number 2017/00509).
Verbal fluency task
Prior to the NIRS measurement recording, participants watched a demonstration video, in which they were asked to remain seated, avoid excessive body or head movements, and focus on a cross displayed during the VFT. The paradigm used in previous studies18 was modified for the English language (Fig. 3). It consisted of a 30 s pre-task period, 60 s task period, and a 70 s post-task period. During the pre- and post-task periods, participants were asked to say “A, B, C, D, E” aloud and repeatedly. During the task period, they were instructed to generate as many words as possible, beginning with A, F and S for 20 s per letter, similar to the conventional VFT59 and VFT protocols used in other fNIRS studies60. The total number of unique words, enunciated within the task period, was recorded as the task performance. Before the actual trial, participants were asked to practice the VFT for a shorter duration, and with the letters H, B and P. This ensured all participants understood the task and responded to the cues correctly during the actual trial.
NIRS measurement
A 52-channel NIRS system (ETG-4000. Hitachi Medical Co., Tokyo, Japan) measured relative oxy-haemoglobin and deoxy-haemoglobin changes using 2 NIR light wavelengths (695 and 830 nm)61. Emitter and detector optodes were arranged 3 cm apart. The area between each emitter and detector pair is called a channel. Anatomically, channels correspond to cortical regions 2–3 cm beneath the skin and scalp surface62. Optodes were placed on the forehead and scalp, with the lowest optodes placed along the T4-Fpz-T3 line, defined by the 10/20 system. This arrangement allowed for haemoglobin changes in the bilateral prefrontal cortex, frontopolar cortex, and the anterior regions of the superior and middle temporal cortices to be measured. These approximate channel locations are based on the anatomical craniocerebral correction of the international 10/20 system. Real coordinates of each channel were acquired using a 3D digitiser (Patriot system, Polhemus, USA) for each subject. Coordinates were superimposed on a cerebral cortex atlas using the statistical parametric mapping for near-infrared spectroscopy (NIRS-SPM) toolbox63,64,65, and did not differ between controls and patients (Supplementary Table 1).
NIRS signal analysis
NIR signals were processed according to the method described by Takizawa et al.18. Oxy-haemoglobin, deoxy-haemoglobin and total haemoglobin were derived from optical densities using the modified Beer-Lambert law. Haemoglobin changes during the task period were normalised by linear fitting between a 10 s baseline at the end of the pre-task period, and a 5 s baseline 50 s into the post-task period (Fig. 3). A moving average factor of 5 was applied to remove short term motion artefacts. An algorithm identifying channels with body movement artefacts, or high and low frequency noise was applied. Artefact channels were not used in further analysis. Previous studies have also established the reliability of synthesising NIRS signals from a cluster of associated channels19. The first cluster of 11 channels is located approximately at the frontopolar and dorsolateral prefrontal cortex. The second cluster of 20 channels is located approximately at the left and right ventrolateral prefrontal cortex, superior temporal cortex, and middle temporal cortex. These clusters are referred to as ‘frontal region’ and ‘temporal region’ respectively (Fig. 4). Oxy-haemoglobin changes measured at the channels for each region are averaged for each subject. Participants with at least 6 available channels in either regions of interest were included. Oxy-haemoglobin changes at each region was quantified using 2 visual spatiotemporal indices, called the integral and centroid values. The integral value is the area under the oxy-haemoglobin curve during the task period, and it is a metric of haemodynamic response magnitude. The centroid value is the time corresponding to the centre of the area under the oxy-haemoglobin curve, and it is a metric of haemodynamic change over time. A small centroid value indicates an earlier haemodynamic response, while a large centroid value indicates a delayed haemodynamic response19.
Statistical analysis
The effect of diagnostic group on continuous and categorical variables were determined using t-test and Pearson’s chi-square test, respectively. Continuous variables are age, years of education, number of words generated, HAM-D score, GAF score, integral values, centroid values and number of available channels. Categorical variables are gender, ethnicity, handedness and family psychiatric history. Integral and/or centroid values that were significantly different between MDD and HCs were used in further statistical analysis.
Variables associated with frontal and temporal region integral and/or centroid values within diagnostic groups were determined using Student’s t-test for dichotomous variables, Kruskal-Wallis test for categorical variables, and Pearson’s correlation for continuous variables. Dichotomous variables are gender, family psychiatric history, past admission to a psychiatric ward and pharmacotherapy. Categorical variables are ethnicity and handedness. Continuous variables are age, years of education, number of words generated, number of available channels in the corresponding region of interest, HAM-D score, GAF score, age at MDD onset, duration of MDD, and equivalent doses of antidepressants, anxiolytics and sedatives, and antipsychotics. Equivalent doses were calculated based on published mean dose ratios. Reference drugs for each class are fluoxetine, diazepam and chlorpromazine, respectively66,67. For patients receiving more than one drug in each class, the combined equivalent dose was calculated.
