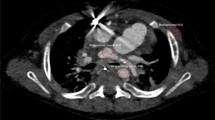
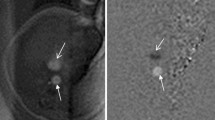
Abstract
Perinatal autopsy is the standard method for investigating fetal death; however, it requires dissection of the fetus. Human fetal microfocus computed tomography (micro-CT) provides a generally more acceptable and less invasive imaging alternative for bereaved parents to determine the cause of early pregnancy loss compared with conventional autopsy techniques. In this protocol, we describe the four main stages required to image fetuses using micro-CT. Preparation of the fetus includes staining with the contrast agent potassium triiodide and takes 3–19 d, depending on the size of the fetus and the time taken to obtain consent for the procedure. Setup for imaging requires appropriate positioning of the fetus and takes 1 h. The actual imaging takes, on average, 2 h 40 min and involves initial test scans followed by high-definition diagnostic scans. Postimaging, 3 d are required to postprocess the fetus, including removal of the stain, and also to undertake artifact recognition and data transfer. This procedure produces high-resolution isotropic datasets, allowing for radio-pathological interpretations to be made and long-term digital archiving for re-review and data sharing, where required. The protocol can be undertaken following appropriate training, which includes both the use of micro-CT techniques and handling of postmortem tissue.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
Examples of data produced by following this protocol are included in the protocol. Further details of the data presented are not publicly available because the information could compromise research participant privacy/consent.
References
Michalski, S. T., Porter, J. & Pauli, R. M. Costs and consequences of comprehensive stillbirth assessment. Am. J. Obstet. Gynecol. 186, 1027–1034 (2002).
MBRRACE-UK. Perinatal confidential enquiry: term, singleton, intrapartum stillbirth and intrapartum-related neonatal death. https://www.npeu.ox.ac.uk/assets/downloads/mbrrace-uk/reports/MBRRACE-UK%20Intrapartum%20Confidential%20Enquiry%20Report%202017%20-%20final%20version.pdf (2017).
Osborn, M., Lowe, J., Cox, P. G., Hargitai, B. & Marton, T. Royal College of Pathologists. Guidelines on autopsy practice: fetal autopsy (2nd trimester fetal loss and termination of pregnancy for congenital anomaly). https://www.rcpath.org/uploads/assets/b20ea503-7799-433c-99160653762f896c/Fetal-autopsy-2nd-trimester-fetal-loss-and-termination-of-pregnancy-for-congenital-anomaly.pdf (2017).
Blokker, B. M., Wagensveld, I. M., Weustink, A. C., Oosterhuis, J. W. & Hunink, M. G. Non-invasive or minimally invasive autopsy compared to conventional autopsy of suspected natural deaths in adults: a systematic review. Eur. Radiol. 26, 1159–1179 (2016).
Blokker, B. M. et al. Conventional autopsy versus minimally invasive autopsy with postmortem MRI, CT, and CT-guided biopsy: comparison of diagnostic performance. Radiology https://doi.org/10.1148/radiol.2018180924 (2018).
Lewis, C. et al. Factors affecting uptake of postmortem examination in the prenatal, perinatal and paediatric setting. BJOG 125, 172–181 (2018).
Osborn, M., Cox, P. G., Hargitai, B. & Marton, T. Royal College of Pathologists. Guidelines on autopsy practice: neonatal death. https://www.rcpath.org/uploads/assets/0a7c073e-c773-4941-a1e998df666e17e3/G168-Guidelines-on-autopsy-practice-Neonatal-death.pdf (2019).
Sieswerda-Hoogendoorn, T. & van Rijn, R. R. Current techniques in postmortem imaging with specific attention to paediatric applications. Pediatr. Radiol. 40, 141–152 (2010).
Royal College of Obstetricians and Gynaecologists & Royal College of Pathologists. Fetal and perinatal pathology: report of a working party. https://www.rcpath.org/uploads/assets/19f28c61-2a55-4eba-a3d9bf652a803424/FetalAndPerinatalPath-Jun01.pdf (2001).
Lewis, C. et al. Availability of less invasive prenatal, perinatal and paediatric autopsy will improve uptake rates: a mixed-methods study with bereaved parents. BJOG 126, 754 (2019).
Kang, X. et al. Parental acceptance of minimally invasive fetal and neonatal autopsy compared with conventional autopsy. Prenat. Diagn. 34, 1106–1110 (2014).
Taher, M. B., Pearson, J., Cohen, M. & Offiah, A. C. Acceptability of post-mortem imaging among Muslim and non-Muslim communities. Br. J. Radiol. 91, 20180295 (2018).
Lewis, C. et al. Minimally invasive autopsy for fetuses and children based on a combination of post-mortem MRI and endoscopic examination: a feasibility study. Health Technol. Assess. 23, 1–104 (2019).
