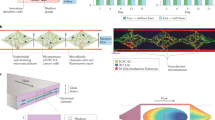
Abstract
Arthritis affects millions of people worldwide. With only a few disease-modifying drugs available for treatment of rheumatoid arthritis and none for osteoarthritis, a clear need exists for new treatment options. Current disease models used for drug screening and development suffer from several disadvantages and, most importantly, do not accurately emulate all facets of human joint diseases. A humanized joint-on-chip (JoC) model or platform could revolutionize research and drug development in rheumatic diseases. A JoC model is a multi-organ-on-chip platform that incorporates a range of engineered features to emulate essential aspects and functions of the human joint and faithfully recapitulates the joint’s physiological responses. In this Review, we propose an architecture for such a JoC platform, discuss the status of the engineering of individual joint tissues and the efforts to combine them in a functional JoC model and identify unresolved issues and challenges in constructing an accurate, physiologically relevant system. The goal is to ultimately obtain a reliable and ready-to-use humanized model of the joint for studying the pathophysiology of rheumatic diseases and screening drugs for treatment of these conditions.
Key points
-
Current in vitro and in vivo models only partly recapitulate the complexity of human arthritic diseases and consequently lack translational power in the development of new disease-modifying treatments.
-
Engineering a miniaturized version of the human joint as a joint-on-chip platform that faithfully emulates key aspects of a healthy joint and in which disease-specific triggers can be introduced could substantially advance research into arthritic diseases.
-
The minimal functional joint-on-chip requires an osteochondral unit and a synovial membrane unit that emulate the composition of the extracellular matrix and appropriate cell types in the respective tissues and that are connected to each other using microfluidic coupling.
-
The minimal joint-on-chip can be extended with additional tissue units, such as those emulating the meniscus, ligaments and Hoffa’s fat pad; inter-organ communication could be achieved by connecting the different tissue units to a motherboard with integrated sensors to enable real-time measurements.
-
Although promising and potentially revolutionary, multiple challenges must still be overcome to produce a reliable joint-on-chip model that could be used in arthritis research and drug development programmes.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
McDonough, C. M. & Jette, A. M. The contribution of osteoarthritis to functional limitations and disability. Clin. Geriatr. Med. 26, 387–399 (2010).
Guo, Q. et al. Rheumatoid arthritis: pathological mechanisms and modern pharmacologic therapies. Bone Res. 6, 15 (2018).
Chen, D. et al. Osteoarthritis: toward a comprehensive understanding of pathological mechanism. Bone Res. 5, 16044 (2017).
Onishi, K. et al. Osteoarthritis: a critical review. Crit. Rev. Phys. Rehabil. Med. 24, 251–264 (2012).
Aletaha, D. & Smolen, J. S. Diagnosis and management of rheumatoid arthritis: a review. JAMA 320, 1360–1372 (2018).
Ghouri, A. & Conaghan, P. G. Update on novel pharmacological therapies for osteoarthritis. Ther. Adv. Musculoskelet. Dis. 11, 1759720–19864492 (2019).
Low, L. A., Mummery, C., Berridge, B. R., Austin, C. P. & Tagle, D. A. Organs-on-chips: into the next decade. Nat. Rev. Drug Discov. 20, 345–361 (2021).
Jensen, C. & Teng, Y. Is it time to start transitioning from 2D to 3D cell culture? Front. Mol. Biosci. 7, 33 (2020).
Kapalczynska, M. et al. 2D and 3D cell cultures — a comparison of different types of cancer cell cultures. Arch. Med. Sci. 14, 910–919 (2018).
Duval, K. et al. Modeling physiological events in 2D vs. 3D cell culture. Physiology 32, 266–277 (2017).
Huh, D., Hamilton, G. A. & Ingber, D. E. From 3D cell culture to organs-on-chips. Trends Cell Biol. 21, 745–754 (2011).
Charlier, E. et al. Chondrocyte dedifferentiation and osteoarthritis (OA). Biochem. Pharmacol. 165, 49–65 (2019).
Bessis, N., Decker, P., Assier, E., Semerano, L. & Boissier, M. C. Arthritis models: usefulness and interpretation. Semin. Immunopathol. 39, 469–486 (2017).
