Abstract
The Newcastle disease virus (NDV) strain AF2240 is an avian avulavirus that has been demonstrated to possess oncolytic activity against cancer cells. However, to illicit a greater anti-cancer immune response, it is believed that the incorporation of immunostimulatory genes such as IL12 into a recombinant NDV backbone will enhance its oncolytic effect. In this study, a newly developed recombinant NDV that expresses IL12 (rAF-IL12) was tested for its safety, stability and cytotoxicity. The stability of rAF-IL12 was maintained when passaged in specific pathogen free (SPF) chicken eggs from passage 1 to passage 10; with an HA titer of 29. Based on the results obtained from the MTT cytotoxic assay, rAF-IL12 was determined to be safe as it only induced cytotoxic effects against normal chicken cell lines and human breast cancer cells while sparing normal cells. Significant tumor growth inhibition (52%) was observed in the rAF-IL12-treated mice. The in vivo safety profile of rAF-IL12 was confirmed through histological observation and viral load titer assay. The concentration and presence of the expressed IL12 was quantified and verified via ELISA assay. In summary, rAF-IL12 was proven to be safe, selectively replicating in chicken and cancer cells and was able to maintain its stability throughout several passages; thus enhancing its potential as an anti-breast cancer vaccine.
Similar content being viewed by others
Introduction
Newcastle disease virus (NDV) belongs to the family of Paramyxoviridae. It has a negative-stranded and non-segmented RNA; with a total viral genome size of 15.9 kb1. It has six proteins that include a nucleocapsid protein (NP), phosphoprotein (P), large protein (L), matrix protein (M), hemagglutinin-neuraminidase glycoprotein (HN) and fusion protein (F)2. Another two additional proteins, V and W are produced during P gene transcription through RNA editing3. NDV strains can be classified into three main pathotypes; velogenic (highly virulent), mesogenic (intermediate virulence) or lentogenic (non-virulent)4. NDV strain AF2240 is a Malaysian viscerotropic velogenic strain that was isolated in the 1960s and is currently used in this country as a challenge virus in vaccine trials3. The virus has been studied extensively for its oncolytic activity in our laboratory (reviewed in Kalyanasundram et al.5). In addition, the AF2240 strain has resulted in several successful in vitro, in vivo and preclinical trials in cancer patients as anticancer agents since 1950s6,7,8,9,10,11.
The development of recombinant NDV has been primarily focused on increasing the oncolytic activity and therapeutic effect. Recombinant NDV expressing several genes such as interleukin 2 (IL2) and interferon-α12, granulocyte/macrophage colony-stimulating factor13, influenza NS1 protein14, tumor necrosis factor, and interferon-γ15 have been extensively studied. In this study, we inserted a human IL12 gene into the NDV genome at the position between M and F genes. IL12 is a heterodimeric cytokine that is produced specifically by phagocytic cells as well as antigen-presenting cells and it is known to enhance anti-tumor immune response16. In addition, IL12 regulates inflammation through the adaptive and innate immune responses by activating natural killer (NK) and T cells; thus boosting the anti-tumor immune response17. A study conducted by Sangro et al.18 proved that an adenovirus encoding IL12 was able to target liver cancer cells through intratumoral injections. In that study, IL12 was able to induce anti-tumor effects while reducing systemic toxicity18.
There are several studies that have investigated the role of IL12 in many types of cancer such as liver, colorectal and pancreatic cancer18, renal cancer19 and gastrointestinal primary malignancy20. The objective of this study was to evaluate the safety, stability and cytotoxic effect of rAF-IL12 against human breast cancer cells in vitro and in vivo for the potential development of an efficacious anti-breast cancer vaccine.
Results
Generation of recombinant NDV (rAF-IL12)
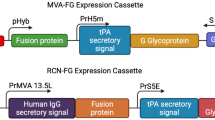
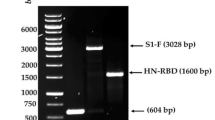
Successful insertion of IL12 gene into the NDV genome was done by cloning the gene between two Nhe1 restriction sites that were introduced between the M and F genes. The presence of the gene was verified by polymerase chain reaction (PCR) through the detection of size differences in the intergenic region of all NDV genes as shown in Fig. S2. The size of M/F region without IL12 is 377 bp, while in the presence of IL12, the size increases to 2 kb14. For further verification of the correct orientation of the IL12 gene, the gene was sequenced and 100% similarity was confirmed.
Stability and pathogenicity assessment of rAF-IL12
The HA titer of rAF-IL12 was stable when propagated in the embryonated chicken eggs from passage 1 with a starting HA unit of 28 up to passage 10 with a final HA unit of 211 (Table 1). The rAF-IL12 of passages 1, 5 and 10 were used in the subsequent studies to investigate if the stability and pathogenicity of the recombinant virus was maintained throughout the respective passages.
Pathogenicity of the rAF-IL12 was determined using the mean death time (MDT) and intracerebral pathogenicity index (ICPI). The rAF-IL12 passages 1, 5, and 10 showed an ICPI of 1.78; indicating towards a velogenic nature (Table 2). However, MDT results indicated towards a more mesogenic nature as compared to that of the velogenic parental AF2240. According to Dortmans et al.21, although MDT provides useful virulence indication, ICPI is a more accurate measure for the virulence of NDV strains.
