Abstract
Mosquito community composition plays a central role in the transmission of zoonotic vector-borne pathogens. We evaluated how the mosquito community affects the seroprevalence of West Nile virus (WNV) in house sparrows along an urbanisation gradient in an area with the endemic circulation of this virus. We sampled 2544 birds and 340829 mosquitoes in 45 localities, analysed in 15 groups, each containing one urban, one rural and one natural area. WNV seroprevalence was evaluated using an epitope-blocking ELISA kit and a micro virus-neutralization test (VNT). The presence of WNV antibodies was confirmed in 1.96% and 0.67% of birds by ELISA and VNT, respectively. The VNT-seropositive birds were captured in rural and natural areas, but not in urban areas. Human population density was zero in all the localities where VNT-positive birds were captured, which potentially explains the low incidence of human WNV cases in the area. The prevalence of neutralizing antibodies against WNV was positively correlated with the abundance of the ornithophilic Culex perexiguus but negatively associated with the abundance of the mammophilic Ochlerotatus caspius and Anopheles atroparvus. These results suggest that the enzootic circulation of WNV in Spain occurs in areas with larger populations of Cx. perexiguus and low human population densities.
Similar content being viewed by others
Introduction
The mosquito-borne West Nile virus (WNV; Flaviviridae) circulates naturally in wild birds1. Occasionally, infected mosquitoes transmit WNV to mammals, which are dead-end hosts of this virus. Most WNV infections in humans are asymptomatic or associated with mild symptoms, and only a small percentage of patients develop more severe neurological diseases such as aseptic meningitis or encephalitis2,3. Nevertheless, in North America, where WNV was first detected in 1999 in New York City, WNV has spread throughout the country and caused hundred of human fatalities2. Contrary to the situation in North America, in Europe WNV infections are usually asymptomatic in birds4,5,6. WNV has been endemic in Spain since at least 2003, with a seroprevalence of up to 42.9% in some bird species7,8,9. However, only six cases of WNV disease in humans have ever been reported, one in 200410, two in 201011 and three in 201612.
Mosquitoes of the genus Culex play a key role in WNV circulation in Europe13,14, although WNV has also been detected in mosquitoes belonging to the genera Aedes, Anopheles and Culiseta14. A multi-species Susceptible-Infectious-Recovered (SIR) transmission model published recently by Roche et al.15 suggests that an increase in vector species richness enhances pathogen transmission due to a concomitant greater abundance of vectors, which translates into more competent vectors for pathogen transmission15. In addition to the differences between mosquito capacity for transmitting WNV (vector competence), blood feeding patterns may determine the contact rate between mosquitoes and susceptible hosts and hence ultimately determine WNV epidemiology16,17,18. Muñoz et al.17 estimated the WNV transmission risk for different mosquito species in southern Spain based on mosquito abundance, vector competence and the fraction of blood meals taken from birds. These authors’ analysis indicated that Cx. perexiguus was the main vector for the enzootic cycle of WNV and that the risk of WNV transmission to humans was very low in the studied area17.
Here, we study the role of the abundance of mosquitoes and species richness explaining the seroprevalence of WNV in wild bird, the house sparrows (Passer domesticus), in southern Spain. Active circulation of WNV occurs here, as is shown by virus isolation from mosquitoes14,19, seroconversions in wild birds20, the presence of antibodies in juvenile birds21, and the incidence of disease in humans and horses11,22. In this region, house sparrows are common hosts of mosquitoes17, competent hosts for WNV23 and may play a key role in WNV amplification and transmission to humans24,25,26. Taking into account the above-mentioned studies, we first compared the prevalence of WNV antibodies in urban, rural and natural areas (defined in terms of human population density) to determine how the distributions of mosquitoes, WNV and people explain the low incidence of WNV in humans in Spain. Secondly, we tested the assumption in the Roche et al.15 model of a positive relationship between vector species richness and vector abundance, as well as the model prediction that vector richness should be positively related to WNV prevalence. Finally, we analysed the relationship between WNV seroprevalence and the abundance of mosquito species that, according to Muñoz et al.17, may contribute in different ways to WNV amplification.
