Abstract
The mouse visual system serves as an accessible model to understand mammalian circuit wiring. Despite rich knowledge in retinal circuits, the long-range connectivity map from distinct retinal ganglion cell (RGC) types to diverse brain neuron types remains unknown. In this study, we developed an integrated approach, called Trans-Seq, to map RGCs to superior collicular (SC) circuits. Trans-Seq combines a fluorescent anterograde trans-synaptic tracer, consisting of codon-optimized wheat germ agglutinin fused to mCherry, with single-cell RNA sequencing. We used Trans-Seq to classify SC neuron types innervated by genetically defined RGC types and predicted a neuronal pair from αRGCs to Nephronectin-positive wide-field neurons (NPWFs). We validated this connection using genetic labeling, electrophysiology and retrograde tracing. We then used transcriptomic data from Trans-Seq to identify Nephronectin as a determinant for selective synaptic choice from αRGC to NPWFs via binding to Integrin α8β1. The Trans-Seq approach can be broadly applied for post-synaptic circuit discovery from genetically defined pre-synaptic neurons.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
Raw scRNA-seq data reported in this paper are available through accession number PRJNA715507. Processed data and scripts for data analysis are available through https://github.com/duanxlab/Trans-Seq.
Code availability
All other data are available in the main manuscript or supplementary materials. AAV plasmids associated with this paper are available at Addgene. Material requests should be directed to X.D.
References
Yemini, E. et al. NeuroPAL: a multicolor atlas for whole-brain neuronal identification in C. elegans. Cell 184, 272–288 (2021).
He, M. & Huang, Z. J. Genetic approaches to access cell types in mammalian nervous systems. Curr. Opin. Neurobiol. 50, 109–118 (2018).
Sanes, J. R. & Zipursky, S. L. Synaptic specificity, recognition molecules, and assembly of neural circuits. Cell 181, 1434–1435 (2020).
Siegert, S. et al. Genetic address book for retinal cell types. Nat. Neurosci. 12, 1197–1204 (2009).
Yonehara, K. et al. The first stage of cardinal direction selectivity is localized to the dendrites of retinal ganglion cells. Neuron 79, 1078–1085 (2013).
Duan, X., Krishnaswamy, A., De la Huerta, I. & Sanes, J. R. Type II cadherins guide assembly of a direction-selective retinal circuit. Cell 158, 793–807 (2014).
Cang, J., Savier, E., Barchini, J. & Liu, X. Visual function, organization, and development of the mouse superior colliculus. Annu. Rev. Vis. Sci. 4, 239–262 (2018).
Martersteck, E. M. et al. Diverse central projection patterns of retinal ganglion cells. Cell Rep. 18, 2058–2072 (2017).
Callaway, E. M. & Luo, L. Monosynaptic circuit tracing with glycoprotein-deleted rabies viruses. J. Neurosci. 35, 8979–8985 (2015).
Lo, L. & Anderson, D. J. A Cre-dependent, anterograde transsynaptic viral tracer for mapping output pathways of genetically marked neurons. Neuron 72, 938–950 (2011).
Zingg, B. et al. AAV-mediated anterograde transsynaptic tagging: mapping corticocollicular input-defined neural pathways for defense behaviors. Neuron 93, 33–47 (2017).
Yoshihara, Y. et al. A genetic approach to visualization of multisynaptic neural pathways using plant lectin transgene. Neuron 22, 33–41 (1999).
Basbaum, A. I. & Menetrey, D. Wheat germ agglutinin-apoHRP gold: a new retrograde tracer for light- and electron-microscopic single- and double-label studies. J. Comp. Neurol. 261, 306–318 (1987).
Duan, X. et al. Subtype-specific regeneration of retinal ganglion cells following axotomy: effects of osteopontin and mTOR signaling. Neuron 85, 1244–1256 (2015).
Brandenberger, R. et al. Identification and characterization of a novel extracellular matrix protein nephronectin that is associated with integrin α8β1 in the embryonic kidney. J. Cell Biol. 154, 447–458 (2001).
Braz, J. M. & Basbaum, A. I. Genetically expressed transneuronal tracer reveals direct and indirect serotonergic descending control circuits. J. Comp. Neurol. 507, 1990–2003 (2008).
Braz, J. M., Rico, B. & Basbaum, A. I. Transneuronal tracing of diverse CNS circuits by Cre-mediated induction of wheat germ agglutinin in transgenic mice. Proc. Natl Acad. Sci. USA 99, 15148–15153 (2002).
Katayama, H., Yamamoto, A., Mizushima, N., Yoshimori, T. & Miyawaki, A. GFP-like proteins stably accumulate in lysosomes. Cell Struct. Funct. 33, 1–12 (2008).
Morrie, R. D. & Feller, M. B. Development of synaptic connectivity in the retinal direction selective circuit. Curr. Opin. Neurobiol. 40, 45–52 (2016).
Tsuriel, S., Gudes, S., Draft, R. W., Binshtok, A. M. & Lichtman, J. W. Multispectral labeling technique to map many neighboring axonal projections in the same tissue. Nat. Methods 12, 547–552 (2015).
Takatoh, J. et al. New modules are added to vibrissal premotor circuitry with the emergence of exploratory whisking. Neuron 77, 346–360 (2013).
Byun, H. et al. Molecular features distinguish ten neuronal types in the mouse superficial superior colliculus. J. Comp. Neurol. 524, 2300–2321 (2016).
Gale, S. D. & Murphy, G. J. Distinct representation and distribution of visual information by specific cell types in mouse superficial superior colliculus. J. Neurosci. 34, 13458–13471 (2014).