In vitro cytotoxicity evaluation of rAF-IL12
To assess the in vitro cytotoxic effect of rAF-IL12 in multiple cell types, MTT assay was conducted using the highest HA unit of each representative virus (as mentioned above). The IC50 (half-maximal inhibitory concentration) results in Fig. 1a represents a chicken embryo fibroblast cell line; DF-1, Fig. 1b,c represent two human breast cancer cell lines; MDA-MB231 and MCF-7, respectively and Fig. 1d represents a non-malignant human breast cell line; MCF-10A, when treated with either rAF-IL12 (passages 1, 5 or 10) or the parental NDV AF2240 for 24, 48 and 72 hours. In general, rAF-IL12 from all tested passages were able to significantly (p < 0.05) induce cytotoxic effects and inhibit the proliferation in DF-1 and both breast cancer cells. A similar pattern was observed in the parental AF2240-treated groups. Othman et al.22 demonstrated that NDV AF2240 strain possess cytotoxic activity effects against various types of cancer. However, rAF-IL12 showed enhanced cytotoxic effects against the breast cancer cell lines as compared to AF2240 (Fig. 1a–c); where the rAF-IL12 IC50 values were significantly lower (p < 0.05).
Cytotoxic effects of rAF-IL12 and the parental strain AF2240 against: (a) DF-1; (b) MDA-MB231; (c) MCF-7 and; (d) MCF-10A at 24, 48 and 72 h post-inoculation. The values are expressed as the mean values ± standard deviation of triplicate experiments. The mean values as indicated using different superscript letters were significantly different at p < 0.05 when compared between parental AF2240- and rAF-IL12 (Passages 1, 5 & 10)-treated groups.
The MTT results prove that rAF-IL12, at each tested passage was able to significantly induce cytotoxicity against DF-1, MDA-MB231 and MCF-7 and was safe as it caused limited cytotoxic effects against the normal MCF-10A breast cell line.
Level of IL12 expression by rAF-IL12
The concentration of IL12 that was secreted by rAF-IL12 passages 1, 5 and 10 in DF-1, MDA-MB231, MCF-7 and MCF-10A cells was measured by an ELISA assay that contained capture antibodies that only recognized the IL-12 heterodimer and not the individual subunits. As demonstrated in Fig. 2, high levels of IL12 were detected in all the rAF-IL12-treated cells.
The expression of human IL-12 in multiple cell lines infected with NDV AF2240 and rAF-IL12 (Passages 1, 5 & 10) determined by ELISA assay at 24, 48 and 72 h. (a) DF-1 cells treated with NDV AF2240 and rAF-IL12 (Passages 1, 5 & 10), (b) MCF-7 cells treated with NDV AF2240 and rAF-IL12 (Passages 1, 5 & 10), (c) MDA-MB231 cells treated with NDV AF2240 and rAF-IL12 (Passages 1, 5 & 10), (d) MCF10A cells treated with NDV AF2240 and rAF-IL12 (Passages 1, 5 & 10). The values are expressed as the mean values ± standard deviation of triplicate experiments. The mean values as indicated using different superscript letters were significantly different at p < 0.05 when compared between parental AF2240- and rAF-IL12 (Passages 1, 5 & 10)-treated groups.
As compared to the parental AF2240, the concentration of IL12 was significantly (p < 0.05) higher in all the tested passages of rAF-IL12 across all tested time points; where the level of IL12 expression was found to be as high as 600 pg/ml at 24 hours post-infection but the expression levels gradually decreased over time. Although there was a significant reduction of IL12 expression in the later time points, the levels were still significantly above 100 pg/ml.
rAF-IL12 significantly inhibits the growth of 4T1 cells in vivo
In this study, 27 Balb/c mice were subcutaneously injected with 4T1 mouse breast cancer cells to develop tumors. The mice were randomly divided into 3 intra-tumoral treatment groups of 9 mice each. The mice in the first group were treated with rAF-IL12 passage 10 (27 HAU), the second group received an equivalent dose of AF2240 and the third group was treated with PBS. The rAF-IL12 demonstrated greater anti-tumor effects as compared to the parental AF2240 (Fig. 3). Tumor size in rAF-IL12-treated mice was significantly reduced as compared to the AF2240 and PBS-treated mice (Fig. 3a). As shown in Fig. 3b, the relative tumor weight after 28 days of treatment was less in rAF-IL12-treated mice as compared to AF2240-treated mice. The rAF-IL12 treatment resulted in 52% growth inhibition, while AF2240 caused 34.5% growth inhibition as compared to the PBS control group. Thus the tumor burden of rAF-IL12 treated group was significantly reduced (p < 0.05) in comparison to that of the AF2240 and control group (Fig. 3c).