Results
In all, 340829 female mosquitoes belonging to 13 species and five genera were trapped. The commonest species was Culex theileri Theobald (n = 282891), followed in descending order by Ochlerotatus caspius Pallas (n = 21155), Culex pipiens Linnaeus (n = 19268), Culex perexiguus Theobald (n = 5939) and Anopheles atroparvus Van Thiel (n = 5387). In addition, 1237 females of the potential WNV vector Culex modestus Ficalbi were captured. The other species were trapped in relatively low numbers and for this reason—and also because they are not involved in the transmission of WNV—were not considered in any of the analyses (with the exception of the species richness calculation). A positive relationship was found between the overall abundance of mosquitoes and the richness of vector species (est = 2.45, z = 6.05, p < 0.001).
Sera obtained from 2544 house sparrows were analysed to detect WNV antibodies. According to the ELISA tests, 50 birds (1.96%) from 18 different localities tested positively (Table 1), while 113 (4.44%) provided doubtful results. Of these birds, 17 (0.67% of the total individuals sampled) had neutralizing antibodies against WNV as confirmed by VNT (Table 1). These 17 WNV-positive birds were captured in five of the 45 studied localities, all of them in rural and natural areas in Huelva province (Fig. 1). WNV seroprevalence in these five localities ranged from 1.6% to 8.5%. Specific USUV-neutralizing antibodies were detected in a single bird (0.04%) captured in a natural area in Seville province. The human population density tended to be lower (0 in all cases) in areas with VNT-positive birds than in areas with negative cases (mean human population = 77.6, range: 0–1,424) (est = −1.90, z = 1.88, p = 0.06).
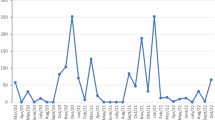
The relationships between ELISA and VNT seroprevalence rates and the number of mosquitoes captured and species richness are summarised in Tables 2 and 3, respectively. Only those variables included in the selected models (those with ∆AIC ≤ 2 compared to the best model) are shown. WNV seroprevalences estimated by ELISA were positively related to mosquito richness and the number of Cx. perexiguus captured but negatively related to the number of Oc. caspius and Cx. theileri captured. Similarly, for the case of the model based on the WNV seroprevalence according to the VNT, the prevalence of neutralizing antibodies against WNV was positively related to the number of Cx. perexiguus captured (Fig. 2) but negatively associated with the number of both the Oc. caspius and An. atroparvus.
Discussion
Both West Nile virus and USUV antibodies were found in wild house sparrows from southern Spain. The seroprevalence of WNV in house sparrows estimated by VNT was positively related to the abundance of Cx. perexiguus but negatively to the abundances of both An. atroparvus and Oc. caspius. These results confirm the important role of Cx. perexiguus in the circulation of WNV in Spain. Transmission risk estimates based on abundances, vector competence and blood meal analyses indicate that the risk of transmission of WNV by Cx. perexiguus is at least an order of magnitude higher than for the other mosquito species analysed17. It is important to note that WNV has been detected in Spain mainly in Cx. perexiguus and Cx. pipiens pools14,19. Moreover, Cx. perexiguus is an abundant ornithophilic mosquito that commonly uses house sparrows as hosts17,27,28.
Interestingly, we found negative relationships between the abundance of two common mosquito species, An. atroparvus and Oc. caspius, and the prevalence of WNV antibodies in wild house sparrows. Both species have a mammal-biased feeding pattern, even though they can feed on birds17,27. Although WNV has been detected in wild collected Oc. caspius29, this species is described as an inefficient vector of WNV by the only experimental study of the vector competence of Oc. caspius conducted to date in Europe13. At least two factors help explain the negative association between these two mosquito species and WNV. Firstly, Oc. caspius prefers saltmarshes as larval breeding sites and An. atroparvus is commonest in sand dunes and scrubland, while Cx. perexiguus is frequently found in rice fields30. Consequently, Oc. caspius and An. atroparvus are probably more abundant in areas where Cx. perexiguus and/or other potential vector species for WNV such as Cx. pipiens and Cx. modestus are rarer. Secondly, the greater abundance of these mosquito species in the study area, where they feed mainly on mammals that are non-competent hosts for WNV, could lead to a reduction in the overall prevalence of WNV in birds. However, we were not able to identify any mechanisms that might support this hypothesis. Due to its mammal-biased diet and low vector competence, we would expect their abundance to have a low—but not negative—effect on WNV amplification. This is mainly because WNV transmission may be maintained by other vector-competent mosquito species present in the area.