Malfait, A. M. & Little, C. B. On the predictive utility of animal models of osteoarthritis. Arthritis Res. Ther. 17, 225 (2015).
Kuyinu, E. L., Narayanan, G., Nair, L. S. & Laurencin, C. T. Animal models of osteoarthritis: classification, update, and measurement of outcomes. J. Orthop. Surg. Res. 11, 19 (2016).
Dolzani, P. et al. Ex vivo physiological compression of human osteoarthritis cartilage modulates cellular and matrix components. PLoS ONE 14, e0222947 (2019).
Kleuskens, M. W. A., van Donkelaar, C. C., Kock, L. M., Janssen, R. P. A. & Ito, K. An ex vivo human osteochondral culture model. J. Orthop. Res. 39, 871–879 (2021).
Cope, P. J., Ourradi, K., Li, Y. & Sharif, M. Models of osteoarthritis: the good, the bad and the promising. Osteoarthritis Cartilage 27, 230–239 (2019).
Sophia Fox, A. J., Bedi, A. & Rodeo, S. A. The basic science of articular cartilage: structure, composition, and function. Sports Health 1, 461–468 (2009).
Burr, D. B. Anatomy and physiology of the mineralized tissues: role in the pathogenesis of osteoarthrosis. Osteoarthritis Cartilage 12, S20–30 (2004).
Oegema, T. R. Jr, Carpenter, R. J., Hofmeister, F. & Thompson, R. C. Jr. The interaction of the zone of calcified cartilage and subchondral bone in osteoarthritis. Microsc. Res. Tech. 37, 324–332 (1997).
Bonewald, L. F. The amazing osteocyte. J. Bone Min. Res. 26, 229–238 (2011).
Feng, X. & Teitelbaum, S. L. Osteoclasts: new insights. Bone Res. 1, 11–26 (2013).
Firestein, G. S. Evolving concepts of rheumatoid arthritis. Nature 423, 356–361 (2003).
Simkin, P. A. Physiology of normal and abnormal synovium. Semin. Arthritis Rheum. 21, 179–183 (1991).
Kurowska-Stolarska, M. & Alivernini, S. Synovial tissue macrophages: friend or foe? RMD Open 3, e000527 (2017).
Brindle, T., Nyland, J. & Johnson, D. L. The meniscus: review of basic principles with application to surgery and rehabilitation. J. Athl. Train. 36, 160–169 (2001).
Lieben, L. Characterization of the infrapatellar fat pad. Nat. Rev. Rheumatol. 13, 571–571 (2017).
Labusca, L. & Zugun-Eloae, F. The unexplored role of intra-articular adipose tissue in the homeostasis and pathology of articular joints. Front. Vet. Sci. 5, 35 (2018).
Bhatia, S. N. & Ingber, D. E. Microfluidic organs-on-chips. Nat. Biotechnol. 32, 760–772 (2014).
Zheng, F. et al. Organ-on-a-chip systems: microengineering to biomimic living systems. Small 12, 2253–2282 (2016).
Bhise, N. S. et al. Organ-on-a-chip platforms for studying drug delivery systems. J. Control. Rel. 190, 82–93 (2014).
Kimura, H., Sakai, Y. & Fujii, T. Organ/body-on-a-chip based on microfluidic technology for drug discovery. Drug Metab. Pharmacokinet. 33, 43–48 (2018).
Wilmer, M. J. et al. Kidney-on-a-chip technology for drug-induced nephrotoxicity screening. Trends Biotechnol. 34, 156–170 (2016).
Kaarj, K. & Yoon, J. Y. Methods of delivering mechanical stimuli to organ-on-a-chip. Micromachines 10, 700 (2019).
Thompson, C. L., Fu, S., Knight, M. M. & Thorpe, S. D. Mechanical stimulation: a crucial element of organ-on-chip models. Front. Bioeng. Biotechnol. 8, 602646 (2020).
Wu, Q. et al. Organ-on-a-chip: recent breakthroughs and future prospects. Biomed. Eng. Online 19, 9 (2020).