Seigneur, E. & Sudhof, T. C. Cerebellins are differentially expressed in selective subsets of neurons throughout the brain. J. Comp. Neurol. 525, 3286–3311 (2017).
Zeisel, A. et al. Molecular architecture of the mouse nervous system. Cell 174, 999–1014 (2018).
Beier, K. T. et al. Circuit architecture of VTA dopamine neurons revealed by systematic input–output mapping. Cell 162, 622–634 (2015).
Peng, Y. R. et al. Satb1 regulates contactin 5 to pattern dendrites of a mammalian retinal ganglion cell. Neuron 95, 869–883 (2017).
Linton, J. M., Martin, G. R. & Reichardt, L. F. The ECM protein nephronectin promotes kidney development via integrinα8β1-mediated stimulation of Gdnf expression. Development 134, 2501–2509 (2007).
Fujiwara, H. et al. The basement membrane of hair follicle stem cells is a muscle cell niche. Cell 144, 577–589 (2011).
Yang, C. F. et al. Sexually dimorphic neurons in the ventromedial hypothalamus govern mating in both sexes and aggression in males. Cell 153, 896–909 (2013).
Hong, Y. K., Kim, I. J. & Sanes, J. R. Stereotyped axonal arbors of retinal ganglion cell subsets in the mouse superior colliculus. J. Comp. Neurol. 519, 1691–1711 (2011).
Chiou, S. H. et al. Pancreatic cancer modeling using retrograde viral vector delivery and in vivo CRISPR/Cas9-mediated somatic genome editing. Genes Dev. 29, 1576–1585 (2015).
Sato, Y. et al. Molecular basis of the recognition of nephronectin by integrin α8β1. J. Biol. Chem. 284, 14524–14536 (2009).
Humphries, J. D., Byron, A. & Humphries, M. J. Integrin ligands at a glance. J. Cell Sci. 119, 3901–3903 (2006).
Epp, A. L. et al. A novel motif in the proximal C-terminus of Pannexin 1 regulates cell surface localization. Sci. Rep. 9, 9721 (2019).
Wright, C. S. Structural comparison of the two distinct sugar binding sites in wheat germ agglutinin isolectin II. J. Mol. Biol. 178, 91–104 (1984).
Linhoff, M. W. et al. An unbiased expression screen for synaptogenic proteins identifies the LRRTM protein family as synaptic organizers. Neuron 61, 734–749 (2009).
Krishnaswamy, A., Yamagata, M., Duan, X., Hong, Y. K. & Sanes, J. R. Sidekick 2 directs formation of a retinal circuit that detects differential motion. Nature 524, 466–470 (2015).
Kebschull, J. M. et al. High-throughput mapping of single-neuron projections by sequencing of barcoded RNA. Neuron 91, 975–987 (2016).
Kim, I. J., Zhang, Y., Meister, M. & Sanes, J. R. Laminar restriction of retinal ganglion cell dendrites and axons: subtype-specific developmental patterns revealed with transgenic markers. J. Neurosci. 30, 1452–1462 (2010).
Cheng, T. W. et al. Emergence of lamina-specific retinal ganglion cell connectivity by axon arbor retraction and synapse elimination. J. Neurosci. 30, 16376–16382 (2010).
Osterhout, J. A., El-Danaf, R. N., Nguyen, P. L. & Huberman, A. D. Birthdate and outgrowth timing predict cellular mechanisms of axon target matching in the developing visual pathway. Cell Rep. 8, 1006–1017 (2014).
Varadarajan, S. G. & Huberman, A. D. Assembly and repair of eye-to-brain connections. Curr. Opin. Neurobiol. 53, 198–209 (2018).
Frisen, J. et al. Ephrin-A5 (AL-1/RAGS) is essential for proper retinal axon guidance and topographic mapping in the mammalian visual system. Neuron 20, 235–243 (1998).
Feldheim, D. A. et al. Genetic analysis of ephrin-A2 and ephrin-A5 shows their requirement in multiple aspects of retinocollicular mapping. Neuron 25, 563–574 (2000).
Sweeney, N. T., James, K. N., Sales, E. C. & Feldheim, D. A. Ephrin-As are required for the topographic mapping but not laminar choice of physiologically distinct RGC types. Dev. Neurobiol. 75, 584–593 (2015).
Ito, S. & Feldheim, D. A. The mouse superior colliculus: an emerging model for studying circuit formation and function. Front. Neural Circuits 12, 10 (2018).
Triplett, J. W. Molecular guidance of retinotopic map development in the midbrain. Curr. Opin. Neurobiol. 24, 7–12 (2014).
Cang, J., Wang, L., Stryker, M. P. & Feldheim, D. A. Roles of ephrin-As and structured activity in the development of functional maps in the superior colliculus. J. Neurosci. 28, 11015–11023 (2008).
Knoll, B. et al. Graded expression patterns of ephrin-As in the superior colliculus after lesion of the adult mouse optic nerve. Mech. Dev. 106, 119–127 (2001).
Ackman, J. B. & Crair, M. C. Role of emergent neural activity in visual map development. Curr. Opin. Neurobiol. 24, 166–175 (2014).
McLaughlin, T., Torborg, C. L., Feller, M. B. & O’Leary, D. D. Retinotopic map refinement requires spontaneous retinal waves during a brief critical period of development. Neuron 40, 1147–1160 (2003).
Xiao, T. et al. Assembly of lamina-specific neuronal connections by Slit bound to type IV collagen. Cell 146, 164–176 (2011).
