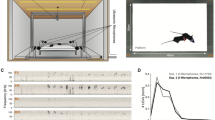
Abstract
Communication plays an integral role in human social dynamics and is impaired in several neurodevelopmental disorders. Mice are used to study the neurobiology of social behavior; however, the extent to which mouse vocalizations influence social dynamics has remained elusive because it is difficult to identify the vocalizing animal among mice involved in a group interaction. By tracking the ultrasonic vocal behavior of individual mice and using an algorithm developed to group phonically similar signals, we showed that distinct patterns of vocalization emerge as male mice perform specific social actions. Mice dominating other mice were more likely to emit different vocal signals than mice avoiding social interactions. Furthermore, we showed that the patterns of vocal expression influence the behavior of the socially engaged partner but do not influence the behavior of other animals in the cage. These findings clarify the function of mouse communication by revealing a communicative ultrasonic signaling repertoire.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Code availability
All code that supports the findings of this study is available from the corresponding author upon reasonable request.
References
Owen-Smith, N. Territoriality in the white rhinoceros (Ceratotherium simum) Burchell. Nature 231, 294–296 (1971).
Pizzari, T. & Birkhead, T. R. Female feral fowl eject sperm of subdominant males. Nature 405, 787–789 (2000).
Chen, P. & Hong, W. Neural circuit mechanisms of social behavior. Neuron 98, 16–30 (2018).
Bahrami, B. et al. Optimally interacting minds. Science 329, 1081–1085 (2010).
Seyfarth, R. M., Cheney, D. L. & Marler, P. Monkey responses to three different alarm calls: evidence of predator classification and semantic communication. Science 210, 801–803 (1980).
Bradbury, J. W. & Vehrencamp, S. L. Principles of Animal Communication (Sinauer Associates, 1998).
Gruters, K. G. & Groh, J. M. Sounds and beyond: multisensory and other non-auditory signals in the inferior colliculus. Front. Neural Circuits 6, 96 (2012).
Marlin, B. J. et al. Oxytocin enables maternal behaviour by balancing cortical inhibition. Nature 520, 499–504 (2015).
Wolpert, D. M., Doya, K. & Kawato, M. A unifying computational framework for motor control and social interaction. Philos. Trans. R. Soc. Lond. B Biol. Sci. 358, 593–602 (2003).
Li, Y. et al. Neuronal representation of social information in the medial amygdala of awake behaving mice. Cell 171, 1176–1190 (2017).
Remedios, R. et al. Social behaviour shapes hypothalamic neural ensemble representations of conspecific sex. Nature 550, 388–392 (2017).
Kingsbury, L. et al. Correlated neural activity and encoding of behavior across brains of socially interacting animals. Cell 178, 429–446 (2019).
Hong, W. et al. Automated measurement of mouse social behaviors using depth sensing, video tracking, and machine learning. Proc. Natl Acad. Sci. USA 112, E5351–E5360 (2015).
Sales, G. D. Ultrasound and mating behaviour in rodents with some observations on other behavioural situations. J. Zool. 168, 149–164 (1972).
Holy, T. E. & Guo, Z. Ultrasonic songs of male mice. PLoS Biol. 3, e386 (2005).
Shepard, K. N. & Liu, R. C. Experience restores innate female preference for male ultrasonic vocalizations. Genes Brain Behav. 10, 28–34 (2011).
Pomerantz, S. M., Nunez, A. A. & Bean, N. J. Female behavior is affected by male ultrasonic vocalizations in house mice. Physiol. Behav. 31, 91–96 (1983).
Mahrt, E. J., Perkel, D. J., Tong, L., Rubel, E. W. & Portfors, C. V. Engineered deafness reveals that mouse courtship vocalizations do not require auditory experience. J. Neurosci. 33, 5573–5583 (2013).
