Abstract
Major depressive disorder (MDD) in the elderly is a risk factor for dementia, but the precise biological basis remains unknown, hampering the search for novel biomarkers and treatments. In this study, we performed metabolomics analysis of cerebrospinal fluid (CSF) from cognitively intact elderly patients (N = 28) with MDD and age- and gender-matched healthy controls (N = 18). The CSF levels of 177 substances were measured, while 288 substances were below the detection limit. Only ascorbic acid was significantly different, with higher levels in the MDD group at baseline. There were no correlations between CSF ascorbic acid levels and clinical variables in MDD patients at baseline. At the 3-year follow-up, there was no difference of CSF ascorbic acid levels between the two groups. There was a negative correlation between CSF ascorbic acid and CSF amyloid-β42 levels in all subjects. However, there were no correlations between ascorbic acid and other biomarkers (e.g., amyloid-β40, total and phosphorylated tau protein). This preliminary study suggests that abnormalities in the transport and/or release of ascorbic acid might play a role in the pathogenesis of late-life depression.
Similar content being viewed by others
Introduction
Late-life depression, one of the most common psychiatric disorders in older adults, is a growing public health concern as the global population ages. Late-life depression is associated with significant functional impairment, high recurrence rates, chronicity, variable treatment response, and high rates of medical comorbidity and mortality1,2,3,4,5,6. Multiple lines of evidence suggest that late-life depression is a risk factor for the development of mild cognitive impairment and dementia, including Alzheimer’s disease (AD) and vascular dementia3, 7,8,9,10. However, the precise molecular mechanisms underlying the relationship between late-life depression and dementia risk remain unknown. A precise understanding of this relationship would likely contribute to improving preventive interventions in the elderly.
Metabolomics is the profiling of small molecule metabolites and provides the potential to characterize specific metabolic phenotypes associated with a disease. Metabolomics has an advantage over other “omics” techniques in that it directly samples the metabolic changes in an organism and integrates information from changes at the gene, transcript, and protein levels, as well as posttranslational modifications10,11,12,13,14. Cerebrospinal fluid (CSF) is arguably the most relevant sampling substrate for the in vivo study of brain disorders as it reflects the metabolic status and the biochemistry of the brain. Metabolomics analyses of CSF in patients and controls therefore have the potential to reveal protein differences linked to the pathogenesis of neuropsychiatric disorders that may have value as biomarkers and might be targets for novel treatments15,16,17,18,19,20,21,22. We reported that the CSF ratio of glutamine/glutamate levels in elderly patients with MDD was significantly higher than that of age-matched healthy controls, and that the increased ratio in patients was significantly decreased after 3-year follow-up in association with decreased depression symptoms over this time period, suggesting that abnormalities in the glutamine-glutamate cycle in the brain play a role in the pathogenesis of late-life depression23. Furthermore, Pomara et al.24, 25 reported state-dependent alterations in CSF amyloid-β42 levels in cognitively intact elderly MDD patients. In agreement, a recent meta-analysis showed significant reduction of CSF amyloid-β42 levels in late-life depression26, suggesting that elderly MDD patients have significant differences in amyloid-β metabolism, with a change in CSF amyloid-β42 levels in the same direction observed in AD patients. However, there is, currently, no report that has evaluated the metabolomics analysis of CSF of elderly MDD patients.
In the present study, we performed metabolomics analysis of CSF samples from elderly cognitive intact patients with MDD and age- and gender-matched healthy controls. Furthermore, we examined protein expression in postmortem brain samples from controls and psychiatric disorders including MDD patients.
Results
As reported previously23,24,25, the two groups did not differ on any relevant clinical or demographic variable with the exception of the mean HAM-D score, which as expected was significantly higher in the MDD group (Table 1). Of note, the proportion of participants with a reported family history of AD was slightly higher in the control group than in the MDD group. The CSF levels of amyloid-β42 in the MDD group were significantly lower than those of the control group, whereas differences in CSF levels of amyloid-β40, total and phosphorylated tau protein did not differ across conditions (Table 1).
