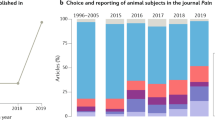
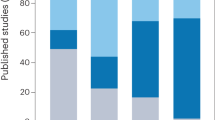
Abstract
For over half a century, male rodents have been the default model organism in preclinical neuroscience research, a convention that has likely contributed to higher rates of misdiagnosis and adverse side effects from drug treatment in women. Studying both sexes could help to rectify these public health problems, but incentive structures in publishing and career advancement deter many researchers from doing so. Moreover, funding agency directives to include male and female animals and human participants in grant proposals lack mechanisms to hold recipients accountable. In this Perspective, we highlight areas of behavioral, cellular and systems neuroscience in which fundamental sex differences have been identified, demonstrating that truly rigorous science must include males and females. We call for a cultural and structural change in how we conduct research and evaluate scientific progress, realigning our professional reward systems and experimental standards to produce a more equitable, representative and therefore translational body of knowledge.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Wu, J. et al. Editor’s Choice—Impact of initial hospital diagnosis on mortality for acute myocardial infarction: a national cohort study. Eur. Heart J. Acute Cardiovasc. Care 7, 139–148 (2018).
Newman-Toker, D. E., Moy, E., Valente, E., Coffey, R. & Hines, A. L. Missed diagnosis of stroke in the emergency department: a cross-sectional analysis of a large population-based sample. Diagnosis 1, 155–166 (2014).
Hinshaw, S. P. et al. Prospective follow-up of girls with attention-deficit/hyperactivity disorder into early adulthood: continuing impairment includes elevated risk for suicide attempts and self-injury. J. Consulting Clin. Psychol. 80, 1041–1051 (2012).
Anderson, G. D. Chapter 1 gender differences in pharmacological response. Int. Rev. Neurobiol. 83, 1–10 (2008).
Beery, A. K. & Zucker, I. Sex bias in neuroscience and biomedical research. Neurosci. Biobehav. Rev. 35, 565–572 (2011).
Mamlouk, G. M., Dorris, D. M., Barrett, L. R. & Meitzen, J. Sex bias and omission in neuroscience research is influenced by research model and journal, but not reported NIH funding. Front. Neuroendocrinol. 57, 100835 (2020).
Woitowich, N. C., Beery, A. & Woodruff, T. A 10-year follow-up study of sex inclusion in the biological sciences. eLife 9, e56344 (2020).
Clayton, J. A. Studying both sexes: a guiding principle for biomedicine. FASEB J. 30, 519–524 (2016).
Woitowich, N. C. & Woodruff, T. K. Implementation of the NIH Sex-Inclusion Policy: attitudes and opinions of study section members. J. Women’s Health 28, 9–16 (2019).
Kokras, N. & Dalla, C. Sex differences in animal models of psychiatric disorders. Br. J. Pharmacol. https://doi.org/10.1111/bph.12710 (2014).
Shansky, R. M. Sex differences in behavioral strategies: avoiding interpretational pitfalls. Curr. Opin. Neurobiol. 49, 95–98 (2018).
Galea, L. A. M., Kavaliers, M. & Ossenkopp, K. P. Sexually dimorphic spatial learning in meadow voles Microtus pennsylvanicus and deer mice Peromyscus maniculatus. J. Exp. Biol. 199, 195–200 (1996).
Roof, R. L. & Havens, M. D. Testosterone improves maze performance and induces development of a male hippocampus in females. Brain Res. 572, 310–313 (1992).
Korol, D. L., Malin, E. L., Borden, K. A., Busby, R. A. & Couper-Leo, J. Shifts in preferred learning strategy across the estrous cycle in female rats. Hormones Behav. 45, 330–338 (2004).
Perrot-Sinal, T. S., Kostenuik, M. A., Ossenkopp, K.-P. & Kavaliers, M. Sex differences in performance in the Morris water maze and the effects of initial nonstationary hidden platform training. Behav. Neurosci. 110, 1309–1320 (1996).
Tronson, N. C. Focus on females: a less biased approach for studying strategies and mechanisms of memory. Curr. Opin. Behav. Sci. 23, 92–97 (2018).