Sonnemans, L. J. P. et al. Dutch guideline for clinical foetal-neonatal and paediatric post-mortem radiology, including a review of literature. Eur. J. Pediatr. 177, 791–803 (2018).
Arthurs, O. J., Taylor, A. M. & Sebire, N. J. Indications, advantages and limitations of perinatal postmortem imaging in clinical practice. Pediatr. Radiol. 45, 491–500 (2015).
Votino, C. et al. Virtual autopsy by computed tomographic angiography of the fetal heart: a feasibility study. Ultrasound Obstet. Gynecol. 39, 679–684 (2012).
Arthurs, O. J. et al. Diagnostic accuracy of post mortem MRI for abdominal abnormalities in foetuses and children. Eur. J. Radiol. 84, 474–481 (2015).
Arthurs, O. J. et al. Diagnostic accuracy of post-mortem MRI for thoracic abnormalities in fetuses and children. Eur. Radiol. 24, 2876–2884 (2014).
Arthurs, O. et al. Diagnostic accuracy and limitations of post-mortem MRI for neurological abnormalities in fetuses and children. Clin. Radiol. 70, 872–880 (2015).
Addison, S., Arthurs, O. J. & Thayyil, S. Post-mortem MRI as an alternative to non-forensic autopsy in foetuses and children: from research into clinical practice. Br. J Radiol. 87, 20130621 (2014).
Thayyil, S. et al. Post-mortem MRI versus conventional autopsy in fetuses and children: a prospective validation study. Lancet 382, 223–233 (2013).
Shelmerdine, S. C., Sebire, N. J. & Arthurs, O. J. Perinatal post mortem ultrasound (PMUS): a practical approach. Insights Imaging 10, 35 (2019).
Tuchtan, L. et al. Diagnosis of congenital abnormalities with post-mortem ultrasound in perinatal death. Diagn. Interv. Imaging 99, 143–149 (2018).
Shelmerdine, S. C., Sebire, N. J. & Arthurs, O. J. Perinatal post-mortem ultrasound (PMUS): radiological-pathological correlation. Insights Imaging 10, 81 (2019).
Shelmerdine, S. C., Hutchinson, J. C., Arthurs, O. J. & Sebire, N. J. Latest developments in post-mortem foetal imaging. Prenat. Diagn. 40, 28–37 (2020).
Kang, X., Carlin, A., Cannie, M., Sanchez, T. C. & Jani, J. C. Fetal postmortem imaging: an overview of current techniques and future perspectives. Am. J. Obstet. Gynecol, https://doi.org/10.1016/j.ajog.2020.04.034 (2020).
Jawad, N. et al. Body weight lower limits of fetal postmortem MRI at 1.5 T. Ultrasound Obstet. Gynecol. 48, 92–97 (2016).
Hutchinson, J. C. et al. Postmortem microfocus computed tomography for early gestation fetuses: a validation study against conventional autopsy. Am. J. Obstet. Gynecol. 218, 445.e441–445.e412 (2018).
Shelmerdine, S. C. et al. Postmortem microfocus computed tomography for noninvasive autopsies: experience in >250 human fetuses. Am. J. Obstet. Gynecol. https://doi.org/10.1016/j.ajog.2020.07.019 (2020).
Shelmerdine, S. C. et al. Characterization of Bardet–Biedl syndrome by postmortem microfocus computed tomography (micro-CT). Ultrasound Obstet. Gynecol. 53, 129–134 (2019).
Hutchinson, J. C. et al. Clinical utility of postmortem microcomputed tomography of the fetal heart: diagnostic imaging vs macroscopic dissection. Ultrasound Obstet. Gynecol. 47, 58–64 (2016).
Dawood, Y., Strijkers, G. J., Limpens, J., Oostra, R. J. & de Bakker, B. S. Novel imaging techniques to study postmortem human fetal anatomy: a systematic review on microfocus-CT and ultra-high-field MRI. Eur. Radiol. https://doi.org/10.1007/s00330-019-06543-8 (2019).
Eloot, L. et al. Quality control of micro-computed tomography systems. Radiat. Prot. Dosim. 139, 463–467 (2010).
Li, K. Z., Gao, Y., Zhang, R., Hu, T. & Guo, B. The effect of a manual instrumentation technique on five types of premolar root canal geometry assessed by microcomputed tomography and three-dimensional reconstruction. BMC Med. Imaging 11, 14 (2011).
Gregg, C. L. & Butcher, J. T. Quantitative in vivo imaging of embryonic development: opportunities and challenges. Differentiation 84, 149–162 (2012).