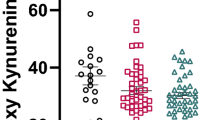
We measured 475 major metabolic compounds from various pathways (e.g., glycolytic system, pentose phosphate pathway, citric acid cycle, urea cycle, polyamine-creatine metabolism pathway, purine metabolism pathway, glutathione metabolism pathway, nicotinamide metabolism pathway, choline metabolism pathway and diverse amino acid metabolism pathways). In this study, we were able to measure 177 metabolites, while 288 were below the detection limit. Interestingly, there was a significant (P = 0.0029) difference in CSF levels of ascorbic acid between the MDD (0.304 ± 0.061 mM, N = 28) and the control groups (0.240 ± 0.075 mM, N = 18) (Table S1) (Table 2) (Fig. 1). In addition, the analysis using covariates (BMI, sex, medication) was also statistically significant (P = 0.0037). There were no significant differences in CSF levels of other substances between the MDD and the control groups at baseline (Table S1). At baseline there were no correlations between CSF ascorbic acid levels and clinical variables in the MDD patients (N = 28). No correlations between CSF ascorbic acid levels and other clinical variables were observed. There was a significant negative correlation (Spearman’s r = −0.31, P = 0.037) between CSF ascorbic acid and CSF amyloid-β42 in the all subjects (N = 46) (Fig. 2). However, there were no significant correlations between ascorbic acid and other biomarkers (e.g., amyloid-β40, total and phosphorylated tau protein) (data not shown). In addition, APOE e4 did not affect CSF levels of ascorbic acid in control and MDD groups (data not shown).
At the 3-year follow-up, there was no difference of CSF ascorbic acid levels between two groups although the HAM-D scores in the elderly MDD patients were significantly decreased (Table 2). Furthermore, there were no correlations between ascorbic acid and other biomarkers (e.g., amyloid-β40, total and phosphorylated tau protein) (data not shown).
Ascorbic acid (vitamin C) is controlled in the brain parenchyma via the sodium dependent vitamin C transporter, SVCT2, which transfers ascorbic acid at the choroid plexus from blood into CSF, and also from extracellular fluid into neurons27. Using Western blot analysis, we measured the expression of SVCT2 in the postmortem samples from the parietal cortex and cerebellum from controls and psychiatric disorders, including MDD, bipolar disorder (BD), and schizophrenia. There was no statistical difference among the four groups (Table 3).
Discussion
In the present study, we found that elderly patients with MDD showed increased CSF levels of ascorbic acid compared to age-matched healthy controls, although CSF levels of other metabolites were not different. Furthermore, we found a negative correlation between CSF ascorbic acid and CSF amyloid-β42 in the all subjects. Given the possible role of CSF amyloid-β42 in the pathogenesis of late-life depression24, 25, 28,29,30, these findings suggest that abnormalities in the brain levels of ascorbic acid might play a role in the depressive symptoms of elderly MDD patients. To our knowledge, this is the first report in the literature of an increase in CSF ascorbic acid in elderly MDD patients, suggesting abnormalities in the transport and/or release of the antioxidant ascorbic acid in the brains of elderly depressed individuals.
Ascorbic acid is a potent water-soluble antioxidant which is not synthesized in the brain. Approximately 40% of ascorbic acid in the brain turns over each day. Ascorbic acid levels are maintained as high as 10 μM in neurons27, 31, suggesting that ascorbic acid is crucial for maintenance of oxidative balance. Under conditions of ascorbic acid deficiency, brain content of ascorbic acid is retained tenaciously, with decreases of less than 2% per day27, 31. Decreased brain levels of ascorbic acid by deficient diet of ascorbic acid may cause dangerous levels of oxidative stress during normal aging, and particularly during inflammatory neurodegenerative diseases including AD. Thus, brain levels of ascorbic acid are under strong homeostatic regulation27, 31. Given the role of oxidative stress and inflammation in late-life depression32,33,34,35,36, it is likely that abnormalities in CSF ascorbic acid associated with oxidative stress may play a role in the pathophysiology of late-life depression.
