Abstract
Many studies have established the high diagnostic accuracy of shear wave elastography (SWE) for the detection of prostate cancer (PCa); however, its utility remains a subject of debate. This meta-analysis sought to appraise the overall accuracy of SWE for the detection of PCa. A literature search of the PubMed, Embase, Cochrane Library, Web of Science and CNKI (China National Knowledge Infrastructure) databases was conducted. In all of the included studies, the diagnostic accuracy of SWE was compared with that of histopathology, which was used as a standard. Data were pooled, and the sensitivity, specificity, area under the curve (AUC), positive likelihood ratio (PLR), negative likelihood ratio (NLR), and diagnostic odds ratio (DOR) were calculated to estimate the accuracy of SWE. The pooled sensitivity and specificity for the diagnosis of PCa by SWE were 0.844 (95% confidence interval: 0.696–0.927) and 0.860 (0.792–0.908), respectively. The AUC was 0.91 (0.89–0.94), the PLR was 6.017 (3.674–9.853), and the NLR was 0.182 (0.085–0.389). The DOR was 33.069 (10.222–106.982). Thus, SWE exhibited high accuracy for the detection of PCa using histopathology as a diagnostic standard. Moreover, SWE may reduce the number of core biopsies needed.
Similar content being viewed by others
Introduction
Prostate cancer (PCa) is a public health problem worldwide. PCa is the most common malignant tumor in adult males, and the incidence rate is increasing1. Moreover, PCa is the second leading cause of cancer death in men. Recently, diagnoses of PCa have relied on levels of prostate-specific antigen (PSA) and digital rectal examination (DRE), although pathologic histology remains the gold standard. However, these approaches potentially lead to under-diagnosis of PCa, whereas biopsy is an invasive method associated with patient discomfort and, in some cases, serious complications.
Although traditional grayscale transrectal ultrasonography (TRUS) is routinely used in diagnosis and to guide biopsy, it is not sufficiently sensitive or specific for biopsy procedures2. Biopsy protocols should be optimized to accurately detect PCa while also reducing the number of prostate biopsy specimens and biopsy-related patient morbidity3. The prostate gland is one of the earliest organs for which elastography was proposed and applied4. PCa is stiffer than normal tissue because of its increased cellularity, which is sometimes found during DRE5,6,7. Transrectal elastosonography (TRES) has already been established to be feasible in guiding biopsies and for improving the detection of prostate lesions8,9,10,11.
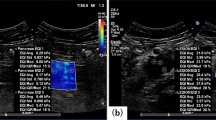
Shear wave elastography (SWE) is a novel real-time imaging technique that represents a substantial advance in ultrasound elastography. When SWE is performed, the transducer automatically generates acoustic radiation force using a special “supersonic” speed that moves multiple focus points following the Mach cone principle. Tissue is then mechanically excited by the Mach cone impulse to generate small, localized tissue displacements (1–10 mm). These displacements have been tracked using a system to calculate the shear wave propagation speed and the quantitative tissue stiffness (i.e., Young’s modulus, kPa)12, 13, which is defined as E = σ/ε, where σ is the applied stress and ε is the strain (the ratio of the resultant deformation of tissue over the original reference length of the medium)14. Previous studies have established that the Young’s modulus of PCa was significantly greater than that of benign prostatic tissue; the sensitivity ranged from 43% to 96.2%, and the specificity ranged from 69.1% to 96.2%14,15,16,17,18,19,20. However, there have been large differences among the results of different studies, and the cut-off value for adequate distinction between PCa and benign tissue remains undetermined. Additionally, another study found that SWE was a poor predictor of malignancy and that the cut-off value had little practical meaning21. Therefore, the present study aimed to perform a meta-analysis to appraise the overall accuracy of SWE for the diagnosis of PCa.
Results
Characteristics of the included studies
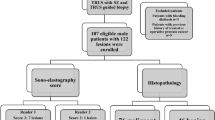
An electronic search identified 286 records. After screening titles and abstracts, we identified 34 studies for full text review. Among these articles, we classified 16 as review articles, 3 as comments, and 5 as other detection methods, whereas 3 had insufficient data for calculation. Ultimately, 7 correlative studies were identified as eligible studies; these were published from 2012 to 2016 (Fig. 1). Among those studies, two were analyzed twice, one according to PSA groupings of 4–20 μg/L and over 20 μg/L and one according to the ultrasonography section (axial vs. sagittal). Ultimately, data from 9 groups were included in this meta-analysis. Additionally, only one study referred to the transition zone, which is rare for PCa, and partial data were not included in the statistical analysis. Among these studies, 2 evaluated the diagnostic accuracy of SWE compared with the histopathology of radical prostatectomy (RP) specimens as a reference standard, whereas 5 compared SWE with the histopathology of TRUS biopsy specimens. The patient clinical features and essential data are summarized in Table 1.