Subsequent stepwise linear regression was performed to verify variables associated with frontal and temporal region integral and/or centroid values. Independent variables included in the model for each diagnostic group are age, gender, years of education, HAM-D score, GAF score, number of words generated and number of available channels in the corresponding region of interest. Additional variables included in the regression analysis for patients with MDD are age at onset, duration of illness, past admission to a psychiatric ward and pharmacotherapy.
Lastly, receiver operating characteristic (ROC) analysis was performed to determine the accuracy of differentiating HCs and patients with MDD based on their frontal and temporal region integral and/or centroid value. All tests were two-tailed, with a significance level of p ≤ 0.05. Data are expressed as mean and standard deviation. The effect size (Cohen’s d) and the 95% confidence interval are reported wherever a statistically significant difference was observed between groups. Statistical analysis was done on SPSS Statistic 21.0 (IBM).
Data availability
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
References
Kessler, R. C. & Bromet, E. J. The epidemiology of depression across cultures. Annual review of public health 34, 119–138, https://doi.org/10.1146/annurev-publhealth-031912-114409 (2013).
Ho, R. C., Mak, K. K., Chua, A. N., Ho, C. S. & Mak, A. The effect of severity of depressive disorder on economic burden in a university hospital in Singapore. Expert review of pharmacoeconomics & outcomes research 13, 549–559, https://doi.org/10.1586/14737167.2013.815409 (2013).
Otte, C. et al. Major depressive disorder. Nature reviews. Disease primers 2, 16065, https://doi.org/10.1038/nrdp.2016.65 (2016).
Bilello, J. A. Seeking an objective diagnosis of depression. Biomarkers in Medicine 10, 861–875, https://doi.org/10.2217/bmm-2016-0076 (2016).
Lai, C. Y. Y., Ho, C. S. H., Lim, C. R. & Ho, R. C. M. Functional near-infrared spectroscopy in psychiatry. BJPsych Advances 23, 324–330, https://doi.org/10.1192/apt.bp.115.015610 (2017).
Ho, C. S., Zhang, M. W. & Ho, R. C. Optical Topography in Psychiatry: A Chip Off the Old Block or a New Look Beyond the Mind-Brain Frontiers? Frontiers in psychiatry 7, 74, https://doi.org/10.3389/fpsyt.2016.00074 (2016).
Gsell, W. et al. The use of cerebral blood flow as an index of neuronal activity in functional neuroimaging: experimental and pathophysiological considerations. Journal of chemical neuroanatomy 20, 215–224 (2000).
Scholkmann, F. et al. A review on continuous wave functional near-infrared spectroscopy and imaging instrumentation and methodology. Neuroimage 85, 6–27 (2014).
Ferrari, M. & Quaresima, V. A brief review on the history of human functional near-infrared spectroscopy (fNIRS) development and fields of application. Neuroimage 63, 921–935 (2012).
Ehlis, A.-C., Schneider, S., Dresler, T. & Fallgatter, A. J. Application of functional near-infrared spectroscopy in psychiatry. Neuroimage 85, 478–488 (2014).
Zhang, H. et al. Near-infrared spectroscopy for examination of prefrontal activation during cognitive tasks in patients with major depressive disorder: a meta-analysis of observational studies. Psychiatry Clin Neurosci 69, 22–33, https://doi.org/10.1111/pcn.12209 (2015).
Pinto-Grau, M., Hardiman, O. & Pender, N. The Study of Language in the Amyotrophic Lateral Sclerosis - Frontotemporal Spectrum Disorder: a Systematic Review of Findings and New Perspectives. Neuropsychology review 28, 251–268, https://doi.org/10.1007/s11065-018-9375-7 (2018).
Duric, V. & Duman, R. S. Depression and treatment response: dynamic interplay of signaling pathways and altered neural processes. Cellular and molecular life sciences: CMLS 70, 39–53, https://doi.org/10.1007/s00018-012-1020-7 (2013).
