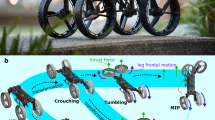
Abstract
The current proliferation of mobile robots spans ecological monitoring, warehouse management and extreme environment exploration, to an individual consumer’s home1,2,3,4. This expanding frontier of applications requires robots to transit multiple environments, a substantial challenge that traditional robot design strategies have not effectively addressed5,6. For example, biomimetic design—copying an animal’s morphology, propulsion mechanism and gait—constitutes one approach, but it loses the benefits of engineered materials and mechanisms that can be exploited to surpass animal performance7,8. Other approaches add a unique propulsive mechanism for each environment to the same robot body, which can result in energy-inefficient designs9,10,11. Overall, predominant robot design strategies favour immutable structures and behaviours, resulting in systems incapable of specializing across environments12,13. Here, to achieve specialized multi-environment locomotion through terrestrial, aquatic and the in-between transition zones, we implemented ‘adaptive morphogenesis’, a design strategy in which adaptive robot morphology and behaviours are realized through unified structural and actuation systems. Taking inspiration from terrestrial and aquatic turtles, we built a robot that fuses traditional rigid components and soft materials to radically augment the shape of its limbs and shift its gaits for multi-environment locomotion. The interplay of gait, limb shape and the environmental medium revealed vital parameters that govern the robot’s cost of transport. The results attest that adaptive morphogenesis is a powerful method to enhance the efficiency of mobile robots encountering unstructured, changing environments.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Information.
References
Miki, T. et al. Learning robust perceptive locomotion for quadrupedal robots in the wild. Sci. Robot. 7, eabk2822 (2022).
Sinatra, N. R. et al. Ultragentle manipulation of delicate structures using a soft robotic gripper. Sci. Robot. 4, eaax5425 (2019).
D’Andrea, R. Guest Editorial: A revolution in the warehouse: a retrospective on Kiva Systems and the grand challenges ahead. IEEE Trans. Autom. Sci. Eng. 9, 638–639 (2012).
Forlizzi, J. & DiSalvo, C. Service robots in the domestic environment: a study of the Roomba vacuum in the home. In HRI ’06: Proc. 1st ACM SIGCHI/SIGART Conference on Human–Robot Interaction 258–265 (ACM, 2006).
Shah, D. et al. Shape changing robots: bioinspiration, simulation, and physical realization. Adv. Mater. 33, 2002882 (2021).
Nygaard, T. F., Martin, C. P., Torresen, J., Glette, K. & Howard, D. Real-world embodied AI through a morphologically adaptive quadruped robot. Nat. Mach. Intell. 3, 410–419 (2021).
Ijspeert, A. J., Crespi, A., Ryczko, D. & Cabelguen, J.-M. From swimming to walking with a salamander robot driven by a spinal cord model. Science 315, 1416–1420 (2007).
Fish, F. E. Advantages of aquatic animals as models for bio-inspired drones over present AUV technology. Bioinspir. Biomim. 15, 025001 (2019).
Yu, J. et al. On a bio-inspired amphibious robot capable of multimodal motion. IEEE/ASME Trans. Mechatron. 17, 847–856 (2012).
Yu, J., Ding, R., Yang, Q., Tan, M. & Zhang, J. Amphibious pattern design of a robotic fish with wheel-propeller-fin mechanisms: amphibious pattern design of a robotic fish. J. Field Robot. 30, 702–716 (2013).
Boxerbaum, A. S. et al. Design, simulation, fabrication and testing of a bio-inspired amphibious robot with multiple modes of mobility. J. Robot. Mechatronics 24, 629–641 (2012).
Lock, R. J., Burgess, S. C. & Vaidyanathan, R. Multi-modal locomotion: from animal to application. Bioinspir. Biomim. 9, 011001 (2013).
Baines, R., Fish, F. & Kramer-Bottiglio, R. in Bioinspired Sensing, Actuation, and Control in Underwater Soft Robotic Systems (eds Paley, D. A. & Wereley, N. M.) Ch. 2 (Springer Nature, 2021).
Dudek, G. et al. AQUA: an amphibious autonomous robot. Computer 40, 46–53 (2007).
Ijspeert, A. J. Amphibious and sprawling locomotion: from biology to robotics and back. Annu. Rev. Control Robot. Auton. Syst. 3, 091919–095731 (2020).
Nyakatura, J. A. et al. Reverse-engineering the locomotion of a stem amniote. Nature 565, 351–355 (2019).
Mazouchova, N., Umbanhowar, P. B. & Goldman, D. I. Flipper-driven terrestrial locomotion of a sea turtle-inspired robot. Bioinspir. Biomim. 8, 026007 (2013).