Doryab, A., Amoabediny, G. & Salehi-Najafabadi, A. Advances in pulmonary therapy and drug development: lung tissue engineering to lung-on-a-chip. Biotechnol. Adv. 34, 588–596 (2016).
Shrestha, J. et al. Lung-on-a-chip: the future of respiratory disease models and pharmacological studies. Crit. Rev. Biotechnol. 40, 213–230 (2020).
Moradi, E., Jalili-Firoozinezhad, S. & Solati-Hashjin, M. Microfluidic organ-on-a-chip models of human liver tissue. Acta Biomater. 116, 67–83 (2020).
Kim, J. et al. Three-dimensional human liver-chip emulating premetastatic niche formation by breast cancer-derived extracellular vesicles. ACS Nano 14, 14971–14988 (2020).
Jellali, R. et al. Long-term human primary hepatocyte cultures in a microfluidic liver biochip show maintenance of mRNA levels and higher drug metabolism compared with Petri cultures. Biopharm. Drug Dispos. 37, 264–275 (2016).
Lee, J. & Kim, S. Kidney-on-a-chip: a new technology for predicting drug efficacy, interactions, and drug-induced nephrotoxicity. Curr. Drug Metab. 19, 577–583 (2018).
Ashammakhi, N., Wesseling-Perry, K., Hasan, A., Elkhammas, E. & Zhang, Y. S. Kidney-on-a-chip: untapped opportunities. Kidney Int. 94, 1073–1086 (2018).
Bein, A. et al. Microfluidic organ-on-a-chip models of human intestine. Cell Mol. Gastroenterol. Hepatol. 5, 659–668 (2018).
Verhulsel, M. et al. Developing an advanced gut on chip model enabling the study of epithelial cell/fibroblast interactions. Lab. Chip 21, 365–377 (2021).
Zhang, Y. S. et al. From cardiac tissue engineering to heart-on-a-chip: beating challenges. Biomed. Mater. 10, 034006 (2015).
Ribas, J. et al. Cardiovascular organ-on-a-chip platforms for drug discovery and development. Appl. Vitr. Toxicol. 2, 82–96 (2016).
Ferraz, M. A. M. M. et al. An oviduct-on-a-chip provides an enhanced in vitro environment for zygote genome reprogramming. Nat. Commun. 9, 4934 (2018).
Kim, S., Kim, W., Lim, S. & Jeon, J. S. Vasculature-on-a-chip for in vitro disease models. Bioengineering 4, 8 (2017).
Moses, S. R., Adorno, J. J., Palmer, A. F. & Song, J. W. Vessel-on-a-chip models for studying microvascular physiology, transport, and function in vitro. Am. J. Physiol. Cell Physiol. 320, C92–C105 (2021).
Doherty, E. L., Aw, W. Y., Hickey, A. J. & Polacheck, W. J. Microfluidic and organ-on-a-chip approaches to investigate cellular and microenvironmental contributions to cardiovascular function and pathology. Front. Bioeng. Biotechnol. 9, 624435 (2021).
Oddo, A. et al. Advances in microfluidic blood-brain barrier (BBB) models. Trends Biotechnol. 37, 1295–1314 (2019).
Virumbrales-Munoz, M. et al. Multiwell capillarity-based microfluidic device for the study of 3D tumour tissue-2D endothelium interactions and drug screening in co-culture models. Sci. Rep. 7, 11998 (2017).
Liu, X. et al. Tumor-on-a-chip: from bioinspired design to biomedical application. Microsyst. Nanoeng. 7, 50 (2021).
Sontheimer-Phelps, A., Hassell, B. A. & Ingber, D. E. Modelling cancer in microfluidic human organs-on-chips. Nat. Rev. Cancer 19, 65–81 (2019).
Picollet-D’hahan, N., Zuchowska, A., Lemeunier, I. & Le Gac, S. Multiorgan-on-a-chip: a systemic approach to model and decipher inter-organ communication. Trends Biotechnol. 39, 788–810 (2021).
Sung, J. H. et al. Recent advances in body-on-a-chip systems. Anal. Chem. 91, 330–351 (2019).