In addition, we observed a positive association between mosquito species richness and the seroprevalence detected by ELISA. The same non-significant tendency was found for WNV neutralizing antibodies detected by VNT. ELISA is a less specific technique than VNT and, consequently, individuals with positive sera for ELISA but negative for VNT have probably been exposed to other unidentified flaviviruses antigenically related to WNV. Using a SIR model, Roche et al.15 concluded that mosquito species richness may increase the transmission success of vector-borne pathogens. However, such an association has never been tested empirically and could be the product of the assumption made in the model that species richness and vector abundance are positively related, a conjecture that, in fact, was supported by our data (see below). Consequently, our results support both the assumption of a positive relationship between vector richness and abundance, and the prediction of a positive relationship between vector richness and pathogen prevalence. Although Cx. perexiguus is the main vector of WNV in the area, other species such as Cx. pipiens and Cx. modestus may contribute significantly to WNV transmission31,32. These mosquito species, in addition to the others that co-exist in the area, could play a role in the transmission of certain flaviviruses. A number of flaviviruses have been isolated from mosquitoes (including Cx. pipiens) in Spain19, which potentially explains the positive correlation found between ELISA seroprevalence and mosquito species richness.
All positive cases of WNV-specific antibodies by VNT in bird sera were found in Huelva province, where evidence of WNV active circulation has existed since 2003, as demonstrated by the molecular detection of the virus in mosquitoes and the seroprevalence found in birds33. In addition, birds with WNV-specific antibodies by VNT were only detected in rural and natural habitats; none of the birds sampled in urban areas (n = 956) were seropositive. Moreover, the negative, marginally significant relationship we found between WNV seroprevalence and human population density may explain why WNV cases in humans are so uncommon in the study area despite the active circulation of the virus between vectors and avian hosts. Our results suggest that WNV, its main vector (Cx. perexiguus) and humans are not all present together in the same places. The seroprevalence of WNV in humans in southern Spain is very low (0.6%) and, mirroring the results for house sparrows in our study, a higher seroprevalence was detected in humans in rural areas than in suburban and urban areas34. Moreover, greater numbers of Cx. perexiguus were captured in natural and rural areas than in urban ones; likewise, the abundance of this species decreases as the percentage of land covered by built-up areas increases35. Indeed, only Cx. pipiens represents a risk for the transmission of WNV in urban areas35.
In conclusion, this study provides evidence of the central role of Cx. perexiguus in the enzootic circulation of WNV in southern Spain. The fact that WNV seropositive birds were found in both natural and rural areas, and tended to be present in areas with lower human densities, may explain the low incidence of WNV in humans in the area despite the local circulation of this virus between mosquitoes and wild birds.
Materials and Methods
Study area
This study was conducted in Andalusia, southern Spain (Fig. 1). This area is characterized by a Mediterranean climate with most precipitations concentrated during winter, while summer represents a long dry season. The study was conducted in 2013 at 45 different sites in Cadiz, Huelva and Seville provinces (southern Spain). The sampling sites (15 in each province) were situated in geographically close groups of three, each with one locality in a natural habitat, one in a rural habitat and one in an urban habitat (Fig. 1). The mean distance between localities within the same triplet was 5,740 m. Selection of the three habitat categories was performed after visual inspection of the areas based on the following criteria: urban habitats contained more densely human-populated areas than the other two habitat types; rural habitats had higher density of livestock than urban and natural areas; and natural habitats were selected on the basis of both lower human and livestock densities than in the other two habitat types, and a generally better conserved landscape.