The tumors harvested from untreated, NDV AF2240 and rAF-IL12 passage 10-treated mice (27 HAU/kg) mice. (a) Representative images, (b) average weight and (c) volume of the tumors harvested from untreated, AF2240 and rAF-IL12 passage 10-treated mice. Each value represents the mean values ± S.E.M. There were 9 mice per group. The values are expressed as the mean values ± standard deviation of triplicate experiments. The mean values as indicated using different superscript letters were significantly different at p < 0.05 when compared between parental AF2240- and rAF-IL12 (Passages 1, 5 & 10)-treated groups.
rAF-IL12 reduced mitosis and significantly augments IL12 cytokine expression
Histological examination of the tumors from the control group identified a high proportion of cancer cells that were mitotic, as demonstrated by the presence of condensed dark purple nuclei that were stained by hematoxylin (indicated by the black arrows) in Fig. 4. However, the number of mitotic cells in the tumors harvested from rAF-IL12-treated mice was reduced as compared to the control group.
The human IL-12 levels were measured in the serum of the rAF-IL12-treated, AF2240-treated and control mice groups. As demonstrated in Fig. 5, the level of IL12 increased significantly (p < 0.05) in the rAF-IL12 passage 10-treated mice as compared to both the AF2240-treated and untreated mice. The level of IL-12 was increased up to 204.07 ± 0.66 pg/mL in the rAF-IL12 passage 10-treated mice.
ELISA-based detection of IL-12 levels in the serum of the control (untreated), NDV AF2240- and rAF-IL12 passage 10-treated mice groups. Each value represents the mean values ± S.E.M for n = 9 mice per group. The values are expressed as the mean values ± standard deviation of triplicate experiments. The mean values as indicated using different superscript letters were significantly different at p < 0.05 when compared between parental AF2240- and rAF-IL12 (Passages 1, 5 & 10)-treated groups.
Quantification of rAF-IL12 in tumor and organs of treated mice
The amount of virus that were detected in the organs and tumors of normal (Fig. 6a) and rAF-IL12-treated mice (Fig. 6b) after 72 hours and the amount of detectable virus detected in rAF-IL12- or AF2240-treated mice after 28 days are demonstrated in Fig. 6c. Interestingly, the virus was present at very low amounts in the red blood cells, liver and kidney, while no virus was detected in the heart and brain after 72 hours in the normal mice. In addition, based on Fig. 6b, there was a significant (p < 0.05) decrease in the amount of virus between 12 hours to 72 hours in the organs of the treated mice. The rAF-IL12 was not detected in the organs of the treated mice after 72 hours. However, the amount of rAF-IL12 significantly (p < 0.05) increased in the tumors of the mice across time. Across all time points, viral copy number of rAF-IL12 was highest in the tumor. Based on Fig. 6c, there was a significant (p < 0.05) decrease in the amount of rAF-IL12 in the treated organs as compared to AF2240. In addition, both viruses were not detected in the brains of the treated mice after 28 days. However, the viral load was substantially increased in the rAFIL12-treated tumors as compared to the AF2240-treated tumors.
Detection of viral copy number in organs and tumor of treated mice using Taqman quantitative real time PCR. Images represent (a) normal (no tumor) and (b) rAF-IL12 treated mice after 3 days using Taqman quantitative real time PCR, c) detection of viral copy number in organs and tumor in NDV rAF-IL12 passage 10- and AF2240-treated mice after 28 days using Taqman quantitative real time PCR. Each value represents the mean values ± S.E.M for n = 9 mice per group. RBC = Red blood cells. The values are expressed as the mean values ± standard deviation of triplicate experiments. The mean values as indicated using different superscript letters were significantly different at p < 0.05 when compared between parental AF2240- and rAF-IL12 (Passages 1, 5 & 10)-treated groups.
Discussion
The recombinant Newcastle disease virus (rNDV) is selectively pathogenic; causing limited harm to humans8. Preclinical studies have been conducted using NDV due to its oncotherapeutic potential; making it a promising non-mainstream oncovirotherapeutic agent8. The virus has been found to be a highly effective oncolytic agent with high selectively against cancer cells23. A previous study identified NDV as an efficient vector to express and deliver foreign genes for vaccination and gene therapy1. Increasingly, the oncotherapeutic potential of using recombinant NDV to carry various different types of genes are being investigated24.
In the present study, the IL12 gene was inserted in between the M and F genes to ensure sufficient IL12 expression. Our results show that the IL12 gene was correctly inserted into the genome of AF2240 between the M and F genes. IL12 (P70) was chosen as the fusion gene candidate due to the heterodimeric cytokine consisting of both light chain p53 and heavy chain p40, IL12A and IL12B, respectively. This was done to avoid competitive suppression of IL12 if the IL12 P40-40 homodimer were used25. To date, there are no reports of using genetically engineered human IL12 expressing-NDV against breast cancer. Human IL12 has been reported to enhance anti-tumor immune response by selectively stimulating human T cells as a powerful agent against tumors16,17,26,27. In this study, the safety, stability and cytotoxic effects of a recombinant NDV, rAF-IL12 was investigated.
Inserting a foreign gene between viral genes can cause changes in the abundance of the mRNAs of the flanking gene28. Hence, it is important to identify the optimum site for the foreign gene to be inserted into the genome of NDV so as to ensure efficient levels of expression of the inserted gene and minimize the impact on NDV replication features. Zhao and Peeters27 conducted a study to test the differences in expression level of a reporter gene encoding human secreted alkaline phosphatase (SEAP) gene when inserted at different sites of the NDV genome. Based on their study, the expression of the SEAP gene that was inserted between NP and P genes was relatively lower as compared to the insertion between the M and F gene when recovered from embryonated eggs at 4 days. The ability of rAF-IL12 to express IL12 in DF-1, MCF-7, MDA-MB231 and MCF-10A was analyzed in this study. High levels of IL12 were detected upon rAF-IL12 treatment across all the tested cell lines. The stability of IL12 gene expression in rAF-IL12 was also confirmed over 10 passages.