Chen, C. S. et al. Divergent strategies for learning in males and females. Curr. Biol. https://doi.org/10.1016/j.cub.2020.09.075 (2020).
Lebron-Milad, K. & Milad, M. R. Sex differences, gonadal hormones and the fear extinction network: implications for anxiety disorders. Biol. Mood Anxiety Disord. 2, 3 (2012).
Fanselow, M. S. Conditioned and unconditional components of post-shock freezing. Pavlovian J. Biol. Sci. 15, 177–182 (1980).
Gruene, T. M., Flick, K., Stefano, A., Shea, S. D. & Shansky, R. M. Sexually divergent expression of active and passive conditioned fear responses in rats. eLife 4, e11352 (2015).
Pellman, B. A., Schuessler, B. P., Tellakat, M. & Kim, J. J. Sexually dimorphic risk mitigation strategies in rats. eNeuro 4, ENEURO.0288-16.2017 (2017).
Greiner, E. M., Müller, I., Norris, M. R., Ng, K. H. & Sangha, S. Sex differences in fear regulation and reward-seeking behaviors in a fear–safety–reward discrimination task. Behav. Brain Res. 368, 111903 (2019).
Wiltschko, A. B. et al. Mapping sub-second structure in mouse behavior. Neuron 88, 1121–1135 (2015).
Nath, T. et al. Using DeepLabCut for 3D markerless pose estimation across species and behaviors. Nat. Protoc. 14, 2152–2176 (2019).
Cahill, L. Why sex matters for neuroscience. Nat. Rev. Neurosci. 7, 477–484 (2006).
de Vries, G. J. Minireview: sex differences in adult and developing brains: compensation, compensation, compensation. Endocrinology 145, 1063–1068 (2004).
Barker, J. M. & Galea, L. A. M. Sex and regional differences in estradiol content in the prefrontal cortex, amygdala and hippocampus of adult male and female rats. Gen. Comp. Endocrinol. 164, 77–84 (2009).
Oberlander, J. G. & Woolley, C. S. 17β-estradiol acutely potentiates glutamatergic synaptic transmission in the hippocampus through distinct mechanisms in males and females. J. Neurosci. 36, 2677–2690 (2016).
Jain, A., Huang, G. Z. & Woolley, C. S. Latent sex differences in molecular signaling that underlies excitatory synaptic potentiation in the hippocampus. J. Neurosci. 39, 1552–1565 (2019).
Liu, S., Seidlitz, J., Blumenthal, J. D., Clasen, L. S. & Raznahan, A. Integrative structural, functional, and transcriptomic analyses of sex-biased brain organization in humans. Proc. Natl Acad. Sci. USA 117, 18788–18798 (2020).
Farrell, M. R., Gruene, T. M. & Shansky, R. M. The influence of stress and gonadal hormones on neuronal structure and function. Hormones Behav. 76, 118–124 (2015).
Shansky, R. M. Estrogen, stress and the brain: progress toward unraveling gender discrepancies in major depressive disorder. Expert Rev. Neurotherapeutics 9, 967–973 (2009).
McEwen, B. S. Plasticity of the hippocampus: adaptation to chronic stress and allostatic load. Ann. N.Y. Acad. Sci. 933, 265–277 (2001).
Radley, J. J. et al. Chronic behavioral stress induces apical dendritic reorganization in pyramidal neurons of the medial prefrontal cortex. Neuroscience 125, 1–6 (2004).
Radley, J. J. et al. Repeated stress alters dendritic spine morphology in the rat medial prefrontal cortex. J. Comp. Neurol. 507, 1141–1150 (2008).
Vyas, A., Mitra, R., Shankaranarayana Rao, B. S. & Chattarji, S. Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. J. Neurosci. 22, 6810–6818 (2002).
Mitra, R., Jadhav, S., McEwen, B. S., Vyas, A. & Chattarji, S. Stress duration modulates the spatiotemporal patterns of spine formation in the basolateral amygdala. Proc. Natl Acad. Sci. USA 102, 9371–9376 (2005).
Kunimatsu, A., Yasaka, K., Akai, H., Kunimatsu, N. & Abe, O. MRI findings in posttraumatic stress disorder. J. Magn. Reson. Imaging https://doi.org/10.1002/jmri.26929 (2019).