Aslanidi, O. V. et al. Application of micro-computed tomography with iodine staining to cardiac imaging, segmentation, and computational model development. IEEE Trans. Med. Imaging 32, 8–17 (2013).
Jacob, R. E. & Carson, J. P. Automated measurement of heterogeneity in CT images of healthy and diseased rat lungs using variogram analysis of an octree decomposition. BMC Med. Imaging 14, 1 (2014).
Al Faraj, A., Shaik, A. S. & Alnafea, M. Intrapulmonary administration of bone-marrow derived M1/M2 macrophages to enhance the resolution of LPS-induced lung inflammation: noninvasive monitoring using free-breathing MR and CT imaging protocols. BMC Med. Imaging 15, 16 (2015).
Chen, K. C., Arad, A., Song, Z. M. & Croaker, D. High-definition neural visualization of rodent brain using micro-CT scanning and non-local-means processing. BMC Med. Imaging 18, 38 (2018).
Thiboutot, J. et al. Current advances in COPD imaging. Acad. Radiol. https://doi.org/10.1016/j.acra.2018.05.023 (2018).
Sanchez, S., Fernandez, V., Pierce, S. E. & Tafforeau, P. Homogenization of sample absorption for the imaging of large and dense fossils with synchrotron microtomography. Nat. Protoc. 8, 1708–1717 (2013).
Kallai, I. et al. Microcomputed tomography-based structural analysis of various bone tissue regeneration models. Nat. Protoc. 6, 105–110 (2011).
Schambach, S. J., Bag, S., Schilling, L., Groden, C. & Brockmann, M. A. Application of micro-CT in small animal imaging. Methods 50, 2–13 (2010).
Arthurs, O. J. et al. Comparison of diagnostic performance for perinatal and paediatric post-mortem imaging: CT versus MRI. Eur. Radiol. 26, 2327–2336 (2016).
Norman, W., Jawad, N., Jones, R., Taylor, A. M. & Arthurs, O. J. Perinatal and paediatric post-mortem magnetic resonance imaging (PMMR): sequences and technique. Br. J. Radiol. 89, https://doi.org/10.1259/bjr.20151028 (2016).
Kang, X. et al. Post-mortem whole-body magnetic resonance imaging of human fetuses: a comparison of 3-T vs. 1.5-T MR imaging with classical autopsy. Eur. Radiol. 27, 3542–3553 (2017).
Staicu, A. et al. Potential clinical benefits and limitations of fetal virtopsy using high-field MRI at 7 Tesla versus stereomicroscopic autopsy to assess first trimester fetuses. Prenat. Diagn. 39, 505–518 (2019).
Thayyil, S. et al. Post-mortem examination of human fetuses: a comparison of whole-body high-field MRI at 9·4 T with conventional MRI and invasive autopsy. Lancet 374, 467–475 (2009).
Pauwels, E., Van Loo, D., Cornillie, P., Brabant, L. & Van Hoorebeke, L. An exploratory study of contrast agents for soft tissue visualization by means of high resolution X-ray computed tomography imaging. J. Microsc. 250, 21–31 (2013).
Dunmore-Buyze, P. J. et al. Three-dimensional imaging of the mouse heart and vasculature using micro-CT and whole-body perfusion of iodine or phosphotungstic acid. Contrast Media Mol. Imaging 9, 383–390 (2014).
Dullin, C. et al. muCT of ex-vivo stained mouse hearts and embryos enables a precise match between 3D virtual histology, classical histology and immunochemistry. PLoS ONE 12, e0170597 (2017).
Walton, L. A. et al. Morphological characterisation of unstained and intact tissue micro-architecture by X-ray computed micro- and nano-tomography. Sci. Rep. 5, 10074 (2015).
Gignac, P. M. & Kley, N. J. Iodine-enhanced micro-CT imaging: methodological refinements for the study of the soft-tissue anatomy of post-embryonic vertebrates. J Exp. Zool. B Mol. Dev. Evol. 322, 166–176 (2014).
Hopkins, T. M. et al. Combining micro-computed tomography with histology to analyze biomedical implants for peripheral nerve repair. J. Neurosci. Methods 255, 122–130 (2015).
Kim, A. J. et al. Microcomputed tomography provides high accuracy congenital heart disease diagnosis in neonatal and fetal mice. Circ. Cardiovasc. Imaging 6, 551–559 (2013).
Metscher, B. D. MicroCT for developmental biology: a versatile tool for high-contrast 3D imaging at histological resolutions. Dev. Dyn. 238, 632–640 (2009).
Metscher, B. D. MicroCT for comparative morphology: simple staining methods allow high-contrast 3D imaging of diverse non-mineralized animal tissues. BMC Physiol. 9, 11 (2009).
Vickerton, P., Jarvis, J. & Jeffery, N. Concentration-dependent specimen shrinkage in iodine-enhanced microCT. J. Anat. 223, 185–193 (2013).