It has been reported that CSF levels of ascorbic acid in humans were higher than blood37,38,39, supporting ascorbic acid as a “nourishing liquor” that constantly surrounds the brain40. At the choroid plexus, ascorbic acid is actively transported across the basolateral membrane by SVCT2 into the epithelium and then released into the CSF31, 39. In this study, we found higher CSF levels of ascorbic acid in elderly patients with MDD, suggesting that higher CSF levels of ascorbic acid depends on both active “carrier” transport processes and “barrier” integrity of the blood-brain barrier. This may be necessary to prevent ascorbic acid from diffusing out of the brain driven by the concentration gradient39. However, we did not find any changes in the expression of SVCT2 in the postmortem brain samples from MDD. Nonetheless, further research on the role of SVCT2 in the transport of ascorbic acid is needed.
It has also been reported that the CSF: plasma ratio of ascorbic acid in AD patients was higher than that in controls39, 41,42,43, suggesting increased consumption of ascorbic acid as a result of oxidative stress in the AD brain, leading to lower plasma levels39. In 32 adults followed for one year with mild-to-moderate AD, the rates of cognitive decline were not explained by CSF or plasma ascorbic acid independently39. Taken together, it seems that increased CSF levels of ascorbic acid in elderly MDD patients may be due to an increased transport of ascorbic acid into CSF from blood although plasma levels of ascorbic acid were not measured in this study. Further precise studies underlying the reasons of higher CSF levels of ascorbic acid are needed.
A recent study using [11C]PK11195 and positron emission tomography demonstrated an increased microglial activation in the brain from patients with late-life depression44, suggesting neuroinflammation (or oxidative stress) in the brain in elderly MDD patients. It is well known that peripheral inflammatory substances (e.g., C-reactive protein, interleukin (IL)-8, IL-6, tumor necrosis factor (TNF)-α) are higher in patients with late-life depression35, 36. Furthermore, immunological biomarkers, such as vascular endothelial growth factor (VEGF), the chemokine eotaxin, TNF-α, interferon-γ, and macropharge inflammatory protein-1α, are associated with brain structure in late-life depression36. Taken together, it is likely that compensatory responses to oxidative stress in the brain of elderly MDD patients may contribute to the increased CSF levels of ascorbic acid although further detailed study on this hypothesis is needed.
We previously reported a reduction of amyloid-β42 in the same MDD patients at baseline24. Interestingly, we found a negative correlation between CSF ascorbic acid and CSF amyloid-β42 in the all subjects, suggesting a close relationship between these two biomarkers. Importantly, CSF ascorbic acid in elderly MDD patients was no longer significantly different from controls after a 3-year period; the loss of significance coincided with reduction in the severity of depressive symptoms, suggesting that abnormalities in CSF ascorbic acid in elderly depression may be state-dependent. Furthermore, Pomara et al.24 reported higher CSF levels of isoprostane, a biomarker for oxidative stress, in the same patients with MDD. However, there was no correlation between CSF ascorbic acid and CSF isoprostane in all the subjects (data not shown). Taken together, these findings highlight that static indices such as levels of ascorbic acid either in CSF or brain reflect dynamic and brain region specific alterations in the oxidative balance.
Humans have lost the ability to synthesize ascorbic acid27. A cross-sectional and prospective study showed that use of ascorbic acid supplement is associated with reduced prevalence and incidence of AD, suggesting the effectiveness of dietary supplementation of ascorbic acid in older adults45. A recent meta-analysis showed that serum levels of ascorbic acid in patients with MDD were lower than controls, and that serum levels of ascorbic acid in patients with MDD were increased after antidepressant therapy46. Therefore, it is possible that supplementation of ascorbic acid in elderly patients with MDD may contribute to reduced prevalence of AD.
