Abstract
Blood pressure (BP), especially systolic BP (SBP), is higher in adult growth hormone deficiency (AGHD) patients than in normal controls. Additionally, obesity is a known risk factor for hypertension, and growth hormone deficiency (GHD) is an important cause of short stature. For children with GHD, attention has been directed solely towards height. Few studies have assessed its potential impact on BP. Here, we investigated the effect of body mass index standard deviation score (BMISDS) on BP in children with short stature. This study included 736 children with short stature divided into two groups based on peak growth hormone (GH) level in GH provocation tests [severe GHD (SGHD) group = 212 children; non-SGHD group = 524 children]. We found that SBP was significantly higher in the SGHD group than in the non-SGHD group (p = 0.045). Additionally, there was a significant positive association between BMISDS and SBP in the SGHD group (β = 3.12, 95% CI: 1.40–4.84, p < 0.001), but no association between these variables was observed in the non-SGHD group. Thus, SGHD patients had a higher SBP than non-SGHD patients. BMISDS is a significant factor for higher SBP in SGHD patients but not in non-SGHD patients.
Similar content being viewed by others
Introduction
Adult growth hormone deficiency (AGHD) is associated with a metabolic profile similar to that of metabolic syndrome, which is defined by the clustering of obesity, dyslipidemia, glucose intolerance, and hypertension1. All these factors may accelerate atherosclerosis and promote higher cardiovascular morbidity, and untreated AGHD is widely accepted to lead to cardiovascular diseases (CVDs), whereas growth hormone (GH) treatment has beneficial metabolic effects in these patients2. On the other hand, the situation in children is less clear, as few studies have investigated metabolic abnormalities and their association with CVD in children. A recent review by De Leonibus C suggested that the development of atheromatous plaques begins early in childhood in the context of GH deficiency (GHD)3. Thus, understanding the risk factors for GHD in children is important for preventing disease and reducing morbidity caused by GHD.
In the general population, obesity is a known risk factor for hypertension. Obesity contributes to the development of hypertension via several interconnected and complex pathways, such as inflammation, oxidative stress, insulin resistance, and neurohormonal and humoral dysfunction4. Obesity is the excessive accumulation or expansion of adipose tissue. Population studies revealed that GH influences body composition5,6 and that GHD is associated with weight gain and a higher body mass index (BMI) in children and adults7. Basic research further showed that GH could reduce adipose tissue mass by regulating adipokine secretion, cellular senescence, the immune cell profile, angiogenesis, and lipid droplet formation8.
Blood pressure (BP), especially systolic BP (SBP), was reported to be higher in AGHD patients than in normal controls9,10. However, these data were obtained from heterogeneous cohorts of adult patients with GHD of varying etiologies and durations. How BP changes in children with GHD is unknown, and the relationship between BMI and BP in children with GHD remains unclear.
GHD is a common cause of linear growth restriction in children, and the diagnosis of GHD mainly relies on clinical manifestations combined with the GH provocation test. There are significant differences in body composition and metabolic traits between patients with severe GHD (SGHD) and the general population11. According to the criteria for SGHD10,11,12, this study defined SGHD as a GH peak <5 ng/ml. In this study, we sought to investigate the relationship between BMI standard deviation score (BMISDS) and SBP in children with SGHD.
Results
Clinical and laboratory characteristics of the subjects
Seven hundred thirty-six patients aged 10.2 ± 3.5 years, including 516 boys and 220 girls, were enrolled in this study. The demographic, anthropometric, and clinical characteristics of the 736 patients stratified according to GH peak in the GH provocation test are shown in Table 1. Two hundred twelve patients were assigned to the SGHD group based on a GH peak lower than 5 ng/ml, and the other 524 patients were assigned to the non-SGHD group. The patients in the SGHD group showed higher SBP (p = 0.035), weight (p < 0.001), BMI (p < 0.001), BMISDS (p < 0.001), insulin-like growth factor-1 standard deviation score (IGF-1SDS) (p = 0.002), triglyceride (TG) (p = 0.023), total cholesterol (TC) (p < 0.001) and low-density lipoprotein cholesterol (LDL-C) (p = 0.008) than the non-SGHD group. However, there was no significant difference between groups in diastolic BP (DBP), sex, age, height, height standard deviation score (HtSDS), IGF-1, insulin-like growth factor-binding protein-3 (IGFBP-3), fasting plasma glucose (FPG), high-density lipoprotein cholesterol (HDL-C) or bone age (BA).