Methodological quality assessment of the included studies
Quality evaluation results for the individual studies are shown in Table 2. The overall risk of bias was low because the index test and reference test characterization were adequate in most studies, and only one equivocal result was reported. In two studies15, 17, it was unclear whether the pathologist was blinded to the SWE results. One study14 used a previously determined cut-off value, which was based on clinical experience and reported in the literature as the SWE reference standard. Another study19 found that SWE was limited as a tool to reliably differentiate benign from malignant prostate tissues.
Diagnostic accuracy
Statistical analysis revealed no heterogeneity arising from a threshold effect, and the Spearman correlation coefficient of sensitivity and 1-specificity was −0.533 (p = 0.139). Ultimately, the diagnostic accuracy of SWE for the diagnosis of PCa was computed based on a pooled sensitivity of 0.844 (95% confidence interval (CI): 0.696–0.927), pooled specificity of 0.860 (95% CI: 0.792–0.908), pooled positive likelihood ratio (PLR) of 6.017 (95% CI: 3.674–9.853), pooled negative likelihood ratio (NLR) of 0.182 (95% CI: 0.085–0.389), and pooled diagnostic odds ratio (DOR) of 33.069 (95% CI: 10.222–106.982). Forest plots of all indices are shown in Fig. 2. An overall high degree of accuracy was revealed by the summary receiver operating characteristic (SROC) curve with an area under the curve (AUC) of 0.91 (95% CI: 0.89–0.94) (Fig. 3). A Fagan nomogram was constructed to illustrate the pre- and post-test probability of SWE to predict PCa based on all 7 studies (Fig. 4). Without taking into account the results of SWE, a PCa episode had a ‘pre-test’ probability of 20% to be detected. With a SWE-positive result for the detection of PCa, there was a 60% ‘post-test’ probability of a subsequent PCa episode. With a negative SWE, the ‘post-test’ probability of PCa dropped to 4%.
Evaluation of publication bias
A Deeks’ funnel plot was generated to explore the potential for publication bias. Based on the symmetric shape of the funnel plot of the pooled DOR (Fig. 5) and the Deeks’ test non-significant value (p = 0.156), we detected no potential publication bias in this meta-analysis.
Discussion
Currently, several methods are used to detect PCa. According to current guidelines22, diagnosis should include PSA level measurement, DRE and TRUS. However, none of these measurements can provide an optimal diagnosis for PCa because of limitations of each approach. PSA has led to many cases of misdiagnosis due to its high sensitivity but low specificity23, resulting in many patients with benign lesions undergoing unnecessary biopsy23, 24. DRE has been used as a screening tool for PCa; however, DRE is examiner-dependent method and is limited to the posterior part of the prostate. TRUS is a safe procedure that can provide effective evidence for the detection of PCa. Unfortunately, TRUS is a non-quantitative method that is associated with subjective measurements and largely depends upon the ability of the physician performing the examination; it has a reported sensitivity of 17–57% and specificity of 40–63%25. Therefore, developing an ideal imaging and detection method for PCa that offers high overall sensitivity and specificity is essential.
An increased cell density of a neoplastic mass leads to changes in tissue elasticity such that the stiffness of normal tissue is significantly different from that of tumor tissue26, 27. Elastography is an imaging technique used for the detection of cancer tissue based on stiffness differences among various tissues28, and it has been shown to be a useful diagnostic method for many organs, such as the thyroid, breast and prostate29,30,31. Most studies have reported a remarkable amelioration in PCa identification using elastography32, 33. The sensitivity of elastography for PCa diagnosis can reach or exceed 90%, which is obviously greater than that of PSA, DRE or TRUS32, 34, 35. However, traditional elastography also has many limitations, mostly due to the lack of uniform repeatability resulting from manual compression and operator dependency, which can introduce extensive variability36,37,38.