Finally, there are some limitations to this preliminary study that should be noted. The main limitation was small sample size, and similar, future studies in late-life depression would likely benefit from larger sample sizes. In this study, we did not use the multiple test correction since the purpose of this study was the global analysis of a number of metabolites as a pilot study. Follow-up study with much more samples should be conducted to validate our pilot findings. Another limitation was that we did not measure plasma ascorbic acid in the subjects of this study. Given the role of CSF: plasma ratio of ascorbic acid39, 42, 43, it is of great interest to study the relationship between CSF: plasma ratio of ascorbic acid and late-life depression.
In conclusion, we found that the CSF levels of ascorbic acid in elderly patients with MDD were significantly higher than that of age-matched healthy controls. These preliminary findings suggest that oxidative imbalance in the brain reflected by abnormalities in CSF ascorbic acid play a role in the pathogenesis of late-life depression. Further studies measuring CSF and plasma levels of ascorbic acid using larger cohorts, particularly cohorts of antidepressant-naïve MDD patients, will be of great interest.
Materials and Methods
Participants
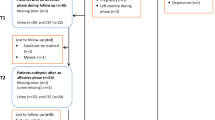
This study was approved by the institutional review boards of the Nathan Kline Institute for Psychiatric Research and the New York University School of Medicine. Metabolomics analysis of this study was approved by Research Ethics Committee of the Graduate School of Medicine, Chiba University. All methods were performed in accordance with the guidelines and regulation of the National Institutes of Health, USA. Participants were volunteers who responded to advertisements in local newspapers and flyers or were recruited from the Memory Education and Research Initiative Program24. All participants provided informed consent prior to examination and received up to $450.00 in compensation. A total of 133 participants completed the baseline evaluation, and 51 of these took part in the optional lumbar puncture procedure. Of these 51 participants, three were excluded because of evidence in their MRI scans of confluent deep or periventricular white matter hyperintensities, defined as one or more hyperintense lesions measuring at least 10 mm in any direction. One individual was excluded because of a Mini-Mental State Examination (MMSE) score below 28. Of the 47 remaining participants, 28 were diagnosed with MDD by a board-certified psychiatrist, leaving 18 comparison subjects. The structural interview for DSM-IV disorders (SCID) was administered by a psychiatrist to establish an MDD diagnosis. Of the 28 patients with MDD, 21 (75%) had recurrent episodes. Table 1 summarizes the demographic and clinical characteristics of the study participants at baseline.
Procedure
The study was conducted over four visits, usually one week apart. The first three visits were conducted at the Nathan Kline Institute for Psychiatric Research and the Clinical and Translational Science Institute, New York University Langone Medical Center. During the first visit, for the purpose of obtaining informed consent, study procedures were explained and participants were informed of their rights. Participants’ medical and psychiatric histories, including family history of AD, were also obtained, and their vital signs were measured. Participants then underwent a psychiatric evaluation, and their global cognitive status was assessed using the MMSE. Additionally, the Hamilton Depression Rating Scale (HAM-D) was administered to rate the severity of current depressive symptoms. Subjects who met the criteria for past MDD but were not currently depressed (i.e., HAM-D score below 16) were included as MDD subjects. Blood was drawn for routine clinical labs and APOE genotyping. During the second visit, participants underwent an MRI scan of the head to quantify the magnitude of vascular brain pathology. During the third visit, subjects underwent a comprehensive neuropsychological assessment, including the Buschke Selective Reminding Test47, the Trail-Making Test, parts A and B48, and the category fluency test49.
Finally, during the fourth visit, a lumbar puncture was performed by a neuroradiologist under guided fluoroscopy in a subset of participants. Prior to the procedure, which was performed between 9:00 a.m. and 10:00 a.m., participants were asked to fast overnight. A total of 15 ml of clear CSF was collected in three polypropylene tubes labeled “A” (first 5 ml), “B” (second 5 ml), and “C” (third 5 ml). The tubes were immediately placed on ice for a maximum of 1 hour until the samples were centrifuged at 4 °C (at 1500 rpm) for 10 minutes. Then, aliquots of 0.25 ml were placed into 1.00-ml polypropylene cryogenic vials and put into Nunc eight-cell storage boxes (Nalge Nunc International, Rochester, N.Y.) at −80 °C. All amyloid-β, tau, and amino acids determinations were performed from tube “C”.