Piluso, S. et al. Mimicking the articular joint with in vitro models. Trends Biotechnol. 37, 1063–1077 (2019).
Longobardi, L. et al. Synovial joints: from development to homeostasis. Curr. Osteoporos. Rep. 13, 41–51 (2015).
Ikada, Y. Challenges in tissue engineering. J. R. Soc. Interface 3, 589–601 (2006).
Fu, Y. et al. Engineering cartilage tissue by co-culturing of chondrocytes and mesenchymal stromal cells. Methods Mol. Biol. 2221, 53–70 (2021).
Gartland, A., Rumney, R. M., Dillon, J. P. & Gallagher, J. A. Isolation and culture of human osteoblasts. Methods Mol. Biol. 806, 337–355 (2012).
Park, D., Lim, J., Park, J. Y. & Lee, S. H. Concise review: stem cell microenvironment on a chip: current technologies for tissue engineering and stem cell biology. Stem Cell Transl. Med. 4, 1352–1368 (2015).
Pittenger, M. F. et al. Mesenchymal stem cell perspective: cell biology to clinical progress. NPJ Regen. Med. 4, 22 (2019).
Augello, A. & De Bari, C. The regulation of differentiation in mesenchymal stem cells. Hum. Gene Ther. 21, 1226–1238 (2010).
George, J., Kuboki, Y. & Miyata, T. Differentiation of mesenchymal stem cells into osteoblasts on honeycomb collagen scaffolds. Biotechnol. Bioeng. 95, 404–411 (2006).
Chen, Q. et al. Fate decision of mesenchymal stem cells: adipocytes or osteoblasts? Cell Death Differ. 23, 1128–1139 (2016).
Somoza, R. A., Welter, J. F., Correa, D. & Caplan, A. I. Chondrogenic differentiation of mesenchymal stem cells: challenges and unfulfilled expectations. Tissue Eng. Part B Rev. 20, 596–608 (2014).
Karagiannis, P. et al. Induced pluripotent stem cells and their use in human models of disease and development. Physiol. Rev. 99, 79–114 (2019).
Guzzo, R. M. & Drissi, H. Differentiation of human induced pluripotent stem cells to chondrocytes. Methods Mol. Biol. 1340, 79–95 (2015).
Jeon, O. H. et al. Human iPSC-derived osteoblasts and osteoclasts together promote bone regeneration in 3D biomaterials. Sci. Rep. 6, 26761 (2016).
Williams, I. M. & Wu, J. C. Generation of endothelial cells from human pluripotent stem cells. Arterioscler. Thromb. Vasc. Biol. 39, 1317–1329 (2019).
Gunhanlar, N. et al. A simplified protocol for differentiation of electrophysiologically mature neuronal networks from human induced pluripotent stem cells. Mol. Psychiatry 23, 1336–1344 (2018).
Nakajima, T. et al. Grafting of iPS cell-derived tenocytes promotes motor function recovery after Achilles tendon rupture. Nat. Commun. 12, 5012 (2021).
Mukherjee, C., Hale, C. & Mukhopadhyay, S. A simple multistep protocol for differentiating human induced pluripotent stem cells into functional macrophages. Methods Mol. Biol. 1784, 13–28 (2018).
Doss, M. X. & Sachinidis, A. Current challenges of iPSC-based disease modeling and therapeutic implications. Cells 8, 403 (2019).
Ben Jehuda, R., Shemer, Y. & Binah, O. Genome editing in induced pluripotent stem cells using CRISPR/Cas9. Stem Cell Rev. Rep. 14, 323–336 (2018).
Adkar, S. S. et al. Step-wise chondrogenesis of human induced pluripotent stem cells and purification via a reporter allele generated by CRISPR-Cas9 genome editing. Stem Cell 37, 65–76 (2019)
Roeder, E., Matthews, B. G. & Kalajzic, I. Visual reporters for study of the osteoblast lineage. Bone 92, 189–195 (2016).
Bader, D. L., Salter, D. M. & Chowdhury, T. T. Biomechanical influence of cartilage homeostasis in health and disease. Arthritis 2011, 979032 (2011).