Ehlis, A.-C., Herrmann, M. J., Plichta, M. M. & Fallgatter, A. J. Cortical activation during two verbal fluency tasks in schizophrenic patients and healthy controls as assessed by multi-channel near-infrared spectroscopy. Psychiatry Research: Neuroimaging 156, 1–13 (2007).
Kubota, Y. et al. Altered prefrontal lobe oxygenation in bipolar disorder: a study by near-infrared spectroscopy. Psychological medicine 39, 1265–1275 (2009).
Yap, K. H. et al. Visualizing Hyperactivation in Neurodegeneration Based on Prefrontal Oxygenation: A Comparative Study of Mild Alzheimer’s Disease, Mild Cognitive Impairment, and Healthy Controls. Frontiers in Aging Neuroscience 9, 287 (2017).
Katzorke, A. et al. Decreased hemodynamic response in inferior frontotemporal regions in elderly with mild cognitive impairment. Psychiatry Research: Neuroimaging 274, 11–18 (2018).
Takizawa, R. et al. Reduced frontopolar activation during verbal fluency task in schizophrenia: a multi-channel near-infrared spectroscopy study. Schizophrenia research 99, 250–262 (2008).
Takizawa, R. et al. Neuroimaging-aided differential diagnosis of the depressive state. Neuroimage 85, 498–507 (2014).
Kawano, M. et al. Correlation between frontal lobe oxy-hemoglobin and severity of depression assessed using near-infrared spectroscopy. Journal of affective disorders 205, 154–158 (2016).
Ohtani, T. et al. Association between longitudinal changes in prefrontal hemodynamic responses and social adaptation in patients with bipolar disorder and major depressive disorder. Journal of affective disorders 176, 78–86 (2015).
Ohi, K. et al. Impact of familial loading on prefrontal activation in major psychiatric disorders: a near-infrared spectroscopy (NIRS) study. Scientific reports 7, 44268 (2017).
Koike, S. et al. Application of functional near infrared spectroscopy as supplementary examination for diagnosis of clinical stages of psychosis spectrum. Psychiatry and clinical neurosciences 71, 794–806 (2017).
Matsumoto, D. & Yoo, S. H. Toward a New Generation of Cross-Cultural Research. Perspectives on psychological science: a journal of the Association for Psychological Science 1, 234–250, https://doi.org/10.1111/j.1745-6916.2006.00014.x (2006).
Eng, N., Vonk, J. M., Salzberger, M. & Yoo, N. A cross-linguistic comparison of category and letter fluency: Mandarin and English. Quarterly Journal of Experimental Psychology, 1747021818765997 (2018).
Wang, J. et al. Right fronto-temporal activation differs between Chinese first-episode and recurrent Major Depression Disorders during a verbal fluency task: A near-infrared spectroscopy study. Psychiatry Research: Neuroimaging 264, 68–75 (2017).
Liu, X. et al. Relationship between the prefrontal function and the severity of the emotional symptoms during a verbal fluency task in patients with major depressive disorder: a multi-channel NIRS study. Progress in Neuro-Psychopharmacology and Biological Psychiatry 54, 114–121 (2014).
Chen, P. Modern Written Chinese in Development. Language in Society 22, 505–537, https://doi.org/10.1017/S0047404500017450 (1993).
Quan, W. et al. Reduced prefrontal activation during a verbal fluency task in Chinese-speaking patients with schizophrenia as measured by near-infrared spectroscopy. Progress in Neuro-Psychopharmacology and Biological Psychiatry 58, 51–58 (2015).
Chou, P.-H. et al. Duration of untreated psychosis and brain function during verbal fluency testing in first-episode schizophrenia: a near-infrared spectroscopy study. Scientific reports 5, 18069 (2015).
Zimmerman, M., Posternak, M. A. & Chelminski, I. Is the cutoff to define remission on the Hamilton Rating Scale for Depression too high? The Journal of nervous and mental disease 193, 170–175 (2005).
Zimmerman, M., Martinez, J. H., Young, D., Chelminski, I. & Dalrymple, K. Severity classification on the Hamilton depression rating scale. Journal of affective disorders 150, 384–388 (2013).
Kriston, L. & von Wolff, A. Not as golden as standards should be: interpretation of the Hamilton Rating Scale for Depression. J Affect Disord 128, 175–177, https://doi.org/10.1016/j.jad.2010.07.011 (2011).
Akiyama, T., Koeda, M., Okubo, Y. & Kimura, M. Hypofunction of left dorsolateral prefrontal cortex in depression during verbal fluency task: A multi-channel near-infrared spectroscopy study. Journal of affective disorders 231, 83–90 (2018).