Becker, C. G., Schweitzer, J., Feldner, J., Schachner, M. & Becker, T. Tenascin-R as a repellent guidance molecule for newly growing and regenerating optic axons in adult zebrafish. Mol. Cell Neurosci. 26, 376–389 (2004).
Yamagata, M. & Sanes, J. R. Versican in the developing brain: lamina-specific expression in interneuronal subsets and role in presynaptic maturation. J. Neurosci. 25, 8457–8467 (2005).
Jiang, Y. et al. In vitro guidance of retinal axons by a tectal lamina-specific glycoprotein Nel. Mol. Cell Neurosci. 41, 113–119 (2009).
Grahovac, J. & Wells, A. Matrikine and matricellular regulators of EGF receptor signaling on cancer cell migration and invasion. Lab. Invest. 94, 31–40 (2014).
Sanes, J. R. & Zipursky, S. L. Design principles of insect and vertebrate visual systems. Neuron 66, 15–36 (2010).
Kay, J. N. et al. Retinal ganglion cells with distinct directional preferences differ in molecular identity, structure, and central projections. J. Neurosci. 31, 7753–7762 (2011).
Trenholm, S., Johnson, K., Li, X., Smith, R. G. & Awatramani, G. B. Parallel mechanisms encode direction in the retina. Neuron 71, 683–694 (2011).
Gale, S. D. & Murphy, G. J. Distinct cell types in the superficial superior colliculus project to the dorsal lateral geniculate and lateral posterior thalamic nuclei. J. Neurophysiol. 120, 1286–1292 (2018).
Gerfen, C. R., Paletzki, R. & Heintz, N. GENSAT BAC Cre-recombinase driver lines to study the functional organization of cerebral cortical and basal ganglia circuits. Neuron 80, 1368–1383 (2013).
Vong, L. et al. Leptin action on GABAergic neurons prevents obesity and reduces inhibitory tone to POMC neurons. Neuron 71, 142–154 (2011).
Taniguchi, H. et al. A resource of Cre driver lines for genetic targeting of GABAergic neurons in cerebral cortex. Neuron 71, 995–1013 (2011).
Network, B. I. C. C. A multimodal cell census and atlas of the mammalian primary motor cortex. Nature 598, 86–102 (2021).
Buffelli, M. et al. Genetic evidence that relative synaptic efficacy biases the outcome of synaptic competition. Nature 424, 430–434 (2003).
Madisen, L. et al. Transgenic mice for intersectional targeting of neural sensors and effectors with high specificity and performance. Neuron 85, 942–958 (2015).
Zimmerman, S. E. et al. Nephronectin regulates mesangial cell adhesion and behavior in glomeruli. J. Am. Soc. Nephrol. 29, 1128–1140 (2018).
Staahl, B. T. et al. Efficient genome editing in the mouse brain by local delivery of engineered Cas9 ribonucleoprotein complexes. Nat. Biotechnol. 35, 431–434 (2017).
Duan, X. et al. Disrupted-In-Schizophrenia 1 regulates integration of newly generated neurons in the adult brain. Cell 130, 1146–1158 (2007).
Peng, Y. R. et al. Binary fate choice between closely related interneuronal types is determined by a Fezf1-dependent postmitotic transcriptional switch. Neuron 105, 464–474 (2020).
Duan, X. et al. Cadherin combinations recruit dendrites of distinct retinal neurons to a shared interneuronal scaffold. Neuron 99, 1145–1154 (2018).
Bai, L. et al. Genetic identification of vagal sensory neurons that control feeding. Cell 179, 1129–1143 (2019).
Kim, J. Y. et al. Viral transduction of the neonatal brain delivers controllable genetic mosaicism for visualising and manipulating neuronal circuits in vivo. Eur. J. Neurosci. 37, 1203–1220 (2013).
Breunig, J. J. et al. Rapid genetic targeting of pial surface neural progenitors and immature neurons by neonatal electroporation. Neural Dev. 7, 26 (2012).
Bei, F. et al. Restoration of visual function by enhancing conduction in regenerated axons. Cell 164, 219–232 (2016).
Satija, R., Farrell, J. A., Gennert, D., Schier, A. F. & Regev, A. Spatial reconstruction of single-cell gene expression data. Nat. Biotechnol. 33, 495–502 (2015).
Becht, E. et al. Dimensionality reduction for visualizing single-cell data using UMAP. Nat. Biotechnol. https://doi.org/10.1038/nbt.4314 (2018).
Wu, Y. E., Pan, L., Zuo, Y., Li, X. & Hong, W. Detecting activated cell populations using single-cell RNA-seq. Neuron 96, 313–329 (2017).
Shang, C. et al. BRAIN CIRCUITS. A parvalbumin-positive excitatory visual pathway to trigger fear responses in mice. Science 348, 1472–1477 (2015).
Shrestha, B. R. et al. Sensory neuron diversity in the inner ear is shaped by activity. Cell 174, 1229–1246 (2018).
Abraira, V. E. et al. The cellular and synaptic architecture of the mechanosensory dorsal horn. Cell 168, 295–310 (2017).