Almqvist, K. F. et al. Treatment of cartilage defects in the knee using alginate beads containing human mature allogenic chondrocytes. Am. J. Sport. Med. 37, 1920–1929 (2009).
Salati, M. A. et al. Agarose-based biomaterials: opportunities and challenges in cartilage tissue engineering. Polymers 12, 1150. (2020).
Jin, R. et al. Enzymatically crosslinked dextran-tyramine hydrogels as injectable scaffolds for cartilage tissue engineering. Tissue Eng. Part A 16, 2429–2440 (2010).
Deshpande, M. C. et al. The effect of poly(ethylene glycol) molecular architecture on cellular interaction and uptake of DNA complexes. J. Control. Rel. 97, 143–156 (2004).
Bougault, C., Paumier, A., Aubert-Foucher, E. & Mallein-Gerin, F. Molecular analysis of chondrocytes cultured in agarose in response to dynamic compression. BMC Biotechnol. 8, 71 (2008).
Benya, P. D. & Shaffer, J. D. Dedifferentiated chondrocytes reexpress the differentiated collagen phenotype when cultured in agarose gels. Cell 30, 215–224 (1982).
Buschmann, M. D., Gluzband, Y. A., Grodzinsky, A. J., Kimura, J. H. & Hunziker, E. B. Chondrocytes in agarose culture synthesize a mechanically functional extracellular matrix. J. Orthop. Res. 10, 745–758 (1992).
Bougault, C. et al. Dynamic compression of chondrocyte-agarose constructs reveals new candidate mechanosensitive genes. PLoS ONE 7, e36964 (2012).
Ashraf, S. & Walsh, D. A. Angiogenesis in osteoarthritis. Curr. Opin. Rheumatol. 20, 573–580 (2008).
Ahearne, M. Introduction to cell-hydrogel mechanosensing. Interface Focus. 4, 20130038 (2014).
Wennink, J. W. H. et al. Injectable hydrogels by enzymatic co-crosslinking of dextran and hyaluronic acid tyramine conjugates. Macromol. Symp. 309–310, 213–221 (2011).
Jin, R. et al. Enzymatically-crosslinked injectable hydrogels based on biomimetic dextran-hyaluronic acid conjugates for cartilage tissue engineering. Biomaterials 31, 3103–3113 (2010).
Occhetta, P. et al. Hyperphysiological compression of articular cartilage induces an osteoarthritic phenotype in a cartilage-on-a-chip model. Nat. Biomed. Eng. 3, 545–557 (2019).
Lee, D., Erickson, A., You, T., Dudley, A. T. & Ryu, S. Pneumatic microfluidic cell compression device for high-throughput study of chondrocyte mechanobiology. Lab. Chip 18, 2077–2086 (2018).
Rosser, J. et al. Microfluidic nutrient gradient-based three-dimensional chondrocyte culture-on-a-chip as an in vitro equine arthritis model. Mater. Today Bio 4, 100023 (2019).
Paggi, C. A., Venzac, B., Karperien, M., Leijten, J. C. H. & Le Gac, S. Monolithic microfluidic platform for exerting gradients of compression on cell-laden hydrogels, and application to a model of the articular cartilage. Sens. Actuat. B Chem. 315, 127917 (2020).
Jusoh, N., Oh, S., Kim, S., Kim, J. & Jeon, N. L. Microfluidic vascularized bone tissue model with hydroxyapatite-incorporated extracellular matrix. Lab. Chip 15, 3984–3988 (2015).
Yuan, H. et al. Osteoinductive ceramics as a synthetic alternative to autologous bone grafting. Proc. Natl Acad. Sci. USA 107, 13614–13619 (2010).
Goncalves, A. M., Moreira, A., Weber, A., Williams, G. R. & Costa, P. F. Osteochondral tissue engineering: the potential of electrospinning and additive manufacturing. Pharmaceutics 13, 983 (2021).
Mansoorifar, A., Gordon, R., Bergan, R. C. & Bertassoni, L. E. Bone-on-a-chip: microfluidic technologies and microphysiologic models of bone tissue. Adv. Funct. Mater. 14, e1702787 (2021).