Noda, T. et al. Frontal and right temporal activations correlate negatively with depression severity during verbal fluency task: a multi-channel near-infrared spectroscopy study. Journal of Psychiatric Research 46, 905–912 (2012).
Pu, S. et al. Suicidal ideation is associated with reduced prefrontal activation during a verbal fluency task in patients with major depressive disorder. Journal of affective disorders 181, 9–17 (2015).
Santor, D. A. & Coyne, J. C. Examining symptom expression as a function of symptom severity: item performance on the Hamilton Rating Scale for Depression. Psychological assessment 13, 127–139 (2001).
Kinou, M. et al. Differential spatiotemporal characteristics of the prefrontal hemodynamic response and their association with functional impairment in schizophrenia and major depression. Schizophrenia research 150, 459–467 (2013).
Pedersen, G., Urnes, O., Hummelen, B., Wilberg, T. & Kvarstein, E. H. Revised manual for the Global Assessment of Functioning scale. European psychiatry: the journal of the Association of European Psychiatrists 51, 16–19, https://doi.org/10.1016/j.eurpsy.2017.12.028 (2018).
Cotter, D. et al. Reduced neuronal size and glial cell density in area 9 of the dorsolateral prefrontal cortex in subjects with major depressive disorder. Cerebral cortex 12, 386–394 (2002).
Arnone, D., McIntosh, A. M., Ebmeier, K. P., Munafo, M. R. & Anderson, I. M. Magnetic resonance imaging studies in unipolar depression: systematic review and meta-regression analyses. European neuropsychopharmacology: the journal of the European College of Neuropsychopharmacology 22, 1–16, https://doi.org/10.1016/j.euroneuro.2011.05.003 (2012).
Okada, G., Okamoto, Y., Morinobu, S., Yamawaki, S. & Yokota, N. Attenuated left prefrontal activation during a verbal fluency task in patients with depression. Neuropsychobiology 47, 21–26, https://doi.org/10.1159/000068871 (2003).
Videbech, P. PET measurements of brain glucose metabolism and blood flow in major depressive disorder: a critical review. Acta psychiatrica Scandinavica 101, 11–20 (2000).
Strawbridge, R., Young, A. H. & Cleare, A. J. Biomarkers for depression: recent insights, current challenges and future prospects. Neuropsychiatric disease and treatment 13, 1245–1262, https://doi.org/10.2147/ndt.s114542 (2017).
Masai, M. et al. Residual symptoms were differentially associated with brain function in remitted patients with major depressive disorders. Yonago acta medica 59, 15 (2016).
Ikeda, E., Shiozaki, K., Ikeda, H., Suzuki, M. & Hirayasu, Y. Prefrontal dysfunction in remitted depression at work reinstatement using near-infrared spectroscopy. Psychiatry Research: Neuroimaging 214, 254–259 (2013).
Masuda, K. et al. Different functioning of prefrontal cortex predicts treatment response after a selective serotonin reuptake inhibitor treatment in patients with major depression. Journal of affective disorders 214, 44–52 (2017).
Tsujii, N. et al. Reduced left precentral regional responses in patients with major depressive disorder and history of suicide attempts. PloS one 12, e0175249 (2017).
Tsujii, N. et al. Right temporal activation differs between melancholia and nonmelancholic depression: a multichannel near-infrared spectroscopy study. Journal of psychiatric research 55, 1–7 (2014).
Tomioka, H. et al. A longitudinal functional neuroimaging study in medication-naive depression after antidepressant treatment. Plos One 10, e0120828 (2015).
Satomura, Y. et al. Severity-dependent and-independent brain regions of major depressive disorder: a long-term longitudinal near-infrared spectroscopy study. Journal of affective disorders (2018).
Goldberg, D. The heterogeneity of “major depression”. World Psychiatry 10, 226–228, https://doi.org/10.1002/j.2051-5545.2011.tb00061.x (2011).
Takamiya, A. et al. High-dose antidepressants affect near-infrared spectroscopy signals: A retrospective study. NeuroImage: Clinical 14, 648–655 (2017).
Lee, Y. et al. Efficacy of antidepressants on measures of workplace functioning in major depressive disorder: A systematic review. Journal of affective disorders 227, 406–415, https://doi.org/10.1016/j.jad.2017.11.003 (2018).