SWE is a technique that uses a sonographic pulse to produce a shear wave in the tissue39, 40. Tissue stiffness is expressed as the Young’s modulus or simply as the ratio of stress generated by tissue deformation41. A previous study showed no significant difference in intra-observer reproducibility among the measurements stratified by prostate gland volume, patient age, or levels of serum PSA42. Compared with quasistatic compression elastography, SWE is much closer to a standard TRUS clinical examination because it does not require any additional compression.
Recently, SWE has been shown to be a useful technique for prostate examination9, 14,15,16,17,18,19, 21, 42. Barr et al.17 reported that SWE showed a high sensitivity of 96.2%, specificity of 96.2%, positive predictive value (PPV) of 69.4%, and negative predictive value (NPV) of 99.6% for the detection of PCa when 37 kPa was used as a cut-off value between benign and malignant lesions. Ahmad et al.14 also showed that the sensitivity and specificity of SWE for PCa detection could each reach 90%. However, Woo et al.19 reported low sensitivity and variable specificity for the diagnostic value of SWE in the detection of PCa, even though the SWE parameters were significantly different between PCa and benign prostate tissues. Additionally, Porsch et al.21 showed that SWE was a poor predictor of malignancy for prostate lesions. Considering these inconsistent results, we believed it necessary to assess the diagnostic value of SWE for the detection of PCa. To the best of our knowledge, this represents the first meta-analysis to evaluate the diagnostic value of SWE for the detection of PCa.
Literature screening was carried out following a strict protocol, and the search ultimately identified 7 relevant studies. Deeks’ funnel plots showed no significant publication bias, and according to the QUADAS-2 questionnaire, the 7 studies were of high quality. Our results showed that SWE had a pooled sensitivity of 84.4% and specificity of 86.0% for the detection of PCa; these values are both higher than those obtained for traditional TRUS32 and real-time elastography for the diagnosis of patients with suspected PCa37. The AUC (0.91) and DOR (33.069) further indicated perfect overall accuracy. Additionally, the PLR value was 6.017 (95% CI: 3.674–9.853), which was clinically meaningful for our measures of diagnostic accuracy.
Currently, the success rate of systematic prostate biopsy varies from 25% to 30%, whereas its false-negative rate ranges from 17% to 21% in patients with a negative initial series of biopsies43, 44. Real-time quantitative SWE imaging has the potential to change the clinical practice of PCa identification and screening by improving the localization of abnormal foci and allowing limited targeted biopsies of suspicious areas, thereby reducing both complications and costs associated with the current standard of care14. Although there was no cut-off-value-related heterogeneity in this meta-analysis, it would be of interest to determine whether the measured stiffness or a specific cut-off value predicts up- or down-grading of these regions. This topic could be the subject of future investigations.
A comprehensive literature search and careful data extraction were performed to avoid bias. Nevertheless, limitations exist in our study. First, we did not carry out subgroup analysis of patients with different measurement locations; previous studies have revealed that the location of tumor foci within the prostate gland can influence the detection rate using TRES5, 16, 45, 46. Although SWE provides much-needed solutions to the ongoing challenge of accurately locating areas of interest in the prostate, it also has the inherent advantage of independence from operator experience and expertise. Second, most studies considered in this meta-analysis used TRUS-guided biopsy data as a reference standard for PCa detection, whereas two studies used histopathology analyses of RP specimens. Although TRUS-guided biopsy is the recommended diagnostic method for most patients suspected of having PCa47, this method performs poorly in locating PCa compared with histopathology of the RP specimen48, and SWE estimates also lack strong correlations with PCa location. Third, we failed to acquire unpublished data, and language limitations might have affected the reliability of our results. Fourth, this meta-analysis did not evaluate the correlation between the stiffness value of a lesion and the Gleason score because of a lack of valid data for extraction despite the fact that the Gleason score is one of the most frequently used histologic grading systems for PCa49.
Based on the findings of this meta-analysis and previous studies, we consider SWE to be a novel and non-invasive imaging technique that is superior to conventional TRUS for the assessment of tissue stiffness to provide information for the detection of PCa and biopsy guidance. The application of SWE might lead to a decrease in the number of biopsy cores. Although SWE does not require any additional compression compared with quasistatic compression elastography and no significant difference in intra-observer reproducibility among the measurements42, practitioners should be trained in its application, and reference standards should be agreed upon for the location of prostate cancer lesions and histopathology. The Gleason score is one of the most frequently used histologic grading systems for PCa, and the prognosis of PCa is closely related to the Gleason score49; thus, multicenter studies with a larger number of cases should be conducted to reveal the correlation between the Gleason score and the tissue stiffness of PCa. In addition, a previous study50 showed that multiparametric MRI (mpMRI) provided the best anatomical and functional imaging of the prostate compared with that of other imaging methods, and a systematic review51 suggested that mpMRI could be used to trigger a targeted repeat biopsy for prostate cancer diagnosis. Future research should be performed to evaluate the correlations between SWE and mpMRI with histopathology as the gold standard.