Acknowledgements
We thank J. Ngai for the Npnt-FlpO line; G. Nachtrab, Q. Wang and X. Chen for the FlpO-dependent rabies tracing system; and C. Gerfen for Ntsr1-GN209 and Grp-KH288 GENSAT mice. We acknowledge A. Basbaum, L. Jan, X. Jin, B. Huang, Y. Kuo, L. Liang, J. Sanes, M. Scanziani, M. Stryker, X. Wei and H. Zeng for their advice on the study. We acknowledge NEI P30EY002162; funding from the RPB unrestricted fund to UCSF-Ophthalmology; NEI F30EY033201 to N.Y.T.; NIDDK RO1DK118032 to D.K.M; NIMH R01MH08188 to J.L.R.; NINDS R01NS077986 to Fan Wang; RBP-CDA, Klingenstein-Simons Neuroscience Fellowship, Whitehall Foundation, E. M. Ziegler Funds for Blindness, Glaucoma Research Foundation (CFC3); and NIH R01EY030138 and R01NS123912 to X.D.
Author information
Authors and Affiliations
Contributions
N.Y.T., Fei Wang and X.D. designed the study. N.Y.T, K.T., C.Y. and E.L.M. designed and performed sc-RNA-seq, with supervision from J.L.R. N.Y.T., K.T., Fan Wang and X.D. designed and optimized the trans-synaptic tracers. N.Y.T. performed all genetics and histology experiments, with the assistance of K.W., A.C.M. and E.J.D. N.Y.T. performed the bioinformatics analysis. Fei Wang and C.Y. performed electrophysiology, with supervision from E.M.U. Fei Wang and N.Y.T. performed retinal tracing experiments. J.T., L.Y. and Fan Wang performed the brain tracing and electrophysiology experiments. The manuscript was written by N.Y.T. and X.D. and included contributions from all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Neuroscience thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Multiple configurations of mWGA-based tracer were examined for anterograde synaptic transfer efficiency in the retinotectal synapses.
The numbers of fluorescence-positive neurons in the SGS from different mWGA-RFP configurations were counted. The percentages were normalized to the highest number as (100%) set by the mWGA-mCherry configuration, which possessed the highest efficiency. The anterograde transfer number was quantified by counting the number of RFP-positive neurons in acute SC slices on the contralateral side (right SC) without fluorescence amplification through immunostaining. We focused on the contralateral (right) SC, as 90% of RGCs project contralaterally. We examined RGC coverage across the retina to ensure there was no retinotopy bias in SC analysis. mWGA-fusion protein configurations were compared under consistent conditions by delivering AAV2 expressing the fusion proteins into the left eyes (Fig. 1a). We used standard AAV Serotype-2 followed by 4 weeks post-injection (wpi) sampling, a commonly established window to examine RGC axonal projections and carry out the optogenetic measurement8. a, Several WGA-fusion proteins were compared side-by-side using an AAV-mediated in vivo screen for highly efficient anterograde transfer from the retina to the brain. b, Comparisons between N-terminal (mCherry-mWGA) and C-terminal fusion (mWGA-mCherry) transfer efficiencies were quantified. c, Transfer efficiencies of different RFP C-terminal fusions, including mWGA-mCherry, mWGA-Ruby3, mWGA-tdTmt, were quantified, n = 7, 5, 6, 5 animals for each condition in the following order, mWGA-mCherry, mCherry-mWGA, mWGA-Ruby3 and mWGA-TdTomato. Statistics for b, two-sided Student’s t-test, **** P < 0.0001; c, one-way ANOVA test. **** P < 0.0001. e-f, (e) Additional samples showing live red-fluorescent labeling of the contralateral SC after acute brain slice preparation 4 weeks post-injection (wpi), indicating transsynaptic transfer onto the recipient neurons enriched in stratum griseum superficiale (SGS) and stratum opticum (SO), but not in stratum griseum intermedium (SGI). f, Magnified view of inset from e showing individual neurons labeled with bright red fluorescent protein from RGC anterograde transfer without signal amplification through immunostaining. The ability to detect native fluorescence is unique to mWGA-mCherry (mWmC) but absent in other mWGA-RFP configurations, such as mWGA-TdTomato shown here (g, h). Scale bars: (e, g, 5 mm; f, h, 50 µm). i-l, Intrinsic electrophysiological properties of mCherry-positive recipient neurons (red, n = 5 animals) and neighboring mCherry-negative neurons (black, n = 7 animals) are similar as showed in Fig. 1m. i, Action potential amplitudes, j, sustained firing rates, k, resting membrane potentials, and l, EPSC frequencies are shown. These intrinsic properties were unperturbed by mWmC-transfer. N.S. not significant. two-sided Student’s t-test. m-p, Retinal vertical sections to show high mWmC coverage across major RGC types, including (m) Spp1 for αRGCs, (n) Cart for ooDSGCs, (o) Melanopsin for ipRGCs, and (p) Foxp2 for F-RGCs. Scale bar: 20 µm. INL: inner nuclear layer; GCL: ganglion cell layer. The percentages of each RGC subclasses were quantified in q, representing a similar fraction of RGC subclasses among all RGCs, n = 5 animals. f-i, Intraocular injections of mWmC lead to efficient monosynaptic transfer to connected neurons in multiple retino-recipient areas, including SC (t,u) and LGN (r, s). By contrast, the secondary relay neurons in V1 (u) or those in the lateral posterior nucleus of the thalamus (LP) (s) do not show mWmC transfer. Scale bars: (r, t, 2 mm; s, u, 20 mm). v, Immunostaining for RFP (mWmC) indicates high efficiency of anterograde transfer onto the recipient neurons in stratum griseum superficiale (SGS) and stratum opticum (SO). mWmC-positive cells are largely NeuN-positive. Dotted-yellow circles indicate mCherry and NeuN double-positive neurons from RGC anterograde transsynaptic transfer. w, mWmC-positive cells are largely GFAP-negative, which were quantified in x. n = 5 animals for each condition. **** P < 0.0001, two-sided Student’s t-test. Scale bars: (v, w, 50 µm). All data in this figure are presented as mean ± s.e.m.