Lu, Y., Ho, C. S., McIntyre, R. S., Wang, W. & Ho, R. C. Effects of vortioxetine and fluoxetine on the level of Brain Derived Neurotrophic Factors (BDNF) in the hippocampus of chronic unpredictable mild stress-induced depressive rats. Brain research bulletin 142, 1–7, https://doi.org/10.1016/j.brainresbull.2018.06.007 (2018).
Hamilton, M. A rating scale for depression. Journal of neurology, neurosurgery, and psychiatry 23, 56 (1960).
American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-IV-TR. (American Psychiatric Association, 2000).
American Psychiatric Association. Diagnostic and statistical manual of mental disorders (DSM-5®). (American Psychiatric Pub, 2013).
Patterson, J. In Encyclopedia of Clinical Neuropsychology (eds. Jeffrey S. Kreutzer, John DeLuca, & Bruce Caplan) 1024-1026 (Springer New York, 2011).
Heinzel, S. et al. Aging-related cortical reorganization of verbal fluency processing: a functional near-infrared spectroscopy study. Neurobiology of aging 34, 439–450, https://doi.org/10.1016/j.neurobiolaging.2012.05.021 (2013).
Yamashita, Y. et al. Noninvasive near-infrared topography of human brain activity using intensity modulation spectroscopy. Optical Engineering 35 (1996).
Okada, E. & Delpy, D. T. Near-infrared light propagation in an adult head model. II. Effect of superficial tissue thickness on the sensitivity of the near-infrared spectroscopy signal. Applied optics 42, 2915–2921 (2003).
Singh, A. K., Okamoto, M., Dan, H., Jurcak, V. & Dan, I. Spatial registration of multichannel multi-subject fNIRS data to MNI space without MRI. Neuroimage 27, 842–851 (2005).
Jang, K.-E. et al. Wavelet minimum description length detrending for near-infrared spectroscopy. Journal of biomedical optics 14, 034004 (2009).
Ye, J. C., Tak, S., Jang, K. E., Jung, J. & Jang, J. NIRS-SPM: statistical parametric mapping for near-infrared spectroscopy. Neuroimage 44, 428–447 (2009).
Hayasaka, Y. et al. Dose equivalents of antidepressants: evidence-based recommendations from randomized controlled trials. Journal of affective disorders 180, 179–184 (2015).
Inada, T. & Inagaki, A. Psychotropic dose equivalence in J apan. Psychiatry and clinical neurosciences 69, 440–447 (2015).
Jichi Medical University. NIRS tools, http://www.jichi.ac.jp/brainlab/tools.html (2010).
Jurcak, V., Tsuzuki, D. & Dan, I. 10/20, 10/10, and 10/5 systems revisited: their validity as relative head-surface-based positioning systems. Neuroimage 34, 1600–1611 (2007).
Acknowledgements
The authors would like to thank all study participants and Hitachi Medical Systems Singapore for their in-kind contribution of the ETG-4000 system. This study was funded by NUS iHeathtech Other Operating Expenses (R-722-000-004-731), the Ministry of Education under HICOE scheme to CISIR, UTP, and the Vingroup Innovation Foundation in project code VINIF.2019.DA14.
Author information
Authors and Affiliations
Contributions
S.F.H. conducted the fNIRS scans, analysed the data and drafted the manuscript. T.T.Q., S.H.H., C.W.C. and C.S.H. recruited participants. R.Y., T.B.T., W.W.T. and B.T. provided advice for fNIRS signal or statistical analysis. R.C.H. designed and supervised the study. All authors contributed to the preparation of this manuscript and approved the final article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Husain, S., Yu, R., Tang, TB. et al. Validating a functional near-infrared spectroscopy diagnostic paradigm for Major Depressive Disorder. Sci Rep 10, 9740 (2020). https://doi.org/10.1038/s41598-020-66784-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-66784-2
This article is cited by
-
Insomniacs show greater prefrontal activation during verbal fluency task compared to non-insomniacs: a functional near-infrared spectroscopy investigation of depression in patients
BMC Psychiatry (2023)
-
An fNIRS investigation of novel expressed emotion stimulations in schizophrenia
Scientific Reports (2023)
-
Association of the time spent on social media news with depression and suicidal ideation among a sample of Lebanese adults during the COVID-19 pandemic and the Lebanese economic crisis
Current Psychology (2023)
-
Perceived social support and depressive symptoms in Chinese patients with ovarian cancer and the mediating role of resilience:a cross-sectional study
Current Psychology (2023)
-
Long-term artificial/natural daytime light affects mood, melatonin, corticosterone, and gut microbiota in rats
Applied Microbiology and Biotechnology (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.