In conclusion, this meta-analysis shows that SWE has high sensitivity and specificity for the detection of PCa and is useful for differentiating between malignant and benign prostate lesions. Thus, we believe that SWE could improve the guiding capability and reduce the unnecessary core biopsies required for diagnosis. Further studies with a multicenter design will be needed to assess the role of SWE in the detection of PCa.
Methods
Search strategy
An independent search of the English and Chinese medical literature using the PubMed (Medicine) database and cross-citation with other databases (i.e., Embase, Cochrane Library databases, Web of Science and CNKI) was performed to identify all studies involving diagnostic tests that estimated the value of SWE for the diagnosis of PCa. Searches were conducted using the following key words: elastography, sonoelastography, and elastosonography combined with prostate. Repeated articles were manually excluded. Unpublished relevant data were also considered, but no studies with such data were found that were appropriate for inclusion. This study was performed by two independent authors. The search was updated until October 23, 2016.
Eligibility and exclusion criteria
All articles were evaluated independently by two authors. A study was included if it met the following criteria: (1) a cross-sectional study that evaluated the ability of SWE to detect PCa; (2) use of histopathology as a diagnostic standard; and (3) reported data (sensitivity and specificity) necessary to calculate the true-positive, false-negative, false-positive and true-negative rates of SWE in the diagnosis of PCa. All of the included studies should have obtained informed consent from study participants and received protocol approval by an ethics committee or institutional review board. Review articles, conference reports, letters, editorial comments, opinions, prefaces, low-quality studies and articles not published in English or Chinese were excluded. All disagreements were resolved by consensus.
Data extraction
All relevant data from the 7 included studies, including first author; year that the study was performed; age of subjects; PSA level; number of patients; number of samples; ultrasound system; cut-off value; and number of true positives, false negatives, false positives and true negatives, were extracted in a unified form. Any divergence from this procedure was resolved by discussion.
Assessments of methodological quality
Methodological quality was evaluated using the revised Quality Assessment of Diagnostic Accuracy Studies (QUADAS-2)52 included in a systematic reviews tool. QUADAS-2 classifies risks for bias into four key domains that encompass patient selection, index test, reference standard, flow and timing. Each domain was assessed in terms of the risk of bias, and patient selection, index test, and reference standard were also assessed for applicability. Two authors independently conducted the quality assessment, and any disagreements were resolved by discussion or appeal to a third author.
Statistical analysis
The statistical software package STATA, version 11.0 (Stata Corporation, College Station, TX, USA), and Meta-Disc, version 1.4 for Windows (XI Cochrane Colloquium, Barcelona, Spain), were used in this study. To research possible heterogeneity resulting from the threshold effect, we calculated Spearman correlation coefficients between sensitivity and 1-specificity. The pooled sensitivity, specificity, AUC, PLR, NLR, DOR, and other related indexes were calculated using STATA. Fagan’s nomogram was used to visualize the detection of SWE for PCa using likelihood ratios to calculate a post-test probability based on Bayesian theorems. We performed Deeks’ funnel plot analysis to check for potential publication bias in our study, with a p-value < 0.1 suggesting statistical significance53.
References
Jemal, A. et al. Global cancer statistics. CA: a cancer journal for clinicians 61, 69–90, doi:10.3322/caac.20107 (2011).
Bjurlin, M. A. et al. Optimization of initial prostate biopsy in clinical practice: sampling, labeling and specimen processing. The Journal of urology 189, 2039–2046, doi:10.1016/j.juro.2013.02.072 (2013).
Rodriguez, L. V. & Terris, M. K. Risks and complications of transrectal ultrasound. Current opinion in urology 10, 111–116, doi:10.1097/00042307-200003000-00011 (2000).
Parker, K. J., Huang, S. R., Musulin, R. A. & Lerner, R. M. Tissue response to mechanical vibrations for “sonoelasticity imaging”. Ultrasound in medicine & biology 16, 241–246 (1990).