Among these participants, MDD patients (N = 19) and comparison control subjects (N = 17) were followed for 3 years. The MDD patients were receiving antidepressant, such as including SSRIs (paroxetine, escitalopram, fluoxetine), SNRIs (venlafaxine, duloxetine), and mirtazapine23, 24. Clinical data, including physical examination, routine laboratory tests, psychiatric evaluations, HAM-D rating scale, cognitive functions, and CSF samples were collected at 3-year follow-up.
Metabolomics analysis of human CSF samples
Metabolomics analyses of CSF samples from the MDD and control groups were performed at the Chemicals Evaluation and Research Institute, Japan (CERI, Tokyo, Japan) using an GC-MS/MS based multiple reaction monitoring metabolomics platform. GC/MS/MS analysis was performed using a GCMS-TQ8030 (Shimadzu Co., Kyoto, Japan) with a fused silica capillary column (BPX-5; 30 m × 0.25 mm inner diameter, film thickness: 0.25 µm; SGE Analytical science by Trajan Scientific Australia Pty Ltd). Quantifications of the metabolites were conducted with the built-in GCMS Solution software (Ver.4.20, Shimadzu) and GC/MS/MS Smart Metabolites Database (Ver. 2.0, Shimadzu). In this study, 475 major metabolic compounds from various pathways (glycolytic system, pentose phosphate pathway, citric acid cycle, urea cycle, polyamine-creatine metabolism pathway, purine metabolism pathway, glutathione metabolism pathway, nicotinamide metabolism pathway, choline metabolism pathway and diverse amino acid metabolism pathway) were selected for metabolomics analysis (Table S1).
Western blot analysis
Postmortem brain samples (parietal cortex and cerebellum) from control, major depressive disorder (MDD), bipolar disorder (BD), and schizophrenia were obtained from the Neuropathology Consortium of the Stanley Medical Research Institute (MD, USA) (Table S2)50,51,52. Tissue samples were homogenized in Laemmli lysis buffer, and total protein levels were measured using the DC protein assay kit (Bio-Rad, Hercules, CA, USA). Aliquots (50 μg of total protein) were incubated for 5 min at 95 °C, with an equal volume of 125 mM Tris/HCl, pH 6.8, 20% glycerol, 0.1% bromophenol blue, 10% β-mercaptoethanol, 4% sodium dodecyl sulfate, and subjected to sodium dodecyl sulfate polyacrylamide gel electrophoresis, using Mini-PROTEAN® TGX Stain-Free™ Gels (AnykD, cat #: 456-8125, Bio-Rad). Proteins were transferred onto polyvinylidenedifluoride (PVDF) membranes using a Trans Blot Mini Cell (Bio-Rad). For immunodetection, the blots were blocked with 3% BSA in TBST (TBS + 0.1% Tween-20) for 1 h at room temperature (RT), and kept with primary antibodies overnight at 4 °C. Membranes were probed using a sodium-ascorbate co-transporter 2 (SVCT2) antibody (cat #: sc-9926, 1: 200, Santa Cruz Biotechnology, CA, USA). The next day, membranes were washed three times in TBST, and incubated with horseradish peroxidase conjugated anti-goat antibody 1 hour, at RT. After final three washes with TBST, the bands were detected using enhanced chemiluminescence (ECL) prime the Western Blotting Detection system (GE Healthcare Bioscience, Tokyo, Japan). The blots then were incubated in the stripping buffer (2% SDS, 100 mM β-mercaptoethanol, 62.5 mM Tris/HCL PH 6.8) for 30 min at 60 °C followed by three time washed with TBST. The stripped blots were kept blocking solution for 1 hour and incubated with the primary antibody directed against β-actin. Images were captured with a Fuji LAS3000-mini imaging system (Fujifilm, Tokyo, Japan), and immunoreactive bands were quantified.