The pathogenicity of the recombinant virus based on MDT and ICPI results was also confirmed. The HA, MDT and ICPI results of rAF-IL12 remained stable and unchanged for each tested passage. Thus, the rAF-IL12 was genetically and pathogenically stable during extensive passages in vivo. Replication and pathogenic features of rAF-IL12 was not distinguishable from that of the parent NDV AF2240 strain, proving that the insertion of IL12 gene in the genome did not result in any significant differences in replication1. NDV AF2240 has been reported to be cytotoxic against breast cancer29. Nevertheless, the effect of rAF-IL12 against breast cancer in vitro has not been reported as yet. The recombinant virus was further tested in several cell lines, including the chicken embryonic fibroblast DF-1 cell line, two human breast cancer cell lines; human breast carcinoma cell line MCF-7 and human breast adenocarcinoma cell line MDA-MB231 and a non-malignant human breast cell line MCF-10A by using MTT cytotoxic assay. The MTT results showed that rAF-IL12 was comparable to the parental AF2240 strain; inducing cell death in a dose-dependent manner. The MTT cytotoxic assay also revealed that rAF-IL12 selectively induced cytotoxic effects (IC50) in both chicken and human breast cancer cell lines without significant toxicity against the normal MCF10A cell line, which makes it an ideal oncotherapeutic30. Our results were similar to a study conducted by Ahmad et al.29, where NDV AF2240 was tested against two normal cell lines; human umbilical vein endothelial cells and normal fibroblast of human breast cell. Based on their MTT assay results, NDV AF2240 did not induce cytotoxic activity against both normal cell lines. In the present study, similar results were obtained as both rAF-IL12 (passages 1, 5 and 10) and parental NDV AF2240 displayed minimal cytotoxic effect against non-malignant MCF-10A breast cell line; whereby the IC50 values were unobtainable.
As compared to NDV AF2240, rAF-IL12 managed to reduce the size and weight of the tumor with higher efficacy. In fact, rAF-IL12 significantly reduced the amount of mitotic figures and number of dividing cells in the tumor tissue as compared to the untreated group. The results suggest that using AF2240 in combination with IL12 expression resulted in a greater anti-tumor effect than using AF2240 alone. A study conducted by Rodriguez-Madoz et al.31 proved the safety of IL12 when expressed by a semliki forest virus (SFV-enhIL-12) to combat against chronic viral hepatitis and hepatocellular carcinoma in woodchucks. In that study, tumor regressed significantly while inducing the cytokine-related immune response against the viral hepatitis and hepatocellular carcinoma without any clinical signs of toxicity in the woodchucks when treated with the SFV-enhIL-12. In this study, the safety of rAF-IL12 was proven based on the viral load titer results of liver, kidney, lung, spleen, heart and brain of the treated mice models. The quantification of rAF-IL12 based on the viral load titer showed a significant (p < 0.05) decrease in the vital organs of the treated mice in comparison to the AF2240 treatment. The detectable viral copy number in the organs after 28 days of treatment (Fig. 6c) can be explained by the twice a week viral treatment. Mice sacrificed on Day 28 of treatment would have been injected with the virus on Day 26; only 48 hours before sacrifice. As demonstrated in Fig. 6b, viral clearance only occurs after 72 hours. In both viral treatment groups, no virus was detected in the brain. In another study, IFN-γ was found to be able to significantly decrease viral load in brain tissues32. The secretion of IFN-γ in the brain is activated by IL12; resulting in enhanced immune effector functions for viral clearance32. We postulate that a similar IL12-induced IFN-γ-mediated increase of viral clearance occurred in the other vital organs; including the heart of the rAFIL12-treated mice. However, the exact mechanism(s) by which viral clearance is mediated in these organs is yet to be elucidated.
Intra-tumoral viral treatment enhances viral spreading to the tumor site; causing natural killer cells and cytotoxic T lymphocytes directed against viral antigens in the infected tumor cells to contribute towards the oncolytic activity33. Besides that, improved oncolysis through a combination of both direct viral infection (intra-tumoral) and immune mechanisms (IL12) has also been demonstrated33. This potentially explains the high viral copy number and viral tropism for tumors. Incidentally, the oncoselectivity of NDV is dependent on various intracellular factors such as the expression of specific proteins as well as the extracellular matrix component of the tissues and is thus cell-dependent33. Nevertheless, the anti-viral response in vivo allows efficient NDV viral clearance, which is essential because the viral vector will no longer be needed after the induction of anti-tumor effects via immunostimulatory cytokine expression34,35. Thus the study highlights the ability of rAF-IL12 as an efficient and safe candidate for breast cancer therapy. In the future, the editing of F gene of rAF-IL12 for clinical use will be considered. Hence, further studies should focus on the determination of the apoptotic and anti-metastatic effects of rAF-IL12 against human breast cancer cells to further substantiate the potential of rAF-IL12 as an oncovirotherapeutic.