Coffey, K. R., Marx, R. G. & Neumaier, J. F. DeepSqueak: a deep learning-based system for detection and analysis of ultrasonic vocalizations. Neuropsychopharmacology 44, 859–868 (2019).
Chabout, J. et al. Adult male mice emit context-specific ultrasonic vocalizations that are modulated by prior isolation or group rearing environment. PLoS One 7, e29401 (2012).
Hanson, J. L. & Hurley, L. M. Female presence and estrous state influence mouse ultrasonic courtship vocalizations. PLoS One 7, e40782 (2012).
Sugimoto, H. et al. A role for strain differences in waveforms of ultrasonic vocalizations during male–female interaction. PLoS One 6, e22093 (2011).
Grimsley, J. M., Monaghan, J. J. & Wenstrup, J. J. Development of social vocalizations in mice. PLoS One 6, e17460 (2011).
Neunuebel, J. P., Taylor, A. L., Arthur, B. J. & Egnor, S. R. Female mice ultrasonically interact with males during courtship displays. eLife 4, e06203 (2015).
Ohayon, S., Avni, O., Taylor, A. L., Perona, P. & Roian Egnor, S. E. Automated multi-day tracking of marked mice for the analysis of social behaviour. J. Neurosci. Methods 219, 10–19 (2013).
Kabra, M., Robie, A. A., Rivera-Alba, M., Branson, S. & Branson, K. JAABA: interactive machine learning for automatic annotation of animal behavior. Nat. Methods 10, 64–67 (2013).
Warren, M. R., Sangiamo, D. T. & Neunuebel, J. P. High channel count microphone array accurately and precisely localizes ultrasonic signals from freely-moving mice. J. Neurosci. Methods 297, 44–60 (2018).
Coen, P. et al. Dynamic sensory cues shape song structure in Drosophila. Nature 507, 233–237 (2014).
Silverman, J. L., Yang, M., Lord, C. & Crawley, J. N. Behavioural phenotyping assays for mouse models of autism. Nat. Rev. Neurosci. 11, 490–502 (2010).
Warren, M. R., Spurrier, M. S., Roth, E. D. & Neunuebel, J. P. Sex differences in vocal communication of freely interacting adult mice depend upon behavioral context. PLoS One 13, e0204527 (2018).
Stern, S., Kirst, C. & Bargmann, C. I. Neuromodulatory control of long-term behavioral patterns and individuality across development. Cell 171, 1649–1662 (2017).
Frank, M. J., Doll, B. B., Oas-Terpstra, J. & Moreno, F. Prefrontal and striatal dopaminergic genes predict individual differences in exploration and exploitation. Nat. Neurosci. 12, 1062–1068 (2009).
Favaro, L., Gamba, M., Gili, C. & Pessani, D. Acoustic correlates of body size and individual identity in banded penguins. PLoS One 12, e0170001 (2017).
Stoeger, A. S. & Baotic, A. Information content and acoustic structure of male African elephant social rumbles. Sci. Rep. 6, 27585 (2016).
Rudorf, S. et al. Neural mechanisms underlying individual differences in control-averse behavior. J. Neurosci. 38, 5196–5208 (2018).
Johnson, K. R., Erway, L. C., Cook, S. A., Willott, J. F. & Zheng, Q. Y. A major gene affecting age-related hearing loss in C57BL/6J mice. Hear. Res. 114, 83–92 (1997).
Portfors, C. V., Roberts, P. D. & Jonson, K. Over-representation of species-specific vocalizations in the awake mouse inferior colliculus. Neuroscience 162, 486–500 (2009).
Galindo-Leon, E. E., Lin, F. G. & Liu, R. C. Inhibitory plasticity in a lateral band improves cortical detection of natural vocalizations. Neuron 62, 705–716 (2009).
Shepard, K. N., Lin, F. G., Zhao, C. L., Chong, K. K. & Liu, R. C. Behavioral relevance helps untangle natural vocal categories in a specific subset of core auditory cortical pyramidal neurons. J. Neurosci. 35, 2636–2645 (2015).