Extended Data Fig. 2 Comparison of different versions of WGA tracers for their anterograde and retrograde transfer capacities within retinotectal synapses.
a-d, a, a schematic drawing of the injection within the retinal circuit. b, Retinal wholemount view to show mWmC coverage from RGC starter neurons (Kcng4-Cre for αRGC subclass neurons) in the ganglion cell layer (GCL, b), with limited retrograde transfer to the inner plexiform layer (INL, c). Scale bar: 50 µm, with the retrograde transfer ratios quantified in (d). n = 9 biologically independent samples. e-g, Orthogonal view (e) of RGC labeling with mWmC (red) without any retrograde transfer onto SACs (green, ChAT). Intraocular injections of mWmC into ChAT-Cre led to labeling of SACs (red) for anterograde and monosynaptic tracing onto ooDSGCs (Cart-positive, green; mWmC, red double-positive) in (f). Scale bar: 50μm (e, f). Additionally, Chat-mWmC labeling does not lead transfer mWmC to SPP1-positive neurons (αRGCs, g), indicating the specificity of anterograde transsynaptic transfer as opposed to trans-cellular transfer within a local area. Scale bar: 50μm(g) 0% Spp1-positive, n = 4 animals. h, Injections of WGA-protein conjugated dye, AAV-expressing ctt-WGA cDNA, and AAV-expressing mWmC into SC lead to efficient anterograde transfer to LP. However, they displayed different levels of retrograde labeling, including bright axonal uptake and faint retrograde transfer. mWmC (j) displayed with very limited retrograde spread onto the retina. This is a significant improvement in limiting retrograde transfer compared to WGA-Alexa555 dye (i) or AAV-ctt-WGA (k). n = 4 animals in each condition. Scale bars: (i, j, k, 50 µm). l, Retrograde transfer ratios were normalized across three configurations of the tracers, using the highest numbers from WGA-555 as 100%. n = 5 biologically independent samples. **** P < 0.0001, two-sided Student’s t-test. m, Average numbers of retinal ganglion cells per retina were quantified and compared across the three conditions. n = 5 biologically independent samples. ****, p < 0.0001, two-sided Student’s t-test. n. Additionally, restricting the starter neurons genetically in SC vGlut2-Cre cells further eliminated the axonal retrograde uptake (compared to global mWmC expression). n = 5 biologically independent samples. ****, pP < 0.0001, two-sided Student’s t-test. o, Retrograde transfer ratios were normalized across two conditions, using the highest numbers from straight mWmC expression as 100%. n = 5 biologically independent samples. **** P< 0.0001, two-sided Student’s t-test. p, GPe, another downstream target of the striatum injection (Fig. 3m, n) was traced here; q, Magnified view of M1 in Fig. 3n from the striatum retrograde tracing showed no retrograde spread from the Striatum. Scale bar p-q, 100 µm. All data in this figure are presented as mean ±s.e.m.
Extended Data Fig. 3 Trans-Seq workflow and bioinformatic prediction for neuronal clustering.
To improve adult cell isolation and survival throughout the Trans-Seq protocol, we adopted well-controlled physiological conditions to preserve adult neurons79 and optimized slice preparation and dissociation conditions. a, Freshly prepared brain slice for contralateral SC dissociations at 4 weeks post-injection (wpi). Bright red fluorescence without immunostaining indicates a high-efficiency anterograde transsynaptic transfer from the retina onto contralateral SC recipient neurons. Fluorescence-activated cell sorting (FACS) enriched mCherry-positive neurons and excluded dead cells, debris, and doublets. b-h, FACS gating parameters to isolate mWmC+ neurons from freshly prepared SC slices reflecting the bimodal distribution of the mCherry signals enriched by mWmC tracer. b, First, cell debris were excluded using forward scatter area (FSC-A) vs. side scatter area (SSC-A) profiles. Second, cell doublets were excluded using double gating composed from c forward scatter height (FSC-W) vs. forward scatter area (FSC-A) and d side scatter height (SSC-W) vs. side scatter area (SSC-A) profiles. e, Third, gating for DAPI-negative live cells was subsequently performed, followed by f the last gating for mCherry-positive cells. g, Sample gatings for both DAPI and mCherry were performed by establishing gating parameters on unstained control SC suspensions and backgating isolated cells. n = 4 animals each preparation. h, mCherry-gating results from mWmC-positive samples, summarizing the fraction of cells passing each of the FACS parameters listed above b-g. 25000-50000 mWmC-positive cells were collected each preparation. Using the scRNA-Seq data of the connected neurons from Pan-RGC tracing, we obtained eight neuronal clusters, including three excitatory SC clusters (ESC1-3) and five inhibitory SC clusters (ISC1-5). i, UMAP plot showing the alignment of all three replicates of Pan-RGC Trans-Seq experiments, showing the even distribution of all neuronal clusters across experimental procedures. j, Contribution of each preparation from i to individual neuronal clusters to show the batch effects across three experiments. These results included all three ESCs and five ISCs, indicating no neuronal clusters were biased across the triplicates. (Red, 1st; Green, 2nd; Blue, 3rd preparation). k-m, QC plots of the sorted mCherry-positive neurons for each neuronal cluster, including ESCs and ISCs, examining the number of genes expressed k, counts of transcripts l, and the mitochondria counts m.
Extended Data Fig. 4 Data analysis of the Pan-RGC Trans-Seq data.