Materials and Method
Plasmids, cells and viruses
The plasmid prAF_poltv5 was obtained from Kavitha Muralitharan (Institute of Bioscience, Universiti Putra Malaysia) and served as the backbone for the modification of the NDV genome and plasmid pUNO1-hIL12 (p40p35) containing IL-12 gene was purchased from Invivogen, USA. The cell lines BHK-21 (ATCC-CCL-10), DF-1 (ATCC-CRL-12203), MCF-7 (ATCC-HTB-22), MDA-MB231 (ATCC-HTB-26), MCF-10A (ATCC-CRL-10317) and 4T1 (CRL-2539) were obtained from the ATCC collection (ATCC, USA). BHK-21 cells were maintained in Glasgow Minimum Essential Media (GMEM) supplemented with 10% Newborn Calf Serum (NCS), 1% tryptose phosphate broth, 1% MEM amino acids and 1% penicillin-streptomycin. Geneticin (G418) was added to the cell culture for selection of cells expressing T7 before transfection (1). MCF-7 and 4T1 cells were maintained in RPMI (Roswell Park Memorial Institute), while DF-1 and MDA-MB231 cells were maintained in DMEM (Dulbecco’s Modified Eagle Medium). All media were supplemented with 10% fetal bovine serum and 1% penicillin-streptomycin. MCF-10A cells on the other hand, were maintained in DMEM F12 media supplemented with hydrocortisone (0.5 μg/mL), insulin (10 μg/mL), hEGF (20 351 ng/mL) and 10% (v/v) FBS (PAA, Austria). All the cells were kept in a 37 °C humidified incubator equipped with 5% CO2. The parental NDV strain, AF2240, a Malaysian strain with a HA unit of 210 was used as a positive control throughout this study. Viruses were purified by 30–60% (w/v) sucrose gradient centrifugation at 275 000 × g for 4 h at 4 °C, and stored in TE buffer (10 mM Tris-Cl pH 8.0, 1 mM EDTA, pH 8.0).
Construction and rescue of the recombinant virus
The IL-12 gene was amplified using a set of flanking primers include Nhe1 restriction site, GS, IS, 3′ untranslated region, optimal Kozak sequence, GE and 5′ UTR of IL12 gene. The IL12 gene was amplified from plasmid pUNO1-hIL12 (p40p35). The primer sequences are listed out in Table 3. A restriction site for Nhe1 was added in the forward primer (also in reverse primer) by mutating the sequences in the 5′ untranslated region of the M gene (5′ untranslated region of IL-12 gene) for cloning purposes. The design for the insertion of transgene is illustrated in Fig. S3. To insert the Nhe1 restriction site between the M and F genes, two sets of primers were used in overlap extension PCR (OE-PCR). The M/F fragment was amplified from the plasmid prAF_poltv5.
The BHK-21 cells were maintained in 6-well plates prior to transfection until 60–80% confluency. About 1 µg of full-length rAF-IL12 plasmid, 0.4 µg of pCI-neo-NP, 0.2 µg of pCI-neo-P and 0.2 µg of pCI-neo-L helper plasmids were transfected into the BHK-21 cell line using Lipofectamine™ 300027,36,37. The culture supernatant was harvested at 24 hours post transfection and inoculated into 9-day old specific pathogen free (SPF) embryonated chicken eggs.
Propagation of rAF-IL12
The rAF-IL12 was propagated in allantoic fluid of 9–11 day-old SPF embryonated chicken eggs at 37 °C for 3–4 days. The allantoic fluid was harvested and the replication of virus was confirmed by the HA assay using 1% chicken red blood cells38. The obtained virus was then diluted 105 fold for inoculation into new eggs. The propagation was conducted up to passage 10. DF-1 cells on 6-well plates were infected with serially diluted virus and incubated under a 1% methylcellulose overlay at 37 °C for approximately 3–6 days. The plaque size of each variant was obtained by calculating the mean area of 10 plaques and expressed as a value relative to that of the strain rAF-IL12. Experiments were conducted with an MOI of 0.01 for both NDV AF2240 and rAF-IL12.
Mean death time
To determine the pathogenicity of rAF-IL12 at passage 1, 5 and 10 as well as the parental virus NDV AF2240 in embryonated chicken eggs, MDT was determined according to the OIE manual39. Briefly, five 10-day-old embryonated chicken eggs were infected with serial 10-fold dilutions of viruses. The eggs were incubated at 37 °C and monitored twice daily for 7 days. The time to kill the embryos was recorded. The highest dilution that killed all the embryos was determined to be the minimum lethal dose. The MDT was calculated as the mean time for the minimum lethal dose to kill the embryos. The MDT has been used to classify NDV strains into the following groups: velogenic (taking under 60 hours to kill); mesogenic (taking between 60 and 90 hours to kill); and lentogenic (taking more than 90 hours to kill).