Neilans, E. G., Holfoth, D. P., Radziwon, K. E., Portfors, C. V. & Dent, M. L. Discrimination of ultrasonic vocalizations by CBA/CaJ mice (Mus musculus) is related to spectrotemporal dissimilarity of vocalizations. PLoS One 9, e85405 (2014).
Tschida, K. et al. A specialized neural circuit gates social vocalizations in the mouse. Neuron 103, 459–472 (2019).
Wang, F. et al. Bidirectional control of social hierarchy by synaptic efficacy in medial prefrontal cortex. Science 334, 693–697 (2011).
Moy, S. S. et al. Sociability and preference for social novelty in five inbred strains: an approach to assess autistic-like behavior in mice. Genes Brain Behav. 3, 287–302 (2004).
Gold, J. I. & Shadlen, M. N. The neural basis of decision making. Annu. Rev. Neurosci. 30, 535–574 (2007).
Krakauer, J. W., Ghazanfar, A. A., Gomez-Marin, A., MacIver, M. A. & Poeppel, D. Neuroscience needs behavior: correcting a reductionist bias. Neuron 93, 480–490 (2017).
Gomez-Marin, A., Paton, J. J., Kampff, A. R., Costa, R. M. & Mainen, Z. F. Big behavioral data: psychology, ethology and the foundations of neuroscience. Nat. Neurosci. 17, 1455–1462 (2014).
Berman, G. J. Measuring behavior across scales. BMC Biol. 16, 23 (2018).
Berman, G. J., Choi, D. M., Bialek, W. & Shaevitz, J. W. Mapping the stereotyped behaviour of freely moving fruit flies. J. R. Soc. Interface 11, 20140672 (2014).
Marques, J. C., Lackner, S., Felix, R. & Orger, M. B. Structure of the zebrafish locomotor repertoire revealed with unsupervised behavioral clustering. Curr. Biol. 28, 181–195 (2018).
Morton, E. S. On the occurrence and significance of motivation-structural rules in some bird and mammal sounds. Am. Naturalist 111, 855–869 (1977).
König, B. Fitness effects of communal rearing in house mice: the role of relatedness versus familiarity. Anim. Behav. 48, 1449–1457 (1994).
Seagraves, K. M., Arthur, B. J. & Egnor, S. E. Evidence for an audience effect in mice: male social partners alter the male vocal response to female cues. J. Exp. Biol. 219, 1437–1448 (2016).
Percival, D. B. & Walden, A. T. Spectral Analysis for Physical Applications (Cambridge University Press, 1993).
Grant, E. C. & Mackintosh, J. H. A comparison of the social postures of some common laboratory rodents. Behaviour 21, 246–259 (1963).
Van Oortmerssen, G. A. Biological significance, genetics and evolutionary origin of variability in behaviour within and between inbred strains of mice (Mus musculus). A behaviour genetic study. Behaviour 38, 1–92 (1971).
Miczek, K. A., Maxson, S. C., Fish, E. W. & Faccidomo, S. Aggressive behavioral phenotypes in mice. Behav. Brain Res. 125, 167–181 (2001).
Weissbrod, A. et al. Automated long-term tracking and social behavioural phenotyping of animal colonies within a semi-natural environment. Nat. Commun. 4, 2018 (2013).
Tabler, J. M. et al. Cilia-mediated Hedgehog signaling controls form and function in the mammalian larynx. eLife 6, e19153 (2017).
Schultz, W. Predictive reward signal of dopamine neurons. J. Neurophysiol. 80, 1–27 (1998).
Lemasson, B. H., Anderson, J. J. & Goodwin, R. A. Collective motion in animal groups from a neurobiological perspective: the adaptive benefits of dynamic sensory loads and selective attention. J. Theor. Biol. 261, 501–510 (2009).