Mosquito and bird sampling and identification
Mosquitoes were captured at the 45 sampling sites in April–December, the period with maximum mosquito activity in southern Spain30,36. We used BG-sentinel traps baited with BG-lure and dry ice as a source of CO2, which is considered an effective method for mosquito diversity and abundance characterization35. At each site, once every 45 days, three traps were operated for 24 hours in each of the three localities of the same triplet. Overall, 135 traps (3 traps x 45 localities), with a mean distance between traps of 119 m (range 20–636 m), were employed during each mosquito trapping session for a total trapping effort of 810 trap nights. Mosquito sampling was conducted during days with favourable weather conditions (e.g. clear nights without rain). This procedure was repeated during 5–6 trapping sessions throughout the study period. Female mosquitoes were identified to species level following the morphological keys in Schaffner et al.37 and Becker et al.38. Mosquitoes belonging to the univittatus complex were identified as Culex perexiguus based on male genitalia (see Harbach39). For the case of samples compromising several thousands of mosquitoes captured per trap per night, we visually identified 500 individuals. These 500 mosquitoes were separated in five groups of 100 individuals, which were weighted to the nearest 0.001 g. This approach was used to estimate the proportion of individuals of each species for the rest of the sample based on the weight of the total number of mosquitoes captured35. Mosquito species richness, which ranged from 2 to 10, was calculated as the number of different species captured at each locality during the sampling period35. For each locality, the mean number of captures of the five commonest mosquito species in the study area (Anopheles atroparvus, Ochlerotatus caspius, Culex theileri, Culex pipiens and Cx. perexiguus) and of Cx. modestus, a potential WNV vector in the area14,17,31, were calculated.
House sparrows were sampled using mist-nests at the same localities during capture sessions in July–October, i.e. immediately after the breeding season to maximize the capture of juvenile birds and to better reflect virus circulation during the season from hatching until capture. Each bird was individually marked with a metal ring, sexed and aged40. A blood sample was taken from the jugular vein of each bird using a sterile syringe and preserved in a cool-box during the fieldwork session. In the laboratory, blood was allowed to clot at 4 °C overnight and was then centrifuged for 10 minutes at 4,000 rpm to separate the serum from the cellular fractions. Serum samples were frozen at −80 °C until further analysis.
WNV antibodies detection
Serum samples from birds were analysed with the epitope-blocking ELISA Kit Ingezim West Nile Compac (INGENASA, Madrid, Spain) to determine the presence of WNV antibodies41. Positive results from ELISA may reflect past infections by WNV or even other unidentified flaviviruses circulating in the area. The cut-off value of this commercial ELISA test is set at 30% percentage of inhibition, while samples showing a percentage of inhibition between 30% and 40% are considered doubtful samples as established by the manufacturer41. All samples producing ELISA positive and/or doubtful results were subsequently analysed by a comparative micro virus-neutralization test (VNT) using WNV (strain Eg-101) and Usutu virus (USUV; strain SAAR1776) since the circulation of these flaviviruses has been demonstrated in the study area42. This confirmatory test allow to differentiate the specific antibodies against WNV from those elicited by other related flaviviruses. Neutralization titres were assigned based on the highest dilution of each serum capable of neutralizing the infection in vitro. Separate VNT were performed using serial (two-fold) dilutions (1:10–1:1280) of each serum sample using a micro-VNT method21. For a given sample, WNV-specific antibody responses were assigned when the observed VNT titres against WNV were at least four times higher than those observed against USUV43.
Human density quantification
We estimated the density of human population in the studied areas as the number of people living in a grid of 250 × 250 m. This information was obtained from the Andalusian Institute of Statistics and Cartography based on the number of residents registered in the local population census on 1 January 2013 (Base de Datos Longitudinal de Población de Andalucía). This variable was log-transformed to normalize its distribution.
Statistical analyses
To estimate WNV seroprevalence we controlled for variables that operate at individual level (i.e. age, sex and date of capture) and others that operate at locality level (i.e. mosquito species richness and abundance of the different mosquito species). For this reason two-step analyses were performed. First, we fitted a generalized linear model to the seroprevalence of WNV using binomial distributed errors and including bird sex (fixed factor: male or female), age (fixed factor: juvenile or adult), month (continuous variable) and locality (fixed factor) as independent variables. Two different models were fitted using the results of ELISA and VNT as the dependent variable, respectively. Second, least square means (lsmeans) were calculated by retaining bird age and sampling locality, the only two significant factors explaining variance between individuals in WNV seroprevalence according to the previous models. This procedure allowed us to calculate both the ELISA and VNT seroprevalences for each of the 45 localities while controlling for the potential confounding effect of bird age. Third, two Linear Mixed-effects Models (LMM) were fitted using the lsmeans for ELISA and VNT seroprevalences as dependent variables. ‘Province’ and ‘triplets’ were included as random factors to account for the geographical stratification of the sampling design, and models were fitted using maximum likelihood and normal distributed errors. The independent variables included in these models were the three habitat categories (fixed factor: urban, rural or natural), the number of captures of each of the six main mosquito species found and species richness (continuous variables). Variance Inflation Factors (VIF) were checked to exclude collinearity between independent variables44 and Akaike’s Information Criterion (AIC) was used to select the best final models for each ELISA and VNT LMM model. Parameters were estimated by model averaging of all models with ∆AIC ≤ 245, which were considered to similarly support the data. To normalize their distribution, the numbers of each mosquito species captured were log-transformed and the distribution of all predictors and model residuals were checked using qq plots in R software. We calculated the respective marginal coefficient of determination (R2) for the fixed and random effects of the models according to Nakagawa & Schielzeth46.