Statistical analysis
First, Student t-test, and Fisher’s exact test were used to compare the MDD and control groups on MMSE scores, years of education, body mass index, age, incidence of diabetes, gender, APOE genotypes, and reported family history of AD (Table 1). Second, Student t-test was used to compare the two diagnostic groups with respect to all remaining CSF variables. Data of 3-year follow-up were analyzed using two-way repeated multivariate analysis of variance (MANOVA). Spearman’s rank correlation coefficient was used. All tests were two-tailed, and statistical significance was established at an α of 0.05, unless differently noted. All analyses were conducted using SPSS 22 (SPSS, Inc., Chicago) and SAS Ver. 9.4 (SAS Institute, Cary, North Carolina, USA).
References
Luijendijk, H. J. et al. Incidence and recurrence of late-life depression. Arch Gen Psychiatry 65, 1394–1401 (2008).
Byers, A. L., Yaffe, K., Covinsky, K. E., Friedman, M. B. & Bruce, M. L. High occurrence of mood and anxiety disorders among older adults: The National Comorbidity Survey Replication. Arch Gen Psychiatry 67, 489–496 (2010).
Diniz, B. S., Butters, M. A., Albert, S. M., Dew, M. A. & Reynoilds, C. F. Late-life depression and risk of vascular dementia and Alzheimer’s disease: systematic review and meta-analysis of community-based cohort studies. Bri J Psychiatry 202, 329–335 (2013).
Aziz, R. & Steffens, D. C. What are the causes of late-life depression? Psychiatr Clin North Am 36, 497–516 (2013).
Forlani, C. et al. Prevalence and gender differences in late-life depression: a population-based study. Am J Geriatr Psychiatry 22, 370–380 (2014).
Bock, J. O. et al. The impact of depressive symptoms on healthcare costs in late life: longitudinal findings from the AgeMooDe study. Am J Geriatr Psychiatry 25, 131–141 (2017).
Ownby, R. L., Crocco, E., Acevedo, A., John, V. & Loewenstein, D. Depression and risk for Alzheimer disease: systematic review, meta-analysis, and metaregression analysis. Arch Gen Psychiatry 63, 530–538 (2006).
Barnes, D. E. et al. Midlife vs late-life depressive symptoms and risk of dementia: differential effects for Alzheimer disease and vascular dementia. Arch Gen Psychiatry 69, 493–498 (2012).
Mirza, S. S. et al. A. 10-year trajectories of depressive symptoms and risk of dementia: a population-based study. Lancet Psychiatry 3, 628–635 (2016).
Holmes, E., Wilson, I. D. & Nicholson, J. K. Metabolic phenotyping in health and disease. Cell 134, 714–717 (2008).
Quinones, M. P. & Kaddurah-Daouk, R. Metabolomics tools for identifying biomarkers for neuropsychiatric diseases. Neurobiol Dis 35, 165–176 (2009).
Davies, S. K. et al. Effect of sleep deprivation on the human metabolome. Proc Natl Acad Sci USA 111, 10761–10766 (2014).
Sethis, S. & Brietzke, E. Omic-based biomarkers: Application of metabolomics in neuropsychiatric disorders. Int J Neuropsychopharmacol 19, pyu096 (2015).
Brennan, L. Metabolomics in nutrition research - a powerful window into nutritional metabolism. Essays Biochem 60, 451–458 (2016).
Wishart, D. et al. The human cerebrospinal metabolome. J Chromatogr B Analyt Technol Biomed Life Sci 871, 164–173 (2008).
Trushina, E., Dutta, T., Persson, X. M., Mielke, M. M. & Petersen, R. C. Identification of altered metabolic pathways in plasma and CSF in mild cognitive impairment and Alzheimer’s disease using metabolomics. PLoS One 8, e63644 (2013).
Blasco, H. et al. Metabolomics in cerebrospinal fluid of patients with amyotrophic lateral sclerosis: an untargeted approach via high-resolution mass spectrometry. J Proteome Res 12, 3746–3754 (2013).