Associations between anthropometric (SBP and DBP) and biochemical variables
Univariate analysis was performed to assess the relationship between each variable and BP. As shown in Table 2, age, height, weight, BMI, BMISDS, IGF-1, IGF-1SDS, IGFBP3, BA, height and TG were significantly positively associated with SBP. It was also shown that age, height, weight, BMI, IGF-1, IGF-1SDS, IGFBP3, and BA were significantly positively associated with DBP, and a significant negative association was found between HtSDS and DBP (p = 0.042).
Effect of BMISDS on SBP in different GH peak groups
Given the above results showing that BMISDS was significantly associated with SBP (β = 1.70, 95% CI 1.00–2.41; p < 0.001) (Table 2), we generated a linear regression model with different GH peak groups. As shown in Table 3, the crude model revealed a significant positive association between BMISDS and SBP in the SGHD group (β = 3.57, 95% CI 2.30–4.85; p < 0.001), but there was no association in the non-SGHD group (β = 0.75, 95% CI −0.12–1.62; p = 0.091). To further confirm the findings, we adjusted the models for TG, TC, HDL-C, LDL-C, FPG, IGF-1SDS and IGFBP3 and found an independent association between BMISDS and SBP in the SGHD group (β = 3.12, 95% CI: 1.40–4.84, p < 0.001) but not in the non-SGHD group (β = 0.94, 95% CI: −0.08, 1.95, p = 0.071). Furthermore, a test of the interaction between GH peak and BMISDS was statistically significant (p = 0.001).
Discussion
In this retrospective cross-sectional study, we analyzed the association between BMISDS and SBP in children with short stature. The results showed that SBP was significantly higher in the SGHD group than in the non-SGHD group. Further regression analysis showed that BMISDS was independently associated with SBP in the SGHD group, but there was no evidence of such an association in the non-SGHD group.
Previous studies have documented that GHD patients have a higher prevalence of obesity than the general population. In the present study, we found that patients with SGHD had a higher BMI than those in the non-SGHD group. In addition, according to the diagnostic criteria for childhood obesity13, the incidence of obesity was 4.8% in the SGHD group and 2.5% in the non-SGHD group, while the incidence rates of leanness in the SGHD and non-SGHD groups were 3.9% and 5.6%, respectively. Although these differences were not statistically significant, the trend of increased obesity in patients with SGHD was obvious, and the finding is consistent with reports in the literature10.
Previous studies have reported that GHD patients have an increased prevalence of hypertension compared with the general population10,14,15,16. However, these results are based on AGHD with different causes and courses. To our knowledge, no similar studies in children with GHD are available. The study presented herein showed that SBP was significantly higher in SGHD children than in non-SGHD children. No difference in DBP was found between the two groups, which is inconsistent with previous findings9,10. However, the underlying mechanism requires further exploration.
Obesity is an established risk factor for hypertension17,18, which is more than twice as prevalent in obese individuals than in nonobese individuals19. BMI is an indicator of obesity. Our study indicated that BMISDS was positively related to SBP by univariate analysis, and further subgroup analysis revealed that SBP was positively related to BMISDS in the SGHD group but not in the non-SGHD group. Moreover, multivariate linear regression analysis demonstrated that BMISDS had an independent effect on SBP in patients with SGHD.
Although the specific mechanism for the relationship between BMISDS and SBP remains unclear, we considered based on our findings that this relationship may differ based on the degree of GHD. A previous study showed that metabolic index did not differ between patients with GH peaks above 7 ng/ml and people with normal GH peaks, while a significant difference existed between patients with SGHD and an unaffected population11. Abdominal fat accumulation is a main metabolic feature of GHD, which may lead to abdominal obesity10. People with abdominal obesity reportedly have a 2–3-fold higher risk of hypertension20,21. Thus, we concluded that increased abdominal fat caused by a higher BMI in patients with SGHD may partly explain the difference in SBP between the SGHD and non-SGHD groups. Given the lack of evidence, related studies should be carried out to explore the potential mechanism.
Some limitations in our study must be noted. First, we did not collect data regarding other potential contributors to SBP, such as diet and family history. We intend to analyze the effects of diet, family history and pubertal state on SBP in a prospective study. Second, due to the cross-sectional design of this study, the present findings showed a positive association between BMISDS and SBP in only children with SGHD. Prospective studies are required to further observe the results after GH treatment.