Finally, two additional LMMs were fitted. One to test the model assumption of Roche et al.15 of a positive correlation between mosquito richness and total abundance, and the other to compare the density of human population, as measured in Ferraguti et al.35, at sampling sites with and without VNT positive birds. All the statistical analyses were conducted in R (v. 2.14.2; R Development Core Team 2005) using the packages vegan, lme4, car, arm, Matrix, Rcpp, MASS, MuMIn and lsmeans.
Ethics statement
Bird sampling and mosquito trapping were performed with the necessary permits issued by the regional Department of the Environment (Consejería de Medio Ambiente, Junta de Andalucía) and in accordance with relevant guidelines and regulations. Procedures were approved by the Ethical Committee of CSIC and complied with current Spanish laws. Surveys and sampling on private land and in private residential areas were conducted with all the necessary permits and consent, and in the presence of owners. This study did not affect any endangered species.
References
Hubálek, Z. & Halouzka, J. West Nile fever–a reemerging mosquito–borne viral disease in Europe. Emerg. Infect. Dis. 5, 643–650 (1999).
Sejvar, J. J. The long–term outcomes of human West Nile virus infection. Clin. Infect. Dis. 44, 1617–24 (2007).
Sambri, V. et al. West Nile virus in Europe: emergence, epidemiology, diagnosis, treatment, and prevention. Clin. Microbiol. Infect. 19, 699–704 (2013).
Barzon, L. et al. Large human outbreak of West Nile virus infection in North–Eastern Italy in 2012. Viruses. 5, 2825–39 (2013).
Petersen, L. R., Brault, A. C. & Nasci, R. S. West Nile virus: review of the literature. JAMA. 310, 308–15 (2013).
Zeller, H. G. & Schuffenecker, I. West Nile virus: an overview of its spread in Europe and the Mediterranean basin in contrast to its spread in the Americas. Eur. J. Clin. Microbiol. Infect. Dis. 23, 147–56 (2004).
Figuerola, J. et al. Size matters: West Nile Virus neutralizing antibodies in resident and migratory birds in Spain. Vet. Microbial. 132, 39–46 (2008).
Höfle, U. et al. West Nile virus in the endangered Spanish imperial eagle. Vet. microbiol. 129, 171–178 (2008).
Rizzoli, A. et al. The challenge of West Nile virus in Europe: knowledge gaps and research priorities. Euro Surveill. 20, pii=21135 (2015).
Kaptoul, D. et al. West Nile virus in Spain: report of the first diagnosed case (in Spain) in a human with aseptic meningitis. Scan. J. Infect. Dis. 39, 70–71 (2007).
García–Bocanegra, I. et al. West Nile fever outbreak in horses and humans, Spain, 2010. Emerg. Infect. Dis. 17, 2397–99 (2011).
European Centre for Disease Prevention and Control (ECDC). Epidemiological update: West Nile virus transmission season in Europe, 2016. Stockholm: ECDC. http://ecdc.europa.eu/en/press/news/_layouts/forms/News_DispForm.aspx?ID=1524&List=8db7286c-fe2d-476c-9133-18ff4cb1b568&Source=http%3A%2F%2Fecdc.europa.eu%2Fen%2FPages%2Fhome.aspx (2016).
Balenghien, T. et al. Vector competence of some French Culex and Aedes mosquitoes for West Nile virus. Vector Borne Zoon. Dis. 8, 589–96 (2008).
Engler, O. et al. European surveillance for West Nile virus in mosquito populations. Int. J. Environ. Res. Public Health. 10, 4869–95 (2013).
Roche, B., Rohani, P., Dobson, A. P. & Guégan, J.–F. The impact of community organization on vector–borne pathogens. Am. Nat. 181, 1–11 (2013).