Intracerebral pathogenicity index
The ICPI was performed according to the OIE manual39 whereby ten 1-day old chicks were inoculated with 50 µl of infected allantoic fluid with an HA titer of more than 16 HA units/50 µl and diluted 10-fold in PBS. The birds were kept under observation for 8 days and examined daily. The virus isolates that scored an ICPI <0.7 were declared as lentogenic and those with an ICPI 1.5 were considered as velogenic strains of NDV. The NDV strains with intermediate ICPI values were designated as mesogenic. All experiments involving experimental animals were approved by the committee of IACUC, Universiti Putra Malaysia (protocol number R062/2016) and conducted following the guidelines.
3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyl tetrazolium bromide (MTT) in vitro cytotoxic assay
The MTT assay was conducted in accordance to Mosmann40 with slight modifications. The cells were seeded in a 96-well plate at a concentration of 0.8 × 105 cells/well and incubated in a 37 °C CO2 incubator overnight. The next day, rAF-IL12 at passage 1, 5 and 10 or the parental virus NDV AF2240 was added to the wells at the highest concentration according to their HA unit. The cell viability was measured at 24, 48 and 72 hours post-treatment. Twenty microliter per well of MTT solution (Calbiochem, USA) was added, followed by 4 h incubation at 37 °C in a 5% CO2 atmosphere. Then, the supernatants were removed from the wells and 100 μL/well of dimethyl sulfoxide (DMSO) (Fisher, USA) was added to solubilize the formazan. The absorbance at 570 nm was measured using an ELISA microplate reader (Biotech Instruments, USA). Triplicates were carried out for each experiment. The following formula was used to determine the percentage of viable cells:
Enzyme-linked immunosorbent assay
IL12 concentration was quantified using the DuoSet ELISA Human IL12 p70 kit (R&D systems, Inc., USA) based on suppliers protocol. Upon viral treatment of the cells, the supernatant was collected and loaded into an antibody pre-coated microplate for 2 hours. The plate was washed with a wash buffer and the TMB substrate solution was added in the wells. After adding the stop solution, the absorbance was read on a μ-Quant ELISA Reader (Bio-Tek Instruments, USA) using 450 nm wavelength and 570 nm reference wavelengths.
In vivo cytotoxicity evaluation of rAF-IL12
A group of 5–6 week old female Balb/c mice obtained from Universiti Putra Malaysia’s Animal House were used in this study. The mice were kept under standard conditions at 25 ± 2 °C on a regular light-dark cycle and provided with standard diet pellets and tap water. The mice were divided into 9 mice per group whereby all experiments were conducted according to the Universiti Putra Malaysia’s ethical guidelines for the care of lab animals (UPM/IACUC/AUP-R061/2017).
Untreated and rAF-IL12-treated mice were injected with 1 × 105 cells per mice of the 4T1 cell line subcutaneously. Once the tumor developed after a period of 2 weeks measuring 1 cm, the mice were treated accordingly; untreated (100 µl of PBS), rAF-IL12 (100 µl of 27 HA unit virus) twice a week at the intra-tumoral site. The dose was selected based on a study by Conrad et al.38. Tumors were measured using vernier calipers twice a week41 and the tumor volume was calculated using the following formula: tumor volume (mm3) = [(width)2 × length]/2. The mice were sacrificed by (exsanguination under) ketamine-xylazine anaesthesia (200 mg ketamine and 20 mg xylazine per kg body weight) at day 28. The sections of organs and tumors were placed in 10% formalin for further histological analysis.
Viral quantitative reverse by RT-qCR
Total RNA was extracted from the organs and tumors by mechanical disruption and using a Qiagen RNAeasy Mini Kit (Qiagen, USA). The RNA was subjected to reverse transcriptase PCR using iScript cDNA Kit (Biorad, USA) (4 µl of 5× iScript Reverse Transcription Supemix, 6 µl of RNA template and 10 µl of nuclease-free water) incubated in a thermal cycler for 5 min at 25 °C, 30 min at 42 °C and 5 min at 85 °C with the reaction component. The synthesized cDNA was subjected to Taqman real time PCR assay using a reverse primer 5′ CGCTGTTGCAACCCCAAG 3′; forward primer 5′ TCCGCAAGATCCAAGGGTCT 3′ and probe 5′ (FAM)-AAGCGTTTCTGTCTCCTTCCTCCA-(BHQ) 3′ with the annealing temperature set at 58 °C using iQ Supermix (Biorad, USA). The cycling program was set up with 95 °C for 5 min followed by 39 cycles of 95 °C for 10 s, 56 °C for 30 s and 72 °C for 20 s.
Histopathology staining
The sections of organs and tumor immersed in 10% formalin were paraffin-embedded, stained with haematoxylin and eosin and viewed under a bright-field inverted microscope (Nikon, Japan). Histological architecture and mitotic cells between groups were compared.
Statistical analysis
The results were analyzed using SPSS Statistics 17 software. The results were expressed as mean ± standard error (SE). Statistically significant differences between the means were determined by One-Way ANOVA followed by Duncan post hoc test. Differences were considered significant when the p ≤ 0.05.
Data Availability
The authors declare that all the data in this manuscript are available.
References
Zhao, H., Janke, M., Fournier, P. & Schirrmacher, V. Recombinant Newcastle disease virus expressing human interleukin-2 serves as a potential candidate for tumor therapy. Virus Res 136, 75–80 (2008).