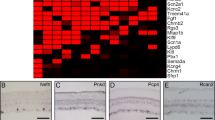
a-d, UMAP plots of Pan-RGC Trans-Seq data show major neuronal markers, including (a) Snap25 and (b) Syt1 as pan-neuronal markers, (c) Slc17a6 (vGluT2) for excitatory neurons including three ESCs, and (d) Gad2 for inhibitory neurons including five ISCs. These markers defined neuronal populations and separated the clusters among three ESCs (Slc17a6-positive, c) and five ISCs (Gad2-positive, d). e-g, We queried previously identified marker genes expressed in SC neuronal subsets (f)22,80. Additionally, several cell-cell adhesion molecules, such as Type II Cdhs (g) and neuropeptides and their putative receptors (e), appeared as good molecular markers for neuronal subsets in the mammalian nervous system81,82. Notably, when we queried the gene expression of Ntsr1 in the Trans-Seq dataset, we found no Ntsr1 expression in any SC neuron cluster (e). Dot-plots as bioinformatics predictions of (e) Neuropeptides and receptors, and (g) Cdhs for SC subset expression.
Extended Data Fig. 5 Additional histology data to validate Trans-Seq data in the SC.
a-c, Validation of Trans-Seq marker gene expression from ESC1 using RNA-Scope in situ hybridization. Npnt (red) is not found in inhibitory neurons marked by Gad2-GFP (a). b, Higher magnification of the boxed area in a. Scale bars: (a, 250 µm; b, 50 µm), quantified in (c). n = 4 animals, per genotype. Data in this figure are presented as mean ± SEM. d-f, Immunohistochemistry using Npnt antibody onto SC excitatory-marking lines. d, Npnt antibody (red) co-localizes with Ntsr1-GN209-YFP (green) for wide-field excitatory neurons. e, f, By contrast, the Npnt antibody (red) does not co-localize with the Grp-KH288-YFP line (green) for narrow-field neurons (e) or the Rorb-YFP line (green) for stellate cells (f). Scale bars: (d-f, 250 µm). g-h, Validation of Trans-Seq marker gene expression from ESC2 using RNA-Scope in situ hybridization. (g) Cdh7 (red)/Slc17a6 (blue) double in situs do not label neurons in the Grp-KH288-YFP transgenic line for narrow-field excitatory neurons at higher magnification (h), n = 4 animals. i-j, Validation of Trans-Seq marker gene expression from ESC3 using RNA-Scope in situ hybridization. (i) Tac1 (red)/Slc17a6 (blue) double in situs do not label neurons in the Rorb-YFP knock-in line primarily for stellate excitatory neurons, at higher magnification (j), n = 4 animals. Red dotted circles in (b, h, j) indicate the absence of in situ signals with GFP staining. Scale bars (h-j, 50 µm). h, Validation of Trans-Seq top marker gene expression from ESC1 using RNA-Scope for in situ hybridization shows that Cbln2 RNA probes (red) are enriched in the Ntsr1-GN209-YFP transgenic line labeling wide-field excitatory neurons (green). Scale bar: 200 µm. n = 3 animals. i, Neuronal distribution of Ntsr1-GN209-YFP transgenic line, including wide-field neurons present in SO and a significant fraction of neurons in the SGI and other deep SC layers. The wide distribution of YFP-positive neurons beyond SO indicates that Ntsr1-GN209-YFP cannot be used to manipulate SO neurons within the SC selectively. Red and blue dotted lines mark the borders of the three zones (SGS, SO, and SGI) within the superficial SC. j, Only wide-field neurons in the SO are Npnt-positive (between red and blue dotted lines), but not the rest of Ntsr1-YFP positive neurons in the SC’s SGI and deep layers. These data further suggest that Npnt is a faithful molecular marker for these neurons within SO. n = 3 animals. Scale bars: (i, 2 mm; j, 500 µm).
Extended Data Fig. 6 Alignment of ESC1-3 clusters from Trans-Seq to a public database of whole-brain excitatory neuron scRNA-seq data.
a, A summary of the three excitatory neuron clusters (ESCs) characterized in Trans-Seq. ESC1-3 were first correlated to a published bioinformatics database for all excitatory neuronal clusters throughout the brain25, indicating that ESC1-3 are characterized as retinorecipient neurons among six excitatory neuron clusters (MEGLU1-6) annotated from the dorsal midbrain. These six clusters were classified as putative SC excitatory clusters among all 11 collicular clusters. ESC1-3 were compared to an immunohistochemical study22 that identified laminar markers but did not link to cell types. ESC1-3 were matched to cell types defined by morphology and physiology23. b-e, Violin plots of candidate marker genes (b, Slc17a6, pan-excitatory neurons), (c, Npnt, ESC1), (d, Tac1, ESC3), and (e, Cdh7, ESC2) among MEGLU1-6. f, Immunohistochemistry of Pou4f2 (green, antibodies) showed no overlap with ESC1 neurons (Npnt-TdTomato, red). Pou4f2 was revealed as an excitatory neuron subset marker for MEGLU3 from a public scRNA-Seq database. A defined Pou4f2-positive population of neurons resides in the SGI. g, Pou4f2-positive SGI neurons did not uptake mWmC following 4wpi of mWmC infection of the RGCs, corroborating the findings from Trans-Seq in defining specific clusters of retino-recipient neurons. Scale bar: 100 µm. h. Quantifications of cell number for Pou4f2-positive and mWmC-positive neuron (red), versus all Pou4f2-positive neurons (black), n = 3 animals, **** P< 0.0001, two-sided Student’s t-test. Data in this figure are presented as mean ± s.e.m. i. A UMAP Plot shows the absence of the MEGLU3 marker (Pou4f2) within Trans-Seq data (Fig. 6).