Crespi, A., Karakasiliotis, K., Guignard, A. & Ijspeert, A. J. Salamandra Robotica II: an amphibious robot to study salamander-like swimming and walking gaits. IEEE Trans. Robot. 29, 308–320 (2013).
Ijspeert, A. J. Biorobotics: using robots to emulate and investigate agile locomotion. Science 346, 196–203 (2014).
Wyneken, J. in The Biology of Sea Turtles Vol. 1 (eds Lutz, P. L. & Musick, J. A.) Ch. 7 (CRC, 1997).
Zani, P. A., Gottschall, J. S. & Kram, R. Giant Galapagos tortoises walk without inverted pendulum mechanical-energy exchange. J. Exp. Biol. 208, 1489–1494 (2005).
Baines, R., Freeman, S., Fish, F. & Kramer-Bottiglio, R. Variable stiffness morphing limb for amphibious legged robots inspired by chelonian environmental adaptations. Bioinspir. Biomim. 15, 025002 (2020).
Blob, R., Mayerl, C., Rivera, A., Rivera, G. & Young, V. On the fence versus all in: insights from turtles for the evolution of aquatic locomotor specializations and habitat transitions in tetrapod vertebrates. Integr. Comp. Biol. 56, 1310–1322 (2016).
Rivera, A. R. V., Wyneken, J. & Blob, R. W. Forelimb kinematics and motor patterns of swimming loggerhead sea turtles (Caretta caretta): are motor patterns conserved in the evolution of new locomotor strategies? J. Exp. Biol. 214, 3314–3323 (2011).
Li, C., Umbanhowar, P. B., Komsuoglu, H., Koditschek, D. E. & Goldman, D. I. Sensitive dependence of the motion of a legged robot on granular media. Proc. Natl Acad. Sci. USA 106, 3029–3034 (2009).
Mazouchova, N., Gravish, N., Savu, A. & Goldman, D. I. Utilization of granular solidification during terrestrial locomotion of hatchling sea turtles. Biol. Lett. 6, 398–401 (2010).
Richefeu, V., El Youssoufi, M. S. & Radjai, F. Shear strength properties of wet granular materials. Phys. Rev. E 73, 051304 (2006).
Simoni, A. & Houlsby, G. T. The direct shear strength and dilatancy of sand and gravel mixtures. Geotech. Geol. Eng. 24, 523–549 (2006).
Kuo, A. D. Choosing your steps carefully. IEEE Robot. Autom Mag. 14, 18–29 (2007).
White, C. H., Lauder, G. V. & Bart-Smith, H. Tunabot Flex: a tuna-inspired robot with body flexibility improves high-performance swimming. Bioinspir. Biomim. 16, 026019 (2021).
Mahadevan, L. Morphogenesis: Geometry, Physics, and Biology (Perimeter Institute for Theoretical Physics, 2021).
Ultee, E., Ramijan, K., Dame, R. T., Briegel, A. & Claessen, D. Stress-induced adaptive morphogenesis in bacteria. Adv. Microb. Physiol. 74, 97–141 (2019).
Justice, S. S., Hunstad, D. A., Cegelski, L. & Hultgren, S. J. Morphological plasticity as a bacterial survival strategy. Nat. Rev. Microbiol. 6, 162–168 (2008).
Kim, S. Y. et al. Reconfigurable soft body trajectories using unidirectionally stretchable composite laminae. Nat. Commun. 10, 3464 (2019).
Kinoshita, C., Fukuoka, T., Narazaki, T., Niizuma, Y. & Sato, K. Analysis of why sea turtles swim slowly: a metabolic and mechanical approach. J. Exp. Biol. 224, jeb236216 (2021).
Butler, P., Milsom, W. & Woakes, A. Respiratory, cardiovascular and metabolic adjustments during steady state swimming in the green turtle, Chelonia mydas. J. Comp. Physiol. B 154, 167–174 (1984).
Baudinette, R. V., Miller, A. M. & Sarre, M. P. Aquatic and terrestrial locomotory energetics in a toad and a turtle: a search for generalisations among ectotherms. Physiol. Biochem. Zool. 73, 672–682 (2000).
Madden, J. D. Mobile robots: motor challenges and materials solutions. Science 318, 1094–1097 (2007).
Davenport, J. Locomotion in hatchling leatherback turtles Dermochelys coriacea. J. Zool. 212, 85–101 (1987).
Eckert, S. A. Swimming speed and patterns of leatherback turtles. J. Exp. Biol. 205, 3689–3697 (2002).