Degenhardt, K., Wright, A. C., Horng, D., Padmanabhan, A. & Epstein, J. A. Rapid 3D phenotyping of cardiovascular development in mouse embryos by micro-CT with iodine staining. Circ. Cardiovasc. Imaging 3, 314–322 (2010).
Lombardi, C. M. et al. Postmortem microcomputed tomography (micro-CT) of small fetuses and hearts. Ultrasound Obstet. Gynecol. 44, 600–609 (2014).
Sandaite, I. et al. Micro-computed tomography of isolated fetal hearts following termination of pregnancy: a feasibility study at 8–12 weeks’ gestation. Prenat. Diagn. https://doi.org/10.1002/pd.5719 (2020).
Sandrini, C. et al. Accuracy of micro-computed tomography in post-mortem evaluation of fetal congenital heart disease. Comparison between post-mortem Micro-CT and conventional autopsy. Front. Pediatr. 7, 92 (2019).
Hutchinson, J. C. et al. Virtual pathological examination of the human fetal kidney using micro-CT. Ultrasound Obstet. Gynecol. 48, 663–665 (2016).
Lombardi, S. et al. Micro-computed tomography: a new diagnostic tool in postmortem assessment of brain anatomy in small fetuses. Neuroradiology 61, 737–746 (2019).
Smith C. M. et al. HoloLens for medical imaging using post-mortem fetal micro-CT data. European Congress of Radiology Abstract. https://doi.org/10.26044/ecr2019/C-0153 (2019).
Shelmerdine, S. C. et al. 3D printing from microfocus computed tomography (micro-CT) in human specimens: education and future implications. Br. J. Radiol. https://doi.org/10.1259/bjr.20180306 (2018).
Acknowledgements
I.C.S. is funded by an NIHR Clinical Doctoral Research Fellowship (ICA-CDRF-2017-03-53). O.J.A. is funded by a National Institute for Health Research (NIHR) Career Development Fellowship (NIHR-CDF-2017-10-037). S.C.S. is supported by a RCUK/ UKRI Innovation Fellowship and Medical Research Council (MRC) Clinical Research Training Fellowship (grant MR/R002118/1), jointly funded by the Royal College of Radiologists (RCR). O.J.A. and N.J.S. receive funding from the Great Ormond Street Hospital Children’s Charity. This article presents independent research, and the views expressed are those of the author(s) and not necessarily those of the funding bodies or the Department of Health and Social Care. The authors acknowledge the help from our mortuary staff at Great Ormond Street Hospital: L. Ward, J. Parmenter, H. McGarrick, B. Czarny and D. Alvarez for their assistance and I. Haig, O. Larkin and B. Smit (Nikon, Tring, UK) for their technical advice. We also thank the parents who consented to and participate in this research.
Author information
Authors and Affiliations
Contributions
I.C.S., S.C.S. and J.C.H. developed and tested the methodology within the paper and drafted the manuscript. N.J.S. and O.J.A. supervised the work. All authors assessed the results, thereby optimizing the technique. All authors edited the paper and approved the final version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Protocols thanks Jorge Murillo-Gonzalez and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Key references using this protocol
Hutchinson, J. C. et al. Ultrasound Obstet Gynecol 47, 58-64 (2016): https://doi.org/10.1002/uog.15764
Hutchinson, J. C. et al. Am. J. Obstet. Gynecol. 218, 445.e441-445.e412 (2018): https://doi.org/10.1016/j.ajog.2018.01.040
Shelmerdine, S. C. et al. Am. J. Obstet. Gynecol. 224, 103.e1–103.e15 (2021): https://doi.org/10.1016/j.ajog.2020.07.019
Supplementary information
Supplementary Information
Supplementary Manual.
Supplementary Data 1
Postmortem examination consent form and guidance given to the consent taker used at Great Ormond Street Hospital, London, UK. Provided in Microsoft Word and pdf format.
Rights and permissions
About this article
Cite this article
Simcock, I.C., Shelmerdine, S.C., Hutchinson, J.C. et al. Human fetal whole-body postmortem microfocus computed tomographic imaging. Nat Protoc 16, 2594–2614 (2021). https://doi.org/10.1038/s41596-021-00512-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41596-021-00512-6
This article is cited by
-
Forensic applications of micro-computed tomography: a systematic review
Clinical and Translational Imaging (2022)
-
Microfocus computed tomography for fetal postmortem imaging: an overview
Pediatric Radiology (2022)
-
Micro-CT yields high image quality in human fetal post-mortem imaging despite maceration
BMC Medical Imaging (2021)
-
A pragmatic evidence-based approach to post-mortem perinatal imaging
Insights into Imaging (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.