Salomon, G. et al. Evaluation of prostate cancer detection with ultrasound real-time elastography: a comparison with step section pathological analysis after radical prostatectomy. European urology 54, 1354–1362, doi:10.1016/j.eururo.2008.02.035 (2008).
Konig, K. et al. Initial experiences with real-time elastography guided biopsies of the prostate. The Journal of urology 174, 115–117, doi:10.1097/01.ju.0000162043.72294.4a (2005).
Ophir, J. et al. Elastography: ultrasonic estimation and imaging of the elastic properties of tissues. Proceedings of the Institution of Mechanical Engineers. Part H, Journal of engineering in medicine 213, 203–233, doi:10.1243/0954411991534933 (1999).
Sumura, M. et al. Initial evaluation of prostate cancer with real-time elastography based on step-section pathologic analysis after radical prostatectomy: a preliminary study. International journal of urology: official journal of the Japanese Urological Association 14, 811–816, doi:10.1111/j.1442-2042.2007.01829.x (2007).
Brock, M. et al. The impact of real-time elastography guiding a systematic prostate biopsy to improve cancer detection rate: a prospective study of 353 patients. The Journal of urology 187, 2039–2043, doi:10.1016/j.juro.2012.01.063 (2012).
van Hove, A. et al. Comparison of image-guided targeted biopsies versus systematic randomized biopsies in the detection of prostate cancer: a systematic literature review of well-designed studies. World journal of urology 32, 847–858, doi:10.1007/s00345-014-1332-3 (2014).
Postema, A., Mischi, M., de la Rosette, J. & Wijkstra, H. Multiparametric ultrasound in the detection of prostate cancer: a systematic review. World journal of urology 33, 1651–1659, doi:10.1007/s00345-015-1523-6 (2015).
Bercoff, J., Tanter, M. & Fink, M. Supersonic shear imaging: a new technique for soft tissue elasticity mapping. IEEE transactions on ultrasonics, ferroelectrics, and frequency control 51, 396–409, doi:10.1109/TUFFC.2004.1295425 (2004).
Franchi-Abella, S., Elie, C. & Correas, J. M. Ultrasound elastography: advantages, limitations and artefacts of the different techniques from a study on a phantom. Diagnostic and interventional imaging 94, 497–501, doi:10.1016/j.diii.2013.01.024 (2013).
Ahmad, S., Cao, R., Varghese, T., Bidaut, L. & Nabi, G. Transrectal quantitative shear wave elastography in the detection and characterisation of prostate cancer. Surgical endoscopy 27, 3280–3287, doi:10.1007/s00464-013-2906-7 (2013).
Mo, Z. et al. Transrectal shear wave elastography combined with transition zone biopsy for detecting prostate cancer. Zhonghua Nan Ke Xue Za Zhi 21, 610–614, doi:10.13263/j.cnki.nja.2015.07.006 (2015).
Rouviere, O. et al. Stiffness of benign and malignant prostate tissue measured by shear-wave elastography: a preliminary study. European radiology 27, 1858–1866, doi:10.1007/s00330-016-4534-9 (2016).
Barr, R. G., Memo, R. & Schaub, C. R. Shear wave ultrasound elastography of the prostate: initial results. Ultrasound quarterly 28, 13–20, doi:10.1097/RUQ.0b013e318249f594 (2012).
Boehm, K. et al. Shear wave elastography for localization of prostate cancer lesions and assessment of elasticity thresholds: implications for targeted biopsies and active surveillance protocols. The Journal of urology 193, 794–800, doi:10.1016/j.juro.2014.09.100 (2015).
Woo, S., Kim, S. Y., Cho, J. Y. & Kim, S. H. Shear wave elastography for detection of prostate cancer: a preliminary study. Korean journal of radiology 15, 346–355, doi:10.3348/kjr.2014.15.3.346 (2014).
Correas, J. M. et al. Prostate cancer: diagnostic performance of real-time shear-wave elastography. Radiology 275, 280–289, doi:10.1148/radiol.14140567 (2015).
Porsch, M. et al. New aspects in shear-wave elastography of prostate cancer. Journal of ultrasonography 15, 5–14, doi:10.15557/JoU.2015.0001 (2015).