In conclusion, children with SGHD presented an elevated SBP compared with non-SGHD children, and in the SGHD group, SBP was positively associated with BMISDS, suggesting that CVD risk factors such as hypertension and obesity should be of concern in children with SGHD.
Subjects and Methods
Study subjects
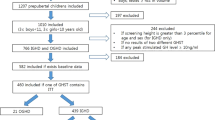
We retrospectively reviewed the medical records of children with short stature from the Department of Endocrinology, Affiliated Hospital of Jining Medical University, between January 2014 and December 2017. The subjects were selected based on the following inclusion criteria: short stature, which was defined as the height of an individual being below the third percentile of the corresponding population for a given age, sex and ethnic group; normal weight and length at birth; and normal prepuberty state. The exclusion criteria included the presence of chronic diseases, chromosomal abnormalities, skeletal dysplasia, genetic metabolic diseases and endocrine system diseases except for GHD, as well as the use of medications that interfere with BP or BMI. Overall, 1313 patients were available during the study period, 736 of whom were eligible for our study and were included, as described in the flow chart (Fig. 1). All enrolled patients were examined for GHD and idiopathic short stature (ISS) based on the GH peak level in provocation tests. A GH peak ≥10 ng/ml indicated a diagnosis of ISS, and a GH peak <10 ng/ml indicated a diagnosis of GHD which included partial GHD (GH peak ≥5 ng/ml) and complete GHD (GH peak <5 ng/ml); all enrolled patients were subdivided into the non-SGHD (partial GHD and ISS; GH peak ≥5 ng/ml) and SGHD groups (complete GHD; GH peak <5 ng/ml).
The study was approved by the Human Ethics Committee of the Affiliated Hospital of Jining Medical University (Shandong, China), and all methods were performed in accordance with the guidelines of the Declaration of Helsinki. All of the families of the patients were informed of the aims of the study, and written informed consent was obtained from the parents of the patients.
Anthropomorphic measurements
Height and weight were measured by a designated individual using the same measuring instrument in the morning with allowable error ranges of 0.1 cm and 0.1 kg, respectively. BP was measured in the seated position by one of two pretrained physicians using an electronic sphygmomanometer with an appropriately sized cuff for the arm of a child, and the average of three measurements was used for analysis. HtSDS was calculated based on the normal range of Chinese children22. BMI was calculated as the ratio between body weight in kilograms and height in meters squared. The stage of puberty was assessed by physical examination according to Tanner staging23; prepuberty was defined24 as a testicular volume less than 4 ml and no pubic hair in boys and as no breast development or pubic hair in girls.
Laboratory measurements
To assess GH secretion, Ldopa (Levodopa Tablets, He Feng, Guang Xi, China; dose: 500 mg for patients weighing more than 30 kg and 250 mg for those weighing less than 30 kg) and insulin (Insulin Injection, Wan Bang, Jiang Su, China; 0.1 U/kg) were administered orally or subcutaneously after overnight fasting. Blood samples were collected at 0, 30, 60, 90, and 120 min after administration to obtain serum GH concentrations at each time point. GH was measured using a chemiluminescence method (ACCESS2, Beckman Coulter; USA) with an analytical sensitivity of 0.010 µg/L. Serum IGF-1 and IGFBP-3 levels were measured by the chemiluminescence immunometric method (DPCIMMULITE 1000 analyzer, SIEMENS, Germany); the intra- and interassay coefficients of variation (CVs) were 3.0% and 6.2% for IGF-1 and 4.4% and 6.6% for IGFBP-3. Measures of liver function (including alanine aminotransferase (ALT), AST, and gamma-glutamyl transferase (GGT)) and kidney function (including Cr, blood urea nitrogen (BUN), and UA), lipid profiles (including TC, HDL-C, LDL-C, and TG), and FPG were obtained by a biochemical autoanalyzer (Cobas c 702, Roche; Shanghai, China). Measures of thyroid function, including free T3 (FT3), free T4 (FT4), thyroid-stimulating hormone (TSH), gonadotropin, cortisol, and adrenocorticotropic hormone (ACTH), were determined by a luminescence immunoassay system (Cobas c 602, Roche; Shanghai, China). IGF-1SDS and BMISDS were calculated according to a previous study22,25.