Torisawa, Y. S. et al. Bone marrow-on-a-chip replicates hematopoietic niche physiology in vitro. Nat. Methods 11, 663–669 (2014).
Bersini, S. et al. A microfluidic 3D in vitro model for specificity of breast cancer metastasis to bone. Biomaterials 35, 2454–2461 (2014).
Chou, D. B. et al. On-chip recapitulation of clinical bone marrow toxicities and patient-specific pathophysiology. Nat. Biomed. Eng. 4, 394–406 (2020).
Yamada, A. et al. Transient microfluidic compartmentalization using actionable microfilaments for biochemical assays, cell culture and organs-on-chip. Lab. Chip 16, 4691–4701 (2016).
Hoemann, C. D., Lafantaisie-Favreau, C. H., Lascau-Coman, V., Chen, G. & Guzman-Morales, J. The cartilage-bone interface. J. Knee Surg. 25, 85–97 (2012).
Simkin, P. A. Consider the tidemark. J. Rheumatol. 39, 890–892 (2012).
Lin, H., Lozito, T. P., Alexander, P. G., Gottardi, R. & Tuan, R. S. Stem cell-based microphysiological osteochondral system to model tissue response to interleukin-1beta. Mol. Pharm. 11, 2203–2212 (2014).
Pirosa, A. et al. An in vitro chondro-osteo-vascular triphasic model of the osteochondral complex. Biomaterials 272, 120773 (2021).
Lin, Z. et al. Osteochondral tissue chip derived from iPSCs: modeling OA pathologies and testing drugs. Front. Bioeng. Biotechnol. 7, 411 (2019).
Moraes, C., Mehta, G., Lesher-Perez, S. C. & Takayama, S. Organs-on-a-chip: a focus on compartmentalized microdevices. Ann. Biomed. Eng. 40, 1211–1227 (2012).
Rothbauer, M. et al. Monitoring tissue-level remodelling during inflammatory arthritis using a three-dimensional synovium-on-a-chip with non-invasive light scattering biosensing. Lab. Chip 20, 1461–1471 (2020).
Ma, H. P. et al. A microfluidic chip-based co-culture of fibroblast-like synoviocytes with osteoblasts and osteoclasts to test bone erosion and drug evaluation. R. Soc. Open Sci. 5, 180528 (2018).
Huh, D. et al. Reconstituting organ-level lung functions on a chip. Science 328, 1662–1668 (2010).
Kim, H. J., Huh, D., Hamilton, G. & Ingber, D. E. Human gut-on-a-chip inhabited by microbial flora that experiences intestinal peristalsis-like motions and flow. Lab. Chip 12, 2165–2174 (2012).
Sinha, R. et al. Endothelial cell alignment as a result of anisotropic strain and flow induced shear stress combinations. Sci. Rep. 6, 29510 (2016).
Petersen, W. & Tillmann, B. Structure and vascularization of the cruciate ligaments of the human knee joint. Anat. Embryol. 200, 325–334 (1999).
Lee, P., Lin, R., Moon, J. & Lee, L. P. Microfluidic alignment of collagen fibers for in vitro cell culture. Biomed. Microdevices 8, 35–41 (2006).
Phan, D. T. T. et al. A vascularized and perfused organ-on-a-chip platform for large-scale drug screening applications. Lab. Chip 17, 511–520 (2017).
Hsu, Y. H., Moya, M. L., Hughes, C. C. W., George, S. C. & Lee, A. P. A microfluidic platform for generating large-scale nearly identical human microphysiological vascularized tissue arrays. Lab. Chip 13, 2990–2998 (2013).
Yang, F. et al. A 3D human adipose tissue model within a microfluidic device. Lab. Chip 21, 435–446 (2021).
Clockaerts, S. et al. The infrapatellar fat pad should be considered as an active osteoarthritic joint tissue: a narrative review. Osteoarthritis Cartilage 18, 876–882 (2010).
Fontanella, C. G. et al. Biomechanical behavior of Hoffa’s fat pad in healthy and osteoarthritic conditions: histological and mechanical investigations. Australas. Phys. Eng. Sci. Med. 41, 657–667 (2018).