Galea, L. A. et al. Sex differences in dendritic atrophy of CA3 pyramidal neurons in response to chronic restraint stress. Neuroscience 81, 689–697 (1997).
Garrett, J. E. & Wellman, C. L. Chronic stress effects on dendritic morphology in medial prefrontal cortex: sex differences and estrogen dependence. Neuroscience 162, 195–207 (2009).
Shansky, R. M. et al. Estrogen promotes stress sensitivity in a prefrontal cortex–amygdala pathway. Cereb. Cortex 20, 2560–2567 (2010).
Blume, S. R., Padival, M., Urban, J. H. & Rosenkranz, J. A. Disruptive effects of repeated stress on basolateral amygdala neurons and fear behavior across the estrous cycle in rats. Sci. Rep. 9, 12292 (2019).
Kessler, R. C. et al. Lifetime prevalence and age-of-onset distributions of mental disorders in the World Health Organization’s World Mental Health Survey Initiative. World Psychiatry 6, 168–176 (2007).
Kessler, R. C., Chiu, W. T., Demler, O., Walters, E. E. & Walters, E. E. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch. Gen. Psychiatry 62, 617–627 (2005).
Ruau, D., Liu, L. Y., Clark, J. D., Angst, M. S. & Butte, A. J. Sex differences in reported pain across 11,000 patients captured in electronic medical records. J. Pain 13, 228–234 (2012).
Steingrímsdóttir, Ó. A., Landmark, T., Macfarlane, G. J. & Nielsen, C. S. Defining chronic pain in epidemiological studies: a systematic review and meta-analysis. Pain 158, 2092–2107 (2017).
Card, T., Canavan, C. & West, J. The epidemiology of irritable bowel syndrome. Clin. Epidemiol. 6, 71 (2014).
Mogil, J. S. & Chanda, M. L. The case for the inclusion of female subjects in basic science studies of pain. Pain 117, 1–5 (2005).
Mogil, J. S. Qualitative sex differences in pain processing: emerging evidence of a biased literature. Nat. Rev. Neurosci. 21, 353–365 (2020).
Will, T. R. et al. Problems and progress regarding sex bias and omission in neuroscience research. eNeuro 4, ENEURO.0278-17.2017 (2017).
Brookoff, D. Chronic pain. 2. The case for opioids. Hospital Pract. 35, 69–84 (2000).
Brookoff, D. Chronic pain. 1. A new disease? Hospital Pract. 35, 45–59 (2000).
Rosen, S. F. et al. Increased pain sensitivity and decreased opioid analgesia in T-cell-deficient mice and implications for sex differences. Pain 160, 358–366 (2019).
Kandasamy, R., Calsbeek, J. J. & Morgan, M. M. Analysis of inflammation-induced depression of home cage wheel running in rats reveals the difference between opioid antinociception and restoration of function. Behav. Brain Res. 317, 502–507 (2017).
Armendariz, A. & Nazarian, A. Morphine antinociception on thermal sensitivity and place conditioning in male and female rats treated with intraplantar complete Freund’s adjuvant. Behav. Brain Res. 343, 21–27 (2018).
Greenspan, J. D. et al. Studying sex and gender differences in pain and analgesia: a consensus report. Pain 132, S26 (2007).
Craft, R. M. Sex differences in opioid analgesia: ‘from mouse to man’. Clin. J. Pain 19, 175–186 (2003).
Wang, X., Traub, R. J. & Murphy, A. Z. Persistent pain model reveals sex difference in morphine potency. Am. J. Physiol. Regul. Integr. Comp. Physiol. 291, R300–R306 (2006).
Doyle, H. H., Eidson, L. N., Sinkiewicz, D. M. & Murphy, A. Z. Sex differences in microglia activity within the periaqueductal gray of the rat: a potential mechanism driving the dimorphic effects of morphine. J. Neurosci. 37, 3202–3214 (2017).
Bernal, S. A., Morgan, M. M. & Craft, R. M. PAG µ opioid receptor activation underlies sex differences in morphine antinociception. Behav. Brain Res. 177, 126–133 (2007).