Statistical analysis
All statistical analyses were performed with R statistical software (https://www.r-project.org;) and EmpowerStats (http://www.empowerstats.com, X&Y Solutions, Inc. Boston MA). Normally distributed variables are expressed as the mean ± standard deviation (SD); non-normally distributed variables are presented as the median (quartile). Categorical data are expressed as percentages. To compare differences between two groups, Student’s t-test was used for normally distributed variables, the Kruskal-Wallis test was used for non-normally distributed variables, and the chi-square test and Fisher’s exact test were used for categorical variables. A univariate model was used to examine whether BMISDS and other anthropometric and biochemical variables were associated with SBP and DBP. Then, the association between BMISDS and SBP in each subgroup defined by GH peak was estimated using a multivariate linear regression model adjusted for pertinent variables, and the interaction of GH peak with BMISDS was tested. A two-tailed P value ≤0.05 was considered to indicate statistical significance in all analyses.
References
Alberti, K. G., Zimmet, P. & Shaw, J. Metabolic syndrome–a new world-wide definition. A Consensus Statement from the International Diabetes Federation. Diabetic medicine: a journal of the British Diabetic Association 23, 469–480, https://doi.org/10.1111/j.1464-5491.2006.01858.x (2006).
Isgaard, J., Arcopinto, M., Karason, K. & Cittadini, A. GH and the cardiovascular system: an update on a topic at heart. Endocrine 48, 25–35, https://doi.org/10.1007/s12020-014-0327-6 (2015).
De Leonibus, C. et al. Growth Hormone Deficiency in Prepubertal Children: Predictive Markers of Cardiovascular Disease. Hormone research in paediatrics 85, 363–371, https://doi.org/10.1159/000444143 (2016).
Cohen, J. B. & Gadde, K. M. Weight Loss Medications in the Treatment of Obesity and Hypertension. Current hypertension reports 21, 16, https://doi.org/10.1007/s11906-019-0915-1 (2019).
Schweizer, R., Martin, D. D., Schonau, E. & Ranke, M. B. Muscle function improves during growth hormone therapy in short children born small for gestational age: results of a peripheral quantitative computed tomography study on body composition. The Journal of clinical endocrinology and metabolism 93, 2978–2983, https://doi.org/10.1210/jc.2007-2600 (2008).
Wollmann, H. A. & Ranke, M. B. Metabolic effects of growth hormone in children. Metabolism: clinical and experimental 44, 97–102 (1995).
Rothermel, J. & Reinehr, T. Metabolic alterations in paediatric GH deficiency. Best practice & research. Clinical endocrinology & metabolism 30, 757–770, https://doi.org/10.1016/j.beem.2016.11.004 (2016).
Berryman, D. E. & List, E. O. Growth Hormone’s Effect on Adipose Tissue: Quality versus Quantity. International journal of molecular sciences 18, https://doi.org/10.3390/ijms18081621 (2017).
Barreto-Filho, J. A. et al. Familial isolated growth hormone deficiency is associated with increased systolic blood pressure, central obesity, and dyslipidemia. The Journal of clinical endocrinology and metabolism 87, 2018–2023, https://doi.org/10.1210/jcem.87.5.8474 (2002).
Lubrano, C. et al. Severe growth hormone deficiency and empty sella in obesity: a cross-sectional study. Endocrine 49, 503–511, https://doi.org/10.1007/s12020-015-0530-0 (2015).
Tidblad, A., Gustafsson, J., Marcus, C., Ritzen, M. & Ekstrom, K. Metabolic differences between short children with GH peak levels in the lower normal range and healthy children of normal height. Growth hormone & IGF research: official journal of the Growth Hormone Research Society and the International IGF Research Society 34, 22–27, https://doi.org/10.1016/j.ghir.2017.04.001 (2017).
Gonzalez, S., Sathyapalan, T., Javed, Z. & Atkin, S. L. Effects of Growth Hormone Replacement on Peripheral Muscle and Exercise Capacity in Severe Growth Hormone Deficiency. Frontiers in endocrinology 9, 56, https://doi.org/10.3389/fendo.2018.00056 (2018).
Brady, T. M. Obesity-Related Hypertension in. Children. Frontiers in pediatrics 5, 197, https://doi.org/10.3389/fped.2017.00197 (2017).