Tucker, V. A. Energetic cost of locomotion in animals. Comp. Biochem. Physiol. 34, 841–846 (1970).
Long, J. H., Schumacher, J., Livingston, N. & Kemp, M. Four flippers or two? Tetrapodal swimming with an aquatic robot. Bioinspir. Biomim. 1, 20–29 (2006).
Chen, Y., Doshi, N., Goldberg, B., Wang, H. & Wood, R. J. Controllable water surface to underwater transition through electrowetting in a hybrid terrestrial–aquatic microrobot. Nat. Commun. 9, 2495 (2018).
Wang, G. et al. Subsea crab bounding gait of leg-paddle hybrid driven shoal crablike robot. Mechatronics 48, 1–11 (2017).
Sellers, W. I., Rose, K. A., Crossley, D. A. & Codd, J. R. Inferring cost of transport from whole-body kinematics in three sympatric turtle species with different locomotor habits. Comp. Biochem. Physiol. A 247, 110739 (2020).
Milana, E. et al. EELWORM: a bioinspired multimodal amphibious soft robot. In 2020 3rd IEEE International Conference on Soft Robotics (RoboSoft) 766–771 (IEEE, 2020).
Kitano, S., Hirose, S., Horigome, A. & Endo, G. TITAN-XIII: sprawling-type quadruped robot with ability of fast and energy-efficient walking. ROBOMECH J. 3, 8 (2016).
Kandhari, A., Wang, Y., Chiel, H. J., Quinn, R. D. & Daltorio, K. A. An analysis of peristaltic locomotion for maximizing velocity or minimizing cost of transport of earthworm-like robots. Soft Robot. 8, 485–505 (2021).
Kau, N., Schultz, A., Ferrante, N. & Slade, P. Stanford Doggo: an open-source, quasi-direct-drive quadruped. In 2019 International Conference on Robotics and Automation (ICRA) 6309–6315 (IEEE, 2019).
Berlinger, F., Saadat, M., Haj-Hariri, H., Lauder, G. V. & Nagpal, R. Fish-like three-dimensional swimming with an autonomous, multi-fin, and biomimetic robot. Bioinspir. Biomim. 16, 026018 (2021).
Kim, K., Spieler, P., Lupu, E.-S., Ramezani, A. & Chung, S.-J. A bipedal walking robot that can fly, slackline, and skateboard. Sci. Robot. 6, eabf8136 (2021).
Bledt, G. et al. MIT Cheetah 3: design and control of a robust, dynamic quadruped robot. In 2018 IEEE/RSJ International Conference on Intelligent Robots and Systems (IROS) 2245–2252 (IEEE, 2018).
Hutter, M. et al. ANYmal—a highly mobile and dynamic quadrupedal robot. In 2016 IEEE/RSJ International Conference on Intelligent Robots and Systems (IROS) 38–44 (IEEE, 2016).
Craig, J. J. Introduction to Robotics: Mechanics & Control (Addison-Wesley, 1986).
Acknowledgements
We thank V. Wilczynski, Deputy Dean of Yale School of Engineering, for use of his pool. This project was sponsored by the Office of Naval Research under award N00014-21-1-2417. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the authors and do not necessarily reflect the views of the Office of Naval Research. R.B. was supported by an NSF Graduate Research Fellowship (DGE-1752134).
Author information
Authors and Affiliations
Contributions
R.B., S.K.P., J.B., T.S. and A.G. designed and built the robot. R.B., S.K.P. and L.R. conducted swimming, terrestrial and transition experiments. R.B., S.K.P., L.R. and F.F. programmed gaits. R.B. conducted CFD simulations and friction tests. R.K.-B. conceived of the project and oversaw the research. All authors contributed to writing the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature thanks Navinda Kottege and Cecilia Laschi for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 System description.
a, Exploded computer-aided-design view, detailing components of robot. Note that the shoulder joints are typically shrouded in rubber bellows, and thus not visible. For clarity, we only depict the bellows on the back right joint in transparent grey. b, 3-DoF kinematic mechanism used to achieve bio-inspired gaits. Symbols θ, ϕ and α are the rotation axes for the swing forward/backward, up/down, and angle of attack motors, respectively.
Extended Data Fig. 2 Robot workspace.
Workspace visualized in the robot’s kinematic simulation. The explored gaits are superimposed atop the cyan workspace in a darker colour. Top two are swimming gaits; bottom two are terrestrial and transition gaits. Chassis beneath the shell is rendered for context. Links are line segments coloured red, green, and blue. The black arrow points in the forward travel direction.
Extended Data Fig. 3 Fabrication of Morphing Limb.