Kilpatrick, A. M., Kramer, L. D., Jones, M. J., Marra, P. P. & Daszak, P. West Nile virus epidemics in North America are driven by shifts in mosquito feeding behavior. PLoS Biol. 4, e82 (2006).
Muñoz, J. et al. Feeding patterns of potential West Nile virus vectors in South–West Spain. PLoS One. 7, e39549 (2012).
Chevalier, V., Tran, A. & Durand, B. Predictive modeling of West Nile Virus transmission risk in the Mediterranean basin: How far from landing?. Int. J. Environ. Res. Public Health. 11, 67–90 (2014).
Vázquez, A. et al. West Nile and Usutu viruses in mosquitoes in Spain, 2008–2009. Am. J. Trop. Med. Hyg. 85, 178–81 (2011).
Figuerola, J., Soriguer, R., Rojo, G., Gómez Tejedor, C. & Jimenez–Clavero, M. A. Seroconversion in wild birds and local circulation of West Nile Virus, Spain. Emerg. Infect. Dis. 13, 1915–17 (2007).
Ferraguti, M. et al. West Nile virus–neutralizing antibodies in wild birds from southern Spain. Epidemiol. Infect. 144, 1907–11 (2016).
García–Bocanegra, I. et al. Seroprevalence and risk factors associated to West Nile virus in horses from Andalusia, Southern Spain. Vet. Microbiol. 160, 341–346 (2012).
del Amo, J. et al. Experimental infection of house sparrows (Passer domesticus) with West Nile virus isolates of Euro–Mediterranean and North American origins. Vet. Res. 45, 33 (2014).
Komar, N. West Nile virus: epidemiology and ecology in North America. Adv. Virus Res. 61, 185–234 (2003).
Godsey, M. S. et al. West Nile virus epizootiology in the southeastern United States, 2001. Vect. Borne Zoon. Dis. 5, 82–89 (2005).
O’Brien, V. A., Meteyer, C. U., Reisen, W. K., Ip, H. S. & Brown, C. R. Prevalence and pathology of West Nile Virus in naturally infected house sparrows, Western Nebraska, 2008. Am. J. Trop. Med. Hyg. 82, 937–44 (2010).
Martínez–de la Puente, J., Ruiz, S., Soriguer, R. & Figuerola, J. Effect of blood meal digestion and DNA extraction protocol on the success of blood meal source determination in the malaria vector Anopheles atroparvus. Malar. J. 12, 109 (2013).
Valinsky, L., Ettinger, C., Bar–Gal, G. K. & Orshan, L. Molecular Identification of bloodmeals from sand flies and mosquitoes collected in Israel. J. Med. Entomol. 51, 678–85 (2014).
Ergunay, K. et al. Serological, molecular and entomological surveillance demonstrates widespread circulation of West Nile Virus in Turkey. PLoS Negl. Trop. Dis. 8, e3028 (2014).
Roiz, D., Ruiz, S., Soriguer, R. & Figuerola, J. Landscape Effects on the Presence, Abundance and diversity of mosquitoes in Mediterranean Wetlands. PLoS One. 10, e0128112 (2015).
Balenghien, T. et al. Evidence of the laboratory vector competence of Culex modestus for West Nile virus. J. Am. Mosq. Control. Assoc. 23, 233–6 (2007).
Brustolin, M. et al. Culex pipiens and Stegomyia albopicta (=Aedes albopictus) populations as vectors for lineage 1 and 2 West Nile virus in Europe. Med. Vet. Entomol. 30, 166–73 (2016).
Sánchez–Gómez, A. et al. Risk mapping of West Nile virus circulation in Spain, 2015. Acta Trop. 169, 163–9 (2017).
Bernabeu–Wittel, M. et al. West Nile virus past infections in the general population of Southern Spain. Enferm. Infec. Microbiol. Clin. 2, 561–5 (2007).
Ferraguti, M. et al. Effects of landscape anthropization on mosquito community composition and abundance. Sci. Rep. 6, 29002 (2016).
Ferraguti, M. et al. Avian Plasmodium in Culex and Ochlerotatus mosquitoes from Southern Spain: effects of season and host-feeding source on parasite dynamics. PLoS One. 8, e66237 (2013).
Schaffner, F. et al. The mosquitoes of Europe, an identification and training programme, CD–ROM (ed. IRD, Montpellier, 2001).