Blasco, H. et al. Untargeted 1H-NMR metabolomics in CSF: toward a diagnostic biomarker for motor neuron disease. Neurology 82, 1167–1174 (2014).
Trupp, M. et al. Metabolite and peptide levels in plasma and CSF differentiating healthy controls from patients with newly diagnosed Parkinson’s disease. J Parkinsons Dis 4, 549–560 (2014).
Gray, E. et al. The longitudinal cerebrospinal fluid metabolomic profile of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Frontotemporal Degener 16, 456–463 (2015).
Wuolikainen, A. et al. Multi-platform mass spectrometry analysis of the CSF and plasma metabolomes of rigorously matched amyotrophic lateral sclerosis, Parkinson’s disease and control subjects. Mol Biosyst 12, 1287–1298 (2016).
Yoshimi, N. et al. Cerebrospinal fluid metabolomics identifies a key role of isocitrate dehydrogenase in bipolar disorder: Evidence in support of mitochondrial dysfunction hypothesis. Mol Psychiatry 21, 1504–1510 (2016).
Hashimoto, K. et al. Abnormality in glutamine-glutamate cycle in the cerebrospinal fluid of cognitively intact elderly individuals with major depressive disorder: 3-year follow-up study. Transl Psychiatry 6, e744 (2016).
Pomara, N. et al. Lower CSF amyloid beta peptides and higher F2-isoprostanes in cognitively intact elderly individuals with major depressive disorder. Am J Psychiatry 169, 523–530 (2012).
Pomara, N. et al. State-dependent alterations in cerebrospinal fluid Aβ42 levels in cognitively intact elderly with late-life major depression. Neuroreport 27, 1068–1071 (2016).
Nascimento, K. K., Silva, K. P., Malloy-Diniz, L. F., Butters, M. A. & Diniz, B. S. Plasma and cerebrospinal fluid amyloid-β levels in late-life depression: A systematic review and meta-analysis. J Psychiatr Res 69, 35–41 (2015).
Rice, M. E. Ascorbate regulation and its neuroprotective role in the brain. Trends Neurosci 23, 209–216 (2000).
Osorio, R. S., Gumb, T. & Pomara, N. Soluble amyloid-β levels and late-life depression. Curr Pharm Des 20, 2547–2554 (2014).
Diniz, B. S. et al. Plasma biosignature and brain pathology related to persistent cognitive impairment in late-life depression. Mol Psychiatry 20, 594–601 (2015).
Mahgoub, N. & Alexopoulos, G. S. Amyloid hypothesis: Is there a role for antiamyloid treatment in late-life depression? Am J Geriatr Psychiatry 24, 239–247 (2016).
Spector, R. Vitamin homeostatis in the central nervous system. New Eng J Med 296, 1293–1398 (1977).
McKinney, B. C., Oh, H. & Sibille, E. Age-by-disease biological interactions: implications for late-life depression. Front Genet 3, 237 (2012).
Tallaksen, C. M., Bøhmer, T. & Bell, H. Concentrations of the water-soluble vitamins thiamin, ascorbic acid, and folic acid in serum and cerebrospinal fluid of healthy individuals. Am J Clin Nutr 56, 559–564 (1992).
Milaneschi, Y. et al. Lipid peroxidation and depressed mood in community-dwelling older men and women. PLoS One 8, e65406 (2013).
Martínez-Cengotitabengoa, M. et al. Peripheral inflammatory parameters in late-life depression: A systematic review. Int J Mol Sci 17, E2022 (2016).
Smagula, S. F. et al. Immunological biomarkers associated with brain structure and executive function in late-life depression: exploratory pilot study. Int J Geriatr Psychiatry, 32, 692–699 (2017).
Quinn, J., Suh, J., Moore, M. M., Kaye, J. & Frei, B. Antioxidants in Alzheimer’s disease-vitamin C delivery to a demanding brain. J Alzheimers Dis 5, 309–313 (2003).