Heidenreich, A. et al. EAU guidelines on prostate cancer. part 1: screening, diagnosis, and local treatment with curative intent-update 2013. European urology 65, 124–137, doi:10.1016/j.eururo.2013.09.046 (2014).
Borley, N. & Feneley, M. R. Prostate cancer: diagnosis and staging. Asian journal of andrology 11, 74–80, doi:10.1038/aja.2008.19 (2009).
Aigner, F. et al. Comparison of real-time sonoelastography with T2-weighted endorectal magnetic resonance imaging for prostate cancer detection. Journal of ultrasound in medicine: official journal of the American Institute of Ultrasound in Medicine 30, 643–649, doi:10.7863/jum.2011.30.5.643 (2011).
Seitz, M. et al. [Imaging procedures to diagnose prostate cancer]. Der Urologe. Ausg. A 46, W1435–1446; quiz W1447–1438, doi:10.1007/s00120-007-1455-x (2007).
Phipps, S., Yang, T. H., Habib, F. K., Reuben, R. L. & McNeill, S. A. Measurement of tissue mechanical characteristics to distinguish between benign and malignant prostatic disease. Urology 66, 447–450, doi:10.1016/j.urology.2005.03.017 (2005).
Krouskop, T. A., Wheeler, T. M., Kallel, F., Garra, B. S. & Hall, T. Elastic moduli of breast and prostate tissues under compression. Ultrasonic imaging 20, 260–274, doi:10.1177/016173469802000403 (1998).
Miyagawa, T. et al. Real-time elastography for the diagnosis of prostate cancer: evaluation of elastographic moving images. Japanese journal of clinical oncology 39, 394–398, doi:10.1093/jjco/hyp026 (2009).
Lyshchik, A. et al. Thyroid gland tumor diagnosis at US elastography. Radiology 237, 202–211, doi:10.1148/radiol.2363041248 (2005).
Itoh, A. et al. Breast disease: clinical application of US elastography for diagnosis. Radiology 239, 341–350, doi:10.1148/radiol.2391041676 (2006).
Rhymer, J. C. Elastography in the detection of prostatic cancer. Clinical radiology 58, 337; author reply 337 (2003).
Brock, M. et al. Comparison of real-time elastography with grey-scale ultrasonography for detection of organ-confined prostate cancer and extra capsular extension: a prospective analysis using whole mount sections after radical prostatectomy. BJU international 108, E217–222, doi:10.1111/j.1464-410X.2011.10209.x (2011).
Dudea, S. M. et al. Value of ultrasound elastography in the diagnosis and management of prostate carcinoma. Medical ultrasonography 13, 45–53 (2011).
Miyanaga, N. et al. Tissue elasticity imaging for diagnosis of prostate cancer: a preliminary report. International journal of urology: official journal of the Japanese Urological Association 13, 1514–1518, doi:10.1111/j.1442-2042.2006.01612.x (2006).
Souchon, R. et al. Monitoring the formation of thermal lesions with heat-induced echo-strain imaging: a feasibility study. Ultrasound in medicine & biology 31, 251–259, doi:10.1016/j.ultrasmedbio.2004.11.004 (2005).
Correas, J. M. et al. Ultrasound elastography of the prostate: state of the art. Diagnostic and interventional imaging 94, 551–560, doi:10.1016/j.diii.2013.01.017 (2013).
Zhang, B. et al. Real-time elastography in the diagnosis of patients suspected of having prostate cancer: a meta-analysis. Ultrasound in medicine & biology 40, 1400–1407, doi:10.1016/j.ultrasmedbio.2014.02.020 (2014).
Good, D. W. et al. Elasticity as a biomarker for prostate cancer: a systematic review. BJU international 113, 523–534, doi:10.1111/bju.12236 (2014).
Barr, R. G. Sonographic breast elastography: a primer. Journal of ultrasound in medicine: official journal of the American Institute of Ultrasound in Medicine 31, 773–783, doi:10.7863/jum.2012.31.5.773 (2012).
Correas, J. M. et al. Update on ultrasound elastography: miscellanea. Prostate, testicle, musculo-skeletal. European journal of radiology 82, 1904–1912, doi:10.1016/j.ejrad.2013.05.031 (2013).
Ophir, J. et al. Elastographic imaging. Ultrasound in medicine & biology 26(Suppl 1), S23–29 (2000).