Sinkovics, J. G. & Horvath, J. C. Newcastle disease virus (NDV): brief history of its oncolytic strains. J Clin Virol 16, 1–15 (2000).
Murulitharan, K., Yusoff, K., Omar, A. R. & Molouki, A. Characterization of Malaysian velogenic NDV strain AF2240-I genomic sequence: a comparative study. Virus genes 46, 431–440 (2013).
Beard, C. W. & Hanson, R. P. Newcastle disease. In Disease of Poultry. Edited by M. S. Hofstad, H. J. Barnes, B. W. Calnek, W. M. Reid & H. W. Yoder. Ames, IA: Iowa State University Press 452–470 (1984).
Kalyanasundram, J., Hamid, A., Yusoff, K. & Chia, S. L. Newcastle disease virus strain AF2240 as an oncolytic virus: A review. Acta tropica 183, 126–133 (2018).
Alabsi, A. M. et al. Effects of Newcastle disease virus strains AF2240 and V4-UPM on cytolysis and apoptosis of leukemia cell lines. Int J Mol Sci 12, 8645–8660 (2011).
Ali, R. et al. Cytolytic effects and apoptosis induction of Newcastle disease virus strain AF2240 on anaplastic astrocytoma brain tumor cell line. Neurochem Res 11, 2051 (2011).
Kumar, S., Nayak, B., Collins, P. L. & Samal, S. K. Evaluation of the Newcastle disease virus F and HN proteins in protective immunity by using a recombinant avian paramyxovirus type 3 vector in chickens. J. Virol 85, 6521–6534 (2011).
Jamal, M. H., Ch’ng, W. C., Yusoff, K. & Shafee, N. Reduced Newcastle disease virus-induced oncolysis in a subpopulation of cisplatin-resistant MCF7 cells is associated with survivin stabilization. Cancer Cell Int 1, 35 (2012).
Lam, H. Y. et al. Immunomodulatory effects of Newcastle disease virus AF2240 strain on human peripheral blood mononuclear cells. Int J Med Sci 12, 1240 (2014).
Pan, Z. et al. Identification of optimal insertion site in recombinant Newcastle disease virus (rNDV) vector expressing foreign gene to enhance its anti-tumor effect. PloS one 10, e0164723 (2016).
Schirrmacher, V. Fifty Years of Clinical Application of Newcastle Disease Virus: Time to Celebrate! Biomedicines 4, 16 (2016).
Janke, M. et al. Recombinant Newcastle disease virus (NDV) with inserted gene coding for GM-CSF as a new vector for cancer immunogene therapy. Gene Therapy 14, 1639 (2007).
Zamarin, D. et al. Enhancement of oncolytic properties of recombinant newcastle disease virus through antagonism of cellular innate immune responses. Mol Ther 17, 697–706 (2009).
Vigil, A. et al. Use of reverse genetics to enhance the oncolytic properties of Newcastle disease virus. Cancer Res 67, 8285–8292 (2007).
Alkayyal, A. A., Mahmoud, A. B. & Auer, R. C. Interleukin-12-expressing oncolytic virus: A promising strategy for cancer immunotherapy. J Taibah Univ Sci 11, 187–193 (2016).
Tugues, S. et al. New insights into IL-12-mediated tumor suppression. Cell Death Differ 22, 237 (2015).
Sangro, B. et al. Phase I trial of intratumoral injection of an adenovirus encoding interleukin-12 for advanced digestive tumors. J Clin Oncol 22, 1389–1397 (2004).
Gollob, J. A. et al. Phase I trial of twice-weekly intravenous interleukin 12 in patients with metastatic renal cell cancer or malignant melanoma: ability to maintain IFN-γ induction is associated with clinical response. Clin Cancer Res 6, 1678–1692 (2000).
Lenzi, R. et al. Phase I study of intraperitoneal recombinant human interleukin 12 in patients with Müllerian carcinoma, gastrointestinal primary malignancies, and mesothelioma. Clin Cancer Res 8, 3686–3695 (2002).
Dortmans, J. C., Koch, G., Rottier, P. J. & Peeters, B. P. Virulence of Newcastle disease virus: what is known so far? Vet Res 42, 122 (2011).
Othman, F., Ideris, A., Motaleb, G. R., Eshak, Z. & Rahmat, A. Oncolytic effect of Newcastle disease virus AF2240 strain on the MCF-7 breast cancer cell line. Yakhteh Medical Journal 12, 17–24 (2010).
Ghrici, M., El Zowalaty, M., Omar, A. R. & Ideris, A. Induction of apoptosis in MCF-7 cells by the hemagglutinin-neuraminidase glycoprotein of Newcastle disease virus Malaysian strain AF2240. Oncol Rep 30, 1035–1044 (2013).
Ren, G. et al. Recombinant Newcastle disease virus encoding IL-12 and/or IL-2 as potential candidate for hepatoma carcinoma therapy. Technol Cancer Res Treat 15, 83–94 (2016).
Colombo, M. P. & Trinchieri, G. Interleukin-12 in anti-tumor immunity and immunotherapy. Cytokine Growth Factor Rev 13, 155–168 (2002).