Bowman, G. L. et al. Ascorbic acid and rates of cognitive decline in Alzheimer’s disease. J Alzheimers Dis 16, 93–98 (2009).
Davson, H. & Segal, H. B. Physiology of the CSF and blood-brain barriers. Boca Raton, CRC, 1996.
Spector, R. & Johanson, C. Micronutrient and urate transport in choroid plexus and kidney: implications for drug therapy. Pharm Res 23, 2515–2524 (2006).
Reiber, H., Ruff, M. & Uhr, M. Ascorbate concentration in human cerebrospinal fluid (CSF) and serum. Intrathecal accumulation and CSF flow rate. Clin Chim Acta 217, 163–173 (1993).
Paraskevas, G. P. et al. Ascorbate in healthy subjects, amyotrophic lateral sclerosis and Alzheimer’s disease. Acta Neurol Scad 96, 88–90 (1997).
Su, L. et al. Neuroinflammatory and morphological changes in late-life depression: the NIMROD study. Bri J Psychiatry 209, 525–526 (2016).
Ancelin, M. L. et al. C-reactive protein gene variants: independent association with late-life depression and circulating protein levels. Transl Psychiatry 5, e499 (2015).
Zandi, P. P. et al. Reduced risk of Alzheimer disease in users of antioxidant vitamin supplements: the Cache County Study. Arch Neurol 61, 82–88 (2004).
Liu, T. et al. A meta-analysis of oxidative stress markers in depression. PLoS One 10, e0138904 (2015).
Buschke, H. Retrieval in human learning. Trans NY Acad Sci 36, 721–729 (1974).
Army Individual Test Battery. Manual of Directions and Scoring. Adjutant General’s Office, War Department; Washington, DC: 1994.
Goodglass, H. & Kaplan, E. Assessment of Aphasia and Related Disorders. Lea & Febiger; Philadelphia: 1972.
Torrey, E. F., Webster, M., Knable, M., Johnston, N. & Yolken, R. H. The Stanley foundation brain collection and neuropathology consortium. Schizophr Res 44, 151–155 (2000).
Ren, Q. et al. Gene deficiency and pharmacological inhibition of soluble epoxide hydrolase confers resilience to repeated social defeat stress. Proc Natl Acad Sci USA 113, E1944–E1952 (2016).
Yang, B., Ren, Q., Zhang, J. C., Chen, Q. X. & Hashimoto, K. Altered expression of BDNF, BDNF pro-peptide, and their precursor proBDNF in the brain and liver tissues from psychiatric disorders: rethinking the brain-liver axis. Transl Psychiatry 7, e1128 (2017).
Acknowledgements
This research was supported by grants from the Strategic Research Program for Brain Sciences from Japan Agency for Medical Research and Development, AMED (to K.H.), and SENSHIN Medical Research Foundation, Japan (to K.H.), the Torsten Söderberg Foundation at the Royal Swedish Academy of Sciences, the Knut and Alice Wallenberg Foundation, the Swedish Research Council and the NIMH (to N.P., R01 MH-080405). We are also thankful to the patients and controls participating in this study. We thank to The Stanley Medical Research Institute for providing the postmortem brain samples.
Author information
Authors and Affiliations
Contributions
K.H. and N.P. designed the research; and K.H., T.I., D.B., J.N., C.R.M., H.Z., K.B., and N.P. coordinated overall experimental goals and reviewed data in addition to participating in writing and reviewing the final manuscript. Y.S. analyzed the data. K.H. wrote the paper. All authors have read and commented on the final manuscript and have agreed to this submission.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hashimoto, K., Ishima, T., Sato, Y. et al. Increased levels of ascorbic acid in the cerebrospinal fluid of cognitively intact elderly patients with major depression: a preliminary study. Sci Rep 7, 3485 (2017). https://doi.org/10.1038/s41598-017-03836-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-03836-0
This article is cited by
-
Mechanistic examination of methimazole-induced hepatotoxicity in patients with Grave’s disease: a metabolomic approach
Archives of Toxicology (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.