Kongsuphol, P. et al. In vitro micro-physiological model of the inflamed human adipose tissue for immune-metabolic analysis in type II diabetes. Sci. Rep. 9, 4887 (2019).
Liu, Y. et al. Adipose-on-a-chip: a dynamic microphysiological in vitro model of the human adipose for immune-metabolic analysis in type II diabetes. Lab. Chip 19, 241–253 (2019).
Loskill, P., Marcus, S. G., Mathur, A., Reese, W. M. & Healy, K. μOrgano: a Lego®-like plug & play system for modular multi-organ-chips. PLoS ONE 10, e0139587 (2015).
Zhang, Y. S. et al. Multisensor-integrated organs-on-chips platform for automated and continual in situ monitoring of organoid behaviors. Proc. Natl Acad. Sci. USA 114, E2293–E2302 (2017).
Ong, L. J. Y. et al. Self-aligning Tetris-Like (TILE) modular microfluidic platform for mimicking multi-organ interactions. Lab. Chip 19, 2178–2191 (2019).
Esch, M. B., Ueno, H., Applegate, D. R. & Shuler, M. L. Modular, pumpless body-on-a-chip platform for the co-culture of GI tract epithelium and 3D primary liver tissue. Lab. Chip 16, 2719–2729 (2016).
Materne, E. M. et al. A multi-organ chip co-culture of neurospheres and liver equivalents for long-term substance testing. J. Biotechnol. 205, 36–46 (2015).
Maschmeyer, I. et al. A four-organ-chip for interconnected long-term co-culture of human intestine, liver, skin and kidney equivalents. Lab. Chip 15, 2688–2699 (2015).
Bortel, E. L., Charbonnier, B. & Heuberger, R. Development of a synthetic synovial fluid for tribological testing. Lubricants 3, 664–686 (2015).
Park, D., Lee, J., Chung, J. J., Jung, Y. & Kim, S. H. Integrating organs-on-chips: multiplexing, scaling, vascularization, and innervation. Trends Biotechnol. 38, 99–112 (2020).
Moraes, C. et al. On being the right size: scaling effects in designing a human-on-a-chip. Integr. Biol. 5, 1149–1161 (2013).
Harink, B., Le Gac, S., Barata, D., van Blitterswijk, C. & Habibovic, P. Microtiter plate-sized standalone chip holder for microenvironmental physiological control in gas-impermeable microfluidic devices. Lab. Chip 14, 1816–1820 (2014).
Palacio-Castaneda, V., Kooijman, L., Venzac, B., Verdurmen, W. P. R. & Le Gac, S. Metabolic switching of tumor cells under hypoxic conditions in a tumor-on-a-chip model. Micromachines 11, 382 (2020).
Sleeboom, J. J. F., Den Toonder, J. M. J. & Sahlgren, C. M. MDA-MB-231 breast cancer cells and their CSC population migrate towards low oxygen in a microfluidic gradient device. Int. J. Mol. Sci. 19, 3047 (2018).
Wilkins, R. J., Browning, J. A. & Ellory, J. C. Surviving in a matrix: membrane transport in articular chondrocytes. J. Membr. Biol. 177, 95–108 (2000).
Hall, A. C., Horwitz, E. R. & Wilkins, R. J. The cellular physiology of articular cartilage. Exp. Physiol. 81, 535–545 (1996).
Arnett, T. R. Extracellular pH regulates bone cell function. J. Nutr. 138, 415S–418S (2008).
Goldie, I. & Nachemson, A. Synovial pH in rheumatoid knee-joints. I. The effect of synovectomy. Acta Orthop. Scand. 40, 634–641 (1969).
Konttinen, Y. T. et al. Acidic cysteine endoproteinase cathepsin K in the degeneration of the superficial articular hyaline cartilage in osteoarthritis. Arthritis Rheum. 46, 953–960 (2002).
Scherer, H. U. & Burmester, G. R. Adaptive immunity in rheumatic diseases: bystander or pathogenic player? Best Pract. Res. Clin. Rheumatol. 25, 785–800 (2011).
Mobasheri, A. et al. Recent advances in understanding the phenotypes of osteoarthritis. F1000Res. 8, 2091 (2019).