Hikosaka, O. The habenula: from stress evasion to value-based decision-making. Nat. Rev. Neurosci. 11, 503–513 (2010).
Thura, D., Cos, I., Trung, J. & Cisek, P. Context-dependent urgency influences speed–accuracy trade-offs in decision-making and movement execution. J. Neurosci. 34, 16442–16454 (2014).
Chang, S. W. et al. Neural mechanisms of social decision-making in the primate amygdala. Proc. Natl Acad. Sci. USA 112, 16012–16017 (2015).
Wiltschko, A. B. et al. Mapping sub-second structure in mouse behavior. Neuron 88, 1121–1135 (2015).
Chabout, J., Sarkar, A., Dunson, D. B. & Jarvis, E. D. Male mice song syntax depends on social contexts and influences female preferences. Front. Behav. Neurosci. 9, 76 (2015).
Acknowledgements
We thank R. Neunuebel, A. Griffin, M. Duncan, M. Smear, R. Egnor, J. Knierim and R. Clein for helpful comments on the manuscript and the staffs of the Life Science Research Facility and the University of Delaware Information Technologies for assistance. We thank R. Egnor and G. Berman for providing software for normalizing vocal signals, D. Kelly for insightful discussions and J. Farmer and J. Quesenberry for help building lab equipment. This work was funded by the National Institutes of Health (2P20GM103653), the University of Delaware Research Foundation and Delaware’s General University Research Program.
Author information
Authors and Affiliations
Contributions
J.P.N. designed the study. D.T.S. and M.R.W. collected and processed the data. D.T.S. and J.P.N. analyzed the data. D.T.S. and J.P.N. wrote the manuscript. M.R.W. provided feedback on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Neuroscience thanks Robert Liu and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–25.
Supplementary Video 1
The video shows a representative example of a male mouse walking. Behaviors were extracted using JAABA. Because increasing the contrast between the white bedding and the black fur of the mouse improves our automated tracking system (Motr), the video recording was intentionally oversaturated. Each recorded male was observed walking (n = 22).
Supplementary Video 2
The video shows a representative example of two male mice circling each other. Mutual circles were observed between males in each recording (males: n = 22; recordings: n = 11).
Supplementary Video 3
The video shows a representative example of two male mice fighting. Fights were observed between males in each recording (males: n = 22; recordings: n = 11).
Supplementary Video 4
The video shows a representative example of a male mouse chasing a female. Each recorded male was observed chasing females (males: n = 22; recordings: n = 11).
Supplementary Video 5
The video shows a representative example of a male mouse running away from the other male. Fleeing was observed for each male recorded (males: n = 22; recordings: n = 11).
Supplementary Video 6
The video shows a representative example of a mouse chasing the other male. Each recorded male was observed chasing the other male (males: n = 22; recordings: n = 11).
Supplementary Video 7
The proportion of each vocal signal type within each behavior was calculated for each mouse. These values were then collapsed across mice. Average ± s.e.m.; n = 3,586 biologically independent samples analyzed.
Rights and permissions
About this article
Cite this article
Sangiamo, D.T., Warren, M.R. & Neunuebel, J.P. Ultrasonic signals associated with different types of social behavior of mice. Nat Neurosci 23, 411–422 (2020). https://doi.org/10.1038/s41593-020-0584-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41593-020-0584-z
This article is cited by
-
Increased gene dosage of RFWD2 causes autistic-like behaviors and aberrant synaptic formation and function in mice
Molecular Psychiatry (2024)
-
High-precision spatial analysis of mouse courtship vocalization behavior reveals sex and strain differences
Scientific Reports (2023)
-
“The song remains the same”: not really! Vocal flexibility in the song of the indris
Animal Cognition (2023)
-
Vocal signals with different social or non-social contexts in two wild rodent species (Mus caroli and Rattus losea)
Animal Cognition (2023)
-
Cross species review of the physiological role of d-serine in translationally relevant behaviors
Amino Acids (2023)