Gazzaruso, C., Gola, M., Karamouzis, I., Giubbini, R. & Giustina, A. Cardiovascular risk in adult patients with growth hormone (GH) deficiency and following substitution with GH–an update. The Journal of clinical endocrinology and metabolism 99, 18–29, https://doi.org/10.1210/jc.2013-2394 (2014).
Gonzalez, S. et al. Effects of human recombinant growth hormone on exercise capacity, cardiac structure, and cardiac function in patients with adult-onset growth hormone deficiency. 45, 1708–1719, https://doi.org/10.1177/0300060517723798 (2017).
Chan, L. et al. Lipid Accumulation Product: a Simple and Accurate Index for Predicting Metabolic Syndrome in Patients with Adult Growth Hormone Deficiency. Experimental and clinical endocrinology & diabetes: official journal, German Society of Endocrinology [and] German Diabetes Association 124, 220–224, https://doi.org/10.1055/s-0035-1569402 (2016).
Garrison, R. J., Kannel, W. B., Stokes, J. 3rd & Castelli, W. P. Incidence and precursors of hypertension in young adults: the Framingham Offspring Study. Preventive medicine 16, 235–251 (1987).
Doll, S., Paccaud, F., Bovet, P., Burnier, M. & Wietlisbach, V. Body mass index, abdominal adiposity and blood pressure: consistency of their association across developing and developed countries. International journal of obesity and related metabolic disorders: journal of the International Association for the Study of Obesity 26, 48–57, https://doi.org/10.1038/sj.ijo.0801854 (2002).
Cutler, J. A. et al. Trends in hypertension prevalence, awareness, treatment, and control rates in United States adults between 1988-1994 and 1999-2004. Hypertension (Dallas, Tex.: 1979) 52, 818–827, https://doi.org/10.1161/hypertensionaha.108.113357 (2008).
Okosun, I. S., Prewitt, T. E. & Cooper, R. S. Abdominal obesity in the United States: prevalence and attributable risk of hypertension. Journal of human hypertension 13, 425–430 (1999).
Seven, E., Thuesen, B. H., Linneberg, A. & Jeppesen, J. L. Abdominal Adiposity Distribution Quantified by Ultrasound Imaging and Incident Hypertension in a General Population. Hypertension (Dallas, Tex.: 1979) 68(1122), 1115, https://doi.org/10.1161/hypertensionaha.116.07306 (2016).
Li, H., Ji, C. Y., Zong, X. N. & Zhang, Y. Q. Height and weight standardized growth charts for Chinese children and adolescents aged 0 to 18 years. Zhonghua er ke za zhi = Chinese journal of pediatrics 47, 487–492 (2009).
Tanner, J. M. & Whitehouse, R. H. Clinical longitudinal standards for height, weight, height velocity, weight velocity, and stages of puberty. Archives of disease in childhood 51, 170–179 (1976).
Wang, P., Ji, B., Shao, Q., Zhang, M. & Ban, B. Association between Insulin-Like Growth Factor-1 and Uric Acid in Chinese Children and Adolescents with Idiopathic Short Stature: A Cross-Sectional Study. BioMed research international 2018, 4259098, https://doi.org/10.1155/2018/4259098 (2018).
Isojima, T. et al. Standardized centile curves and reference intervals of serum insulin-like growth factor-I (IGF-I) levels in a normal Japanese population using the LMS method. Endocrine journal 59, 771–780 (2012).
Acknowledgements
This study was supported by the Jining Science and Technology Bureau (No. 2017SMNS007). We would like to thank the biochemical laboratory of the Affiliated Hospital of Jining Medical University for measuring all biochemical indicators.
Author information
Authors and Affiliations
Contributions
Y.Y.L. and B.B. contributed to the study conception and design; data acquisition, analysis, and interpretation; and drafting and critical revision of the manuscript. Y.H.Z. and M.Z. contributed to the study conception and design; data analysis and interpretation; and critical revision of the manuscript. W.L.Y., B.L.J. and H.P. contributed to the study design and data interpretation and critically revised the manuscript. All authors provided final approval and agree to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Li, Y., Zhang, Y., Zhang, M. et al. Growth hormone peak modifies the effect of BMI on increased systolic blood pressure in children with short stature. Sci Rep 9, 7879 (2019). https://doi.org/10.1038/s41598-019-44299-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-44299-9
This article is cited by
-
Association between systolic blood pressure and uric acid in Chinese children and adolescents with idiopathic short stature: a cross-sectional study
Journal of Human Hypertension (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.