Loyd, D. R., Wang, X. & Murphy, A. Z. Sex differences in μ-opioid receptor expression in the rat midbrain periaqueductal gray are essential for eliciting sex differences in morphine analgesia. J. Neurosci. 28, 14007–14017 (2008).
Krzanowska, E. K., Znamensky, V., Wilk, S. & Bodnar, R. J. Antinociceptive and behavioral activation responses elicited by d-pro2-dndomorphin-2 in the ventrolateral periaqueductal gray are sensitive to sex and gonadectomy differences in rats. Peptides 21, 705–715 (2000).
Krzanowska, E. K. & Bodnar, R. J. Morphine antinociception elicited from the ventrolateral periaqueductal gray is sensitive to sex and gonadectomy differences in rats. Brain Res. 821, 224–230 (1999).
Selley, D. E. et al. Effect of strain and sex on μ opioid receptor-mediated G-protein activation in rat brain. Brain Res. Bull. 60, 201–208 (2003).
Fullerton, E. F., Rubaharan, M., Karom, M. C., Hanberry, R. I. & Murphy, A. Z. Advanced age attenuates the antihyperalgesic effect of morphine and decreases μ-opioid receptor expression and binding in the rat midbrain periaqueductal gray in male and female rats. Neurobiol. Aging 98, 78–87 (2021).
Basbaum, A. I., Clanton, C. H. & Fields, H. L. Opiate and stimulus produced analgesia: functional anatomy of a medullospinal pathway. Proc. Natl Acad. Sci. USA 73, 4685–4688 (1976).
Basbaum, A. I., Clanton, C. H. & Fields, H. L. Three bulbospinal pathways from the rostral medulla of the cat: an autoradiographic study of pain modulating systems. J. Comp. Neurol. 178, 209–224 (1978).
Basbaum, A. I. & Fields, H. L. The origin of descending pathways in the dorsolateral funiculus of the spinal cord of the cat and rat: further studies on the anatomy of pain modulation. J. Comp. Neurol. 187, 513–531 (1979).
Loyd, D. R., Morgan, M. M. & Murphy, A. Z. Morphine preferentially activates the periaqueductal gray–rostral ventromedial medullary pathway in the male rat: a potential mechanism for sex differences in antinociception. Neuroscience 147, 456–468 (2007).
Loyd, D. R. & Murphy, A. Z. Sex differences in the anatomical and functional organization of the periaqueductal gray–rostral ventromedial medullary pathway in the rat: a potential circuit mediating the sexually dimorphic actions of morphine. J. Comp. Neurol. 496, 723–738 (2006).
Watkins, L. R., Hutchinson, M. R., Johnston, I. N. & Maier, S. F. Glia: novel counter-regulators of opioid analgesia. Trends Neurosci. 28, 661–669 (2005).
Hutchinson, M. R. et al. Possible involvement of Toll-like receptor 4/myeloid differentiation factor-2 activity of opioid inactive isomers causes spinal proinflammation and related behavioral consequences. Neuroscience 167, 880–893 (2010).
Hutchinson, M. R. et al. Proinflammatory cytokines oppose opioid-induced acute and chronic analgesia. Brain Behav. Immun. 22, 1178–1189 (2008).
Hutchinson, M. R. et al. Opioid-induced glial activation: mechanisms of activation and implications for opioid analgesia, dependence, and reward. Scientific World Journal 7, 98–111 (2007).
Doyle, H. H. & Murphy, A. Z. Sex differences in innate immunity and its impact on opioid pharmacology. J. Neurosci. Res. 95, 487–499 (2017).
Sorge, R. E. et al. Spinal cord Toll-like receptor 4 mediates inflammatory and neuropathic hypersensitivity in male but not female mice. J. Neurosci. 31, 15450–15454 (2011).
Sorge, R. E. et al. Different immune cells mediate mechanical pain hypersensitivity in male and female mice. Nat. Neurosci. 18, 1081–1083 (2015).
Mogil, J. S. Sex differences in pain and pain inhibition: multiple explanations of a controversial phenomenon. Nat. Rev. Neurosci. 13, 859–866 (2012).