Extended Data Fig. 7 Cre-dependent mWmC for RGC type-specific circuit mapping.
a, Genetic design of AAV vector for Cre-dependent mWmC (AAV2-CAG-DIO-mWmC-WPRE) expression to achieve neuronal subtype-specific anterograde transsynaptic tracing. b-e, mWmC infection can achieve efficient and restricted expression in RGC starter neuron subclasses: Kcng4-Cre is a driver for αRGC subtypes (Spp1-positive, b); and Cart-Cre is a driver for all ooDSGC subtypes (Cart-positive, d). Notably, these Cre-dependent mWmC expressions are restricted to the starter neurons without retrograde spread into the inner retina (that is, no bipolar cells and amacrine cells uptake mWmC), scale bars: 20 µm. The specificities of the Cre-drivers were quantified in c (Kcng4-Cre) and e (Cart-Cre), respectively. n = 5 animals, for each of the Cre line. Data in this figure are presented as mean ± s.e.m. f, mWmC demonstrated a bimodal distribution of red fluorescence in recipient neurons. Thresholding for the high fluorescence intensity population allowed neurons across tissue slices to be classified as transferred or un-transferred. g-i, RGC-subclass specific mWmC anterograde transfer properties can be quantified in the postsynaptic neurons. Anterograde transsynaptic tracing from different starter retinal ganglion cell (RGC) types was compared (g, αRGCs; h, ooDSGCs as Fig. 7) and quantified using this workflow. This quantification (i) demonstrated the differential distribution of SC neurons receiving mWmC transfer from RGC starter cells, supporting the electrophysiology and genetic data from Fig. 7. n = 5 animals, per genotype Scale bar: 100 µm. (Black line is mean intensity curve, gray lines are each example. Red line is mean intensity curve, light red lines are each example).
Extended Data Fig. 8 Characterizations of Nephronectin-positive-wide-field neurons (NPWFs) and their differential inputs from αRGCs versus ooDSGCs.
a, Sample DIC and fluorescent microscope images showing the targeted postsynaptic Npnt-FlpO-TdTomato neurons for connectivity tests. At least 30 times each experiment was repeated independently with similar results. Scale bar: 20 µm. b, The majority of the labeled Npnt-FlpO-TdTomato neurons are positive for Npnt antibody immunostaining (96.5 ± 2.5% double-positive neurons among all TdTomato-positive neurons in b, n = 3 animals). The dotted line marks the pial surface. c, Npnt-FlpO-TdTomatao neurons have their somata positioning right above the top boundary of the SGI (dotted lines) marked by the vAChT. Scale bars: (b and c, 100 µm). d-g, Current-clamp recordings of NPWFs in response to (d) step-stimulation, 50pA per step for 1 second, 10 steps per sweep, and (e) ramp-stimulation, 2.5pA/s for 20 seconds per trail, 3 trails per sweep. f, Voltage-clamp recordings of spontaneous EPSCs. g, A sample spontaneous ESPC trace from f. h, Single-cell morphologies of NPWFs (ESC1) were reconstructed after physiological recording and intracellular dye filling showing the dendritic complexity. Scale bar: 100 µm. i, j, SC-specific Npnt expression using immunohistochemistry (i) was absent in the SC-specific conditional knockout (j). Scale bar: 200 µm. k-m, Sample immunohistochemistry images showing differential mWmC transfer from distinct RGC starter neurons onto retinorecipient neurons in the SC (mWmC, mCherry signal in red). k, Percentages of Npnt-positive wide-field neurons (NPWFs, ESC1) within connected neurons of the αRGCs and ooDSGCs. Data in this figure are presented as mean ±s.e.m. NPWFs were enriched in αRGC tracing datasets (77.0 ± 8.2% double-positive neurons among all Npnt-positive cells in l, n = 5 animals) but largely absent in ooDSGC tracing datasets (4.5 ± 2.1% double-positive cells among all Npnt-positive neurons in m, n = 5 animals). ****P < 0.0001, two-sided Student’s t-test. l, αRGC ouputomes labeled with Kcng4:mWmC. ESC1 neurons were immunostained for Npnt. m, ooDSGC tracing datasets in SC labeled with Cart:mWmC. ESC1 neurons stained with Npnt. Bottom panels are magnified view of inset from l and m labeled by solid square line.
Extended Data Fig. 9 Physiological and histological characterization of the selective RGC synapses onto NPWF neurons (ESC1).