Cohen, C. A., Shea, A. A., Heffron, C. L., Schmelz, E. M. & Roberts, P. C. Interleukin-12 immunomodulation delays the onset of lethal peritoneal disease of ovarian cancer. J Interferon Cytokine Res 36, 62–73 (2016).
Zhao, H. & Peeters, B. P. Recombinant Newcastle disease virus as a viral vector: effect of genomic location of foreign gene on gene expression and virus replication. J. Gen. Virol 84, 781–788 (2003).
Ling, P. et al. Human IL-12 p40 homodimer binds to the IL-12 receptor but does not mediate biologic activity. J Immunol 154, 116–127 (1995).
Ahmed, I., Ahmad, U., Keong, Y. Y., Manna, N. A. & Othman, F. Induction of nitric oxide and TNF-α in Newcastle disease virus (NDV) AF2240 infected RAW 264.7 macrophages and their cytotoxic activity on MDA-MB-231 breast cancer cell line. J Cancer Sci Ther 6, 478–482 (2014).
Liu, T. C., Galanis, E. & Kirn, D. Clinical trial results with oncolytic virotherapy: a century of promise, a decade of progress. Nat Rev Clin Oncol 4, 101–117 (2007).
Rodriguez-Madoz, J. R. et al. Semliki forest virus expressing interleukin-12 induces antiviral and antitumoral responses in woodchucks with chronic viral hepatitis and hepatocellular carcinoma. J Virol 83, 12266–12278 (2009).
Park, S. R. S., Widness, M., Levine, A. D., & Patterson, C. E. T cell, IL-12, and IFN-γ Driven Viral Clearance in Measles Virus-Infected Brain Tissue. J Virol (2011).
Tayeb, S., Zakay-Rones, Z. & Panet, A. Therapeutic potential of oncolytic Newcastle disease virus: a critical review. Oncolytic Virother 4, 49 (2015).
Barajas, M. et al. Gene therapy of orthotopic hepatocellular carcinoma in rats using adenovirus coding for interleukin 12. Hepatology 33, 52–61 (2001).
Rangaswamy, U. S. et al. Newcastle disease virus establishes persistent infection in tumor cells in vitro: contribution of the cleavage site of fusion protein and second sialic acid binding site of hemagglutinin-neuraminidase. J Virol, 00770 (2017).
Peeters, B. P., de Leeuw, O. S., Koch, G. & Gielkens, A. L. Rescue of Newcastle disease virus from cloned cDNA: evidence that cleavability of the fusion protein is a major determinant for virulence. J Virol 73, 5001–5009 (1999).
Yu, Y. et al. Rescue of virulent class I Newcastle disease virus variant 9a5b-D5C1. J Virol 9, 120 (2012).
Conrad, S. J. et al. Oncolytic tanapoxvirus expressing FliC causes regression of human colorectal cancer xenografts in nude mice. J Exp Clin Cancer Res 34, 19 (2015).
Stear, M. J. OIE Manual of Diagnostic Tests and Vaccines for Terrestrial Animals (Mammals, Birds and Bees) 5th Edn. Volumes 1 & 2. World Organization for Animal Health 2004. ISBN 92 9044 622 6.€ 140.-. Parasitology 130(6), 727–727 (2005).
Mosmann, T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 65, 55–63 (1983).
Zi-Bo, L. I., Zhao-Jun, Z. E. N. G., Qian, C. H. E. N., Sai-Qun, L. U. O. & Wei-Xin, H. U. Recombinant AAV-mediated HSVtk gene transfer with direct intratumoral injections and Tet-On regulation for implanted human breast cancer. BMC Cancer 6, 66 (2006).
Acknowledgements
This study was supported by the Ministry Energy, Science, Technology, Environment and Climate Change (MESTECC) Malaysia Flagship Fund, reference number: FP0514B0021-2(DSTIN).
Author information
Authors and Affiliations
Contributions
N.B.A., S.W.T., S.K.Y., A.V. and K.Y. designed the research. Z.M.A., M.A.C.A., S.U.F.S.N. and J.K. performed the research. Z.M.A., M.A.C.A., N.B.A., S.W.T., S.K.Y., K.Y., S.L.C., A.V. and S.C.C. analyzed the data. Z.M.A. and M.A.C.A. wrote the paper. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mohamed Amin, Z., Che Ani, M.A., Tan, S.W. et al. Evaluation of a Recombinant Newcastle Disease Virus Expressing Human IL12 against Human Breast Cancer. Sci Rep 9, 13999 (2019). https://doi.org/10.1038/s41598-019-50222-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-50222-z
This article is cited by
-
Green synthesis of oncolytic Newcastle disease virus-loaded thiolated chitosan nanoformulation for CD44 targeted delivery and sustained release of virus in cervical cancer xenografts
Cancer Nanotechnology (2023)
-
Interaction of reproductive tract infections with estrogen exposure on breast cancer risk and prognosis
BMC Women's Health (2023)
-
Viruses as tools in gene therapy, vaccine development, and cancer treatment
Archives of Virology (2022)
-
Oncolytic viruses for triple negative breast cancer and beyond
Biomarker Research (2021)
-
Viroimmunotherapy for breast cancer: promises, problems and future directions
Cancer Gene Therapy (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.