Vale, R. D. Accelerating scientific publication in biology. Proc. Natl Acad. Sci. USA 112, 13439–13446 (2015).
Cordero, R. J. B., de León-Rodriguez, C. M., Alvarado-Torres, J. K., Rodriguez, A. R. & Casadevall, A. Life science’s average publishable unit (APU) has increased over the past two decades. PLoS ONE 11, e0156983 (2016).
Snyder, S. H. Science interminable: blame Ben? Proc. Natl Acad. Sci. USA 110, 2428–2429 (2013).
van Dijk, D., Manor, O. & Carey, L. B. Publication metrics and success on the academic job market. Curr. Biol. 24, R516–R517 (2014).
Directorate-General for Research and Innovation (European Commission). Interim evaluation of Horizon 2020 Commission staff working document. https://ec.europa.eu/research/evaluations/pdf/archive/h2020_evaluations/swd(2017)220-in-depth-interim_evaluation-h2020.pdf (European Commission, 2018).
Kalpazidou Schmidt, E. & Ovseiko, P. V. Link Horizon Europe funding to real steps to gender equality. Nature 584, 525 (2020).
Shansky, R. M. Are hormones a ‘female problem’ for animal research? Science 364, 825–826 (2019).
Arnegard, M. E., Whitten, L. A., Hunter, C. & Clayton, J. A. Sex as a biological variable: a 5-year progress report and call to action. J. Women’s Health 29, 858–864 (2020).
Prendergast, B. J., Onishi, K. G. & Zucker, I. Female mice liberated for inclusion in neuroscience and biomedical research. Neurosci. Biobehav. Rev. 40C, 1–5 (2014).
Becker, J. B., Prendergast, B. J. & Liang, J. W. Female rats are not more variable than male rats: a meta-analysis of neuroscience studies. Biol. Sex Differences 7, 34 (2016).
Machida, T., Yonezawa, Y. & Noumura, T. Age-associated changes in plasma testosterone levels in male mice and their relation to social dominance or subordinance. Hormones Behav. 15, 238–245 (1981).
Tannenbaum, C., Ellis, R. P., Eyssel, F., Zou, J. & Schiebinger, L. Sex and gender analysis improves science and engineering. Nature 575, 137–146 (2019).
Becker, J. B. & Chartoff, E. Sex differences in neural mechanisms mediating reward and addiction. Neuropsychopharmacology 44, 166–183 (2019).
McCarthy, M. M., Arnold, A. P., Ball, G. F., Blaustein, J. D. & de Vries, G. J. Sex differences in the brain: the not so inconvenient truth. J. Neurosci. 32, 2241–2247 (2012).
Beltz, A. M., Beery, A. K. & Becker, J. B. Analysis of sex differences in pre-clinical and clinical data sets. Neuropsychopharmacology 44, 2155–2158 (2019).
Galea, L. A. M., Choleris, E., Albert, A. Y. K., McCarthy, M. M. & Sohrabji, F. The promises and pitfalls of sex difference research. Front. Neuroendocrinol. 56, 100817 (2020).
Miller, L. R. et al. Considering sex as a biological variable in preclinical research. FASEB J. 31, 29–34 (2017).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Neuroscience thanks Liisa Galea, Margaret McCarthy and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shansky, R.M., Murphy, A.Z. Considering sex as a biological variable will require a global shift in science culture. Nat Neurosci 24, 457–464 (2021). https://doi.org/10.1038/s41593-021-00806-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41593-021-00806-8
This article is cited by
-
Subregion and sex differences in ethanol activation of cholinergic and glutamatergic cells in the mesopontine tegmentum
Scientific Reports (2024)
-
Paving the way towards medicines for women and men
European Journal of Clinical Pharmacology (2024)
-
Complex alterations in inflammatory pain and analgesic sensitivity in young and ageing female rats: involvement of ASIC3 and Nav1.8 in primary sensory neurons
Inflammation Research (2024)
-
Are we moving the dial? Canadian health research funding trends for women’s health, 2S/LGBTQ + health, sex, or gender considerations
Biology of Sex Differences (2023)
-
Sex influences the effects of social status on socioemotional behavior and serotonin neurochemistry in rhesus monkeys
Biology of Sex Differences (2023)