a, Sample EPSCs from NPWF neurons driven by αRGCs with bath-applied TTX + 4-AP (blue trace) and after bath perfusion of CNQX + APV (red trace). Blue dots indicate 2 ms of the blue LED light. b, Quantification of the average amplitudes before and after adding pharmacological blockers in αRGC-NPWF connected pairs. Current amplitudes before adding any drugs are shown in black dots and bars; blue dots and bars show current amplitude after adding TTX + 4-AP; red dots and bars show current amplitude after adding CNQX + APV. n = 8 animals, N.S., no significance, * P< 0.05, two-sided Student’s t-test. The dots are amplitudes of individual cells, and the bar is the average of all cells. c, Among all the ooDSGCs and NPWF, connected pairs, only four cells showed a very small evoked current (black dots and bars). These small currents were blocked by bath-applied TTX and 4-AP (blue dots and bars), suggesting that the evoked currents are not monosynaptic inputs from ooDSGCs. The dots are amplitudes of individual cells, and the bar is the average of the three cells. n = 4 cells, *P < 0.05, two-sided Student’s t-test. d, Sample images of NPWFs in the SC showing high efficiency of Flp-dependent EGFP-2a-TVA-2a-oG (green) and RdGV: Rabies-Cherry (red) overlap. Scale bar: 100 µm. e–j, Vertical sections of contralateral retinas following rabies tracing indicating αRGC subtypes (e and h) OFF-transient αRGCs, (f and i) OFF-sustained αRGCs, and (g and j) On-sustained αRGCs, were retrogradely labeled. Scale bar: 50 µm. (e–g),vAChT staining (green) shows RGC dendritic sublaminations within the retina; (h–j), Spp1 staining (green) to confirm cell identities as αRGCs. k, Quantifications of different RGC subclasses among mCherry+ neurons including αRGCs, ooDSGCs, and other RGC types. l, Magnified view of Fig. 7o to show retrogradely labeled RGCs (red) highly overlap with αRGCs (Spp1-positive, green) in filled arrowheads, but not ooDSGCs (Satb1-positive, blue) in open arrowheads, as quantified in Fig. 7n. n = 6 animals, *** P< 0.005, two-sided Student’s t-test. Scale bar: 100 µm. m, n, Sample images with axons of m, αRGCs, and n, ooDSGCs labeled by YFP (green) in relative position to the dendrites of NPWF neurons (Npnt-RFPs). n = 4 animals each genotype, Scale bar: 100 µm. All data in this figure are presented as mean ± s.e.m.
Extended Data Fig. 10 Validation of genetic and molecular reagents for Npnt mutant analysis.
a, The AAV vector design for FlpO-dependent Caspase3-TEV expression, modified from (Addgene #45580)30. b, c, Confirmation of successfully eliminating ESC1s using AAV-fDIO-Caspase3 in Npnt-FlpO by comparing control b and Caspase3-TEV injections c. The dotted line marks the pial surface. Scale bar: 100 µm. d, Quantifications of Caspase3-deletion of NPWFs efficiencies, compared to control side. n = 5 animals. e, Quantifications of the superficial SC thickness subject to NPWF eliminations. n = 5 animals, per genotype, N.S., no significance, two-sided Student’s t-test. f, The AAV vector design for Cre overexpression. g-h, Confirmation of SC-specific knockout of Npnt using Npnt f/f conditional mutants by comparing controls (g) and mutants (h), n = 3 animals. Scale bar: 100 µm. No obvious cell-autonomous migration or morphological changes of labeled wide-field neurons were observed after Npnt knockout. i. Sample images showing comprehensive Cre coverage across different sublaminae of SC subject to neonatal AAV injections. Anti-Cre staining was displayed here in the green channel. j, Quantifications of AAV-Cre mediated Npnt knockout efficiency, compared to the control side. n = 5 animals. k, The lentiviral vector design for Itga8 knockout based on CRISPR-V2-system to simultaneously express sgRNA/Cas9-2a-Cherry (Addgene Plasmid #99154). l, m, Confirmation of the lentivirus-mediated Itga8 knockout in the retina (m) compared with control (l). Scale bar: 50 µm. n, Quantifications of sgRNA-mediated retinal Itga8 knockdown efficacies, compared to control side. n = 6 animals, Several sgRNAs were evaluated, and an efficient sgRNA targeting the fourth exon of Npnt was selected (See Methods). Cas9/sgRNA-mediated Npnt deletion was validated by immunostaining with Npnt antibody. We performed neonatal injections of AAV-encoding for sgRNA targeting Npnt or control sgRNA-non-cutter and Flp-dependent RFP expression (AAV-sgNpnt-FDIO-mCherry; or AAV-sg-Noncutter-FDIO-mCherry;) into Kcng4-YFP; Npnt-FlpO; H11Cas9 mice. o, The AAV vector is carrying sgRNAs and Frt-dependent mCherry. This vector can simultaneously knock down endogenous Npnt while labeling the NPWF neurons using Npnt-FlpO dependent RFP reporter. The Cas9 was genetically harbored in the H11-Cas9 line via a genetic cross to Npnt-FlpO. p, q, Npnt in the SC was knocked down (q) compared to sg-Non-cutter control (p). Scale bar: 100 µm. The percentages of Npnt knockdown were quantified in (r), n = 4 animals, per genotype, ****P< 0.0001, two-sided Student’s T-test. All data in this figure are presented as mean ± s.e.m.
Supplementary information
Rights and permissions
About this article
Cite this article
Tsai, N.Y., Wang, F., Toma, K. et al. Trans-Seq maps a selective mammalian retinotectal synapse instructed by Nephronectin. Nat Neurosci 25, 659–674 (2022). https://doi.org/10.1038/s41593-022-01068-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41593-022-01068-8
This article is cited by
-
Substance P in the medial amygdala regulates aggressive behaviors in male mice
Neuropsychopharmacology (2024)
-
A Non-canonical Excitatory PV RGC–PV SC Visual Pathway for Mediating the Looming-evoked Innate Defensive Response
Neuroscience Bulletin (2024)
-
Ascertaining cells’ synaptic connections and RNA expression simultaneously with barcoded rabies virus libraries
Nature Communications (2022)
-
An HSV-1-H129 amplicon tracer system for rapid and efficient monosynaptic anterograde neural circuit tracing
Nature Communications (2022)
-
High-density electrode recordings reveal strong and specific connections between retinal ganglion cells and midbrain neurons
Nature Communications (2022)