Becker, N. et al. Mosquitoes and their control (2nd ed.) Berlin Heidelberg, (Springer Verlag, 2010).
Harbach, R. E. The identity of Culex perexiguus Theobald versus ex. univittatus Theobald in southern Europe. Eur. Mosq. Bull. 4, 7 (1999).
Svensson, L. Identification guide to European passerines. Thetford, UK (British Trust for Ornithology, 2006).
Sotelo, E. et al. Development and evaluation of a new epitope-blocking ELISA for universal detection of antibodies to West Nile virus. J Virol Methods 174, 35–41 (2011).
Jurado–Tarifa, E. et al. Monitoring of West Nile virus, Usutu virus and Meaban virus in waterfowl used as decoys and wild raptors in southern Spain. Comp. Immunol. Microbiol. Infect. Dis. 49, 58–64 (2016).
Thrusfield, M. V. Veterinary epidemiology (3rd ed.) Oxford (Blackwell, 2005).
Zuur, A. F., Ieno, E. N. & Elphick, C. S. A protocol for data exploration to avoid common statistical problems. Methods in Ecology and Evolution 1, 3–14 (2010).
Symonds, M. R. E. & Moussalli, A. A brief guide to model selection, multimodel inference and model averaging in behavioural ecology using Akaike’s information criterion. Behav. Ecol. Sociobiol. 65, 13 (2011).
Nakagawa, S. & Schielzeth, H. Ageneral and simple method for obtaining R2 from generalized linear mixed-effects models. Methods Ecol. Evol. 4, 133–142 (2013).
Acknowledgements
This study was funded by projects CGL2015-65055-P from the Spanish Ministry of Science and Innovation and P11-RNM-7038 from the Junta de Andalucía. JMP was partially supported by a 2017 Leonardo Grant for Researchers and Cultural Creators, BBVA Foundation. The Foundation accepts no responsibility for the opinions, statements and contents included in the project and/or the results thereof, which are entirely the responsibility of the authors. M.F. and J.M.P. were partially funded by a FPU grant and a Juan de la Cierva contract, respectively. The CSIC Ethics Committee approved (9 March 2012) the procedures used in this study. Special thanks are due to Alberto Pastoriza, Manuel Vázquez, Manolo Lobón, Óscar González, Carlos Moreno, Cristina Pérez, Esmeralda Pérez, Juana Moreno Fernandez and Antonio Magallanes Martín de Oliva for their help during the fieldwork and with mosquito capture and identification, and to Francisco M. Miranda, Olaya García, María del Carmen Barbero Ameller and Antonio Sanz (INGENASA) for their help with the laboratory analyses. We are grateful to all the landowners and to the Consejeria de Medio Ambiente for allowing us to work on their properties. Two anonymous reviewers provided constructive comments on a previous version of the manuscript. The English text was revised by Michael Lockwood.
Author information
Authors and Affiliations
Contributions
J.M.P., M.F., R.S. and J.F. designed the research; J.M.P., M.F., D.R., J.F. and S.R. collected samples and performed the experiments; J.M.P., M.F., M.Á.J.-C., F.L. and J.F. analyzed data; R.S., M.Á.J.-C. and J.F., contributed to the reagents, materials and analysis tools. J.M.P., M.F., D.R., S.R., E.P.-R., R.S., M.Á.J.-C., F.L. and J.F. wrote the paper.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Martínez-de la Puente, J., Ferraguti, M., Ruiz, S. et al. Mosquito community influences West Nile virus seroprevalence in wild birds: implications for the risk of spillover into human populations. Sci Rep 8, 2599 (2018). https://doi.org/10.1038/s41598-018-20825-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-20825-z
This article is cited by
-
Does land-use and land cover affect vector-borne diseases? A systematic review and meta-analysis
Landscape Ecology (2023)
-
Updated occurrence and bionomics of potential malaria vectors in Europe: a systematic review (2000–2021)
Parasites & Vectors (2022)
-
First detection of Aedes japonicus in Spain: an unexpected finding triggered by citizen science
Parasites & Vectors (2019)
-
Evidence that Passerine Birds Act as Amplifying Hosts for Usutu Virus Circulation
EcoHealth (2019)
-
West Nile virus transmission and human infection risk in Veneto (Italy): a modelling analysis
Scientific Reports (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.