Woo, S., Kim, S. Y., Lee, M. S., Cho, J. Y. & Kim, S. H. Shear wave elastography assessment in the prostate: an intraobserver reproducibility study. Clinical imaging 39, 484–487, doi:10.1016/j.clinimag.2014.11.013 (2015).
Singh, H. et al. Predictors of prostate cancer after initial negative systematic 12 core biopsy. The Journal of urology 171, 1850–1854, doi:10.1097/01.ju.0000119667.86071.e7 (2004).
Mian, B. M. et al. Predictors of cancer in repeat extended multisite prostate biopsy in men with previous negative extended multisite biopsy. Urology 60, 836–840, doi:10.1016/S0090-4295(02)01950-7 (2002).
Pallwein, L. et al. Prostate cancer diagnosis: value of real-time elastography. Abdominal imaging 33, 729–735, doi:10.1007/s00261-007-9345-7 (2008).
Tsutsumi, M. et al. The impact of real-time tissue elasticity imaging (elastography) on the detection of prostate cancer: clinicopathological analysis. International journal of clinical oncology 12, 250–255, doi:10.1007/s10147-007-0669-7 (2007).
Smith, R. A., Cokkinides, V. & Eyre, H. J. Cancer screening in the United States, 2007: a review of current guidelines, practices, and prospects. CA: a cancer journal for clinicians 57, 90–104 (2007).
Muntener, M. et al. Prognostic significance of Gleason score discrepancies between needle biopsy and radical prostatectomy. European urology 53, 767–775, discussion 775–766, doi:10.1016/j.eururo.2007.11.016 (2008).
Barr, R. G. et al. WFUMB Guidelines and Recommendations on the Clinical Use of Ultrasound Elastography: Part 5. Prostate. Ultrasound in medicine & biology 43, 27–48, doi:10.1016/j.ultrasmedbio.2016.06.020 (2017).
Pokorny, M. R. et al. Prospective study of diagnostic accuracy comparing prostate cancer detection by transrectal ultrasound-guided biopsy versus magnetic resonance (MR) imaging with subsequent MR-guided biopsy in men without previous prostate biopsies. European urology 66, 22–29, doi:10.1016/j.eururo.2014.03.002 (2014).
Haider, M. A., Yao, X., Loblaw, A. & Finelli, A. Multiparametric Magnetic Resonance Imaging in the Diagnosis of Prostate Cancer: A Systematic Review. Clinical oncology 28, 550–567, doi:10.1016/j.clon.2016.05.003 (2016).
Whiting, P. F. et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Annals of internal medicine 155, 529–536, doi:10.7326/0003-4819-155-8-201110180-00009 (2011).
Deeks, J. J., Macaskill, P. & Irwig, L. The performance of tests of publication bias and other sample size effects in systematic reviews of diagnostic test accuracy was assessed. Journal of clinical epidemiology 58, 882–893, doi:10.1016/j.jclinepi.2005.01.016 (2005).
Author information
Authors and Affiliations
Contributions
Liang Sang and Xue-mei Wang conceived and designed the study; Liang Sang and Yun-fei Cai performed the literature search and selection; Liang Sang, Yun-fei Cai and Dong-yang Xu analyzed the data; Yun-fei Cai prepared the figures; Liang Sang wrote the paper; and Xue-mei Wang revised the paper. All authors agree and approve the final version of the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sang, L., Wang, Xm., Xu, Dy. et al. Accuracy of shear wave elastography for the diagnosis of prostate cancer: A meta-analysis. Sci Rep 7, 1949 (2017). https://doi.org/10.1038/s41598-017-02187-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-02187-0
This article is cited by
-
Novel non-MRI imaging techniques for primary diagnosis of prostate cancer: micro-ultrasound, contrast-enhanced ultrasound, elastography, multiparametric ultrasound, and PSMA PET/CT
Prostate Cancer and Prostatic Diseases (2024)
-
Preoperative imaging accuracy in size determination of prostate cancer in men undergoing radical prostatectomy for clinically localised disease
Insights into Imaging (2023)
-
Role of the elastography strain ratio using transrectal ultrasonography in the diagnosis of prostate cancer and clinically significant prostate cancer
Scientific Reports (2022)
-
Automated multiparametric localization of prostate cancer based on B-mode, shear-wave elastography, and contrast-enhanced ultrasound radiomics
European Radiology (2020)
-
Shear-wave elastography: role in clinically significant prostate cancer with false-negative magnetic resonance imaging
European Radiology (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.