Fabrication can be broken down into two main tracks: elastomeric actuators (top), and Joule-heating variable-stiffness material (bottom). We fabricate components for each of the limbs’ two halves, A and B. Actuators and variable-stiffness material components for A and B come together in the final step, in which they are hinged together via a sewed joint. The limb cross-section is displayed in the inset. Figure and caption adapted from ref. 22.
Extended Data Fig. 4 Test rig for evaluating force profiles of gaits.
Coordinate system defines positive direction of forces measured via the multi-axis load cell. Adjustable fixtures allowed us to tune the robot’s offset from the pool sides and bottom, as well as its submerged depth.
Extended Data Fig. 5 Representative data for front-right shoulder during paddling and flapping gaits.
Top row gives a robot schematic with superimposed trajectory. Second row gives commanded and actual encoder positions, where the solid line is the actual achieved position. Third row plots the angular velocity. Fourth row is the amperage. Line colour and legend symbols match those of Fig. 1b.
Extended Data Fig. 6 CFD results.
Simulation results of lift and drag forces on the flipper mode of the morphing limb, at 0.3 m/s. Inset depicts definition of Φ with respect to the flow direction.
Extended Data Fig. 7 Representative data from all shoulder joints for creeping gait on the porcelain substrate.
Top row presents snapshots of the robot at key parts of the gait. Graphs underneath give commanded and actual encoder positions, where the solid line is the actual achieved position. Line colour and legend symbols match those of Fig. 1b.
Extended Data Fig. 8 Additional creep data.
a, Left to right: commanded and actual encoder positions, angular velocity, and amperage for front-right shoulder joint. The solid line is the actual achieved position. Line colour and legend symbols match those of Fig. 1b. b, Representative data from motion capture of back-left distal tip of limb during creep gait. Spikes in Z correspond to the limb swinging out, whereas the other portions are the pivot or where the limb serves only to balance the robot. Notice the amplitude and frequency of vibrations occurring at these times, which give an indication of surface normals and roughness. At these sections, we calculated the stability metric to grasp the effect of substrates on COT when creeping. Here, the series are not normalized to all start at 0, for ease of viewing.
Extended Data Fig. 9 Representative data from front-right shoulder joint for crawl gaits across the three substrates.
Top row gives a robot schematic with superimposed trajectory. Second row gives commanded and actual encoder positions, where the solid line is the actual achieved position. Third row plots the angular velocity. Fourth row is the amperage. Line colour and legend symbols match those of Fig. 1b.
Extended Data Fig. 10 Example data from friction tests.
Force versus displacement for elastomer, PLA, or bellows with 500 g weight placed atop them, over the various substrates. Clouds indicate one standard deviation from the mean, over 7 trials, with the solid line as the mean.
Supplementary information
Supplementary Video 1
Aquatic testing. We demonstrate how ART can perform multiple types of aquatic gait using the hydrodynamically favourable flipper shape.
Supplementary Video 2
Land locomotion. ART is capable of traversing multiple substrates. Here we show footage from porcelain, concrete and granite substrates, both in the lab and outdoors on Yale campus.
Supplementary Video 3
Transition-substrate tests. An upright creep gait lacks stability on granular and highly fluidized media. ART adapts to a splayed crawling gait, distributing its weight, to traverse such substrates.
Supplementary Video 4
Morphing sequence. We demonstrate how the morphing limb undergoes drastic, reversible shape change.
Supplementary Video 5
Variable-stiffness composite functionality underwater. The morphing limb is waterproof and able to switch between leg and flipper modes underwater.
Supplementary Video 6
Transition field testing. We combined favourable shape-gait policies from terrestrial and aquatic environments to study terrestrial-to-aquatic transitions at an ocean inlet.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Baines, R., Patiballa, S.K., Booth, J. et al. Multi-environment robotic transitions through adaptive morphogenesis. Nature 610, 283–289 (2022). https://doi.org/10.1038/s41586-022-05188-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-022-05188-w
This article is cited by
-
An Underwater Biomimetic Robot that can Swim, Bipedal Walk and Grasp
Journal of Bionic Engineering (2024)
-
Multibody dynamic modeling and motion analysis of flexible robot considering contact
Multibody System Dynamics (2024)
-
Deformation and Locomotion of Untethered Small-Scale Magnetic Soft Robotic Turtle with Programmable Magnetization
Journal of Bionic Engineering (2024)
-
Soft-robotic green sea turtle (Chelonia mydas) developed to replace animal experimentation provides new insight into their propulsive strategies
Scientific Reports (2023)
-
Embedded shape morphing for morphologically adaptive robots
Nature Communications (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.