Morsink, M. A. J., Willemen, N. G. A., Leijten, J., Bansal, R. & Shin, S. R. Immune organs and immune cells on a chip: an overview of biomedical applications. Micromachines 11, 849 (2020).
Torisawa, Y. S. et al. Modeling hematopoiesis and responses to radiation countermeasures in a bone marrow-on-a-chip. Tissue Eng. Part C. Methods 22, 509–515 (2016).
Bruce, A. et al. Three-dimensional microfluidic tri-culture model of the bone marrow microenvironment for study of acute lymphoblastic leukemia. PLoS ONE 10, e0140506 (2015).
Ramadan, Q. & Ting, F. C. In vitro micro-physiological immune-competent model of the human skin. Lab. Chip 16, 1899–1908 (2016).
Ramadan, Q. et al. NutriChip: nutrition analysis meets microfluidics. Lab. Chip 13, 196–203 (2013).
Mondadori, C. et al. Recapitulating monocyte extravasation to the synovium in an organotypic microfluidic model of the articular joint. Biofabrication 13, 045001 (2021).
Hamza, B. & Irimia, D. Whole blood human neutrophil trafficking in a microfluidic model of infection and inflammation. Lab. Chip 15, 2625–2633 (2015).
Han, S. et al. A versatile assay for monitoring in vivo-like transendothelial migration of neutrophils. Lab. Chip 12, 3861–3865 (2012).
Grässel, S. The role of peripheral nerve fibers and their neurotransmitters in cartilage and bone physiology and pathophysiology. Arthritis Res. Ther. 16, 485 (2014).
Eitner, A., Pester, J., Nietzsche, S., Hofmann, G. O. & Schaible, H. G. The innervation of synovium of human osteoarthritic joints in comparison with normal rat and sheep synovium. Osteoarthritis Cartilage 21, 1383–1391 (2013).
Gribi, S., du Bois de Dunilac, S., Ghezzi, D. & Lacour, S. P. A microfabricated nerve-on-a-chip platform for rapid assessment of neural conduction in explanted peripheral nerve fibers. Nat. Commun. 9, 4403 (2018).
Sharma, A. D. et al. Engineering a 3D functional human peripheral nerve in vitro using the Nerve-on-a-Chip platform. Sci. Rep. 9, 8921 (2019).
Park, S. E. et al. A three-dimensional in vitro model of the peripheral nervous system. NPG Asia Mater. 13, 2 (2021).
Kundu, A. et al. Fabrication and characterization of 3D Printed, 3D microelectrode arrays for interfacing with a peripheral nerve-on-a-chip. ACS Biomater. Sci. Eng. 7, 3018–3029 (2021).
Marzioch, J. et al. On-chip photodynamic therapy — monitoring cell metabolism using electrochemical microsensors. Lab. Chip 18, 3353–3360 (2018).
Rivera, K. R., Yokus, M. A., Erb, P. D., Pozdin, V. A. & Daniele, M. Measuring and regulating oxygen levels in microphysiological systems: design, material, and sensor considerations. Analyst 144, 3190–3215 (2019).
Bonk, S. M. et al. Design and characterization of a sensorized microfluidic cell-culture system with electro-thermal micro-pumps and sensors for cell adhesion, oxygen, and pH on a glass chip. Biosensors 5, 513–536 (2015).
Kieninger, J., Weltin, A., Flamm, H. & Urban, G. A. Microsensor systems for cell metabolism — from 2D culture to organ-on-chip. Lab. Chip 18, 1274–1291 (2018).
Grist, S. M., Chrostowski, L. & Cheung, K. C. Optical oxygen sensors for applications in microfluidic cell culture. Sensors 10, 9286–9316 (2010).
Zhu, J. et al. An integrated adipose-tissue-on-chip nanoplasmonic biosensing platform for investigating obesity-associated inflammation. Lab. Chip 18, 3550–3560 (2018).
Ragab, G., Elshahaly, M. & Bardin, T. Gout: an old disease in new perspective — a review. J. Adv. Res. 8, 495–511 (2017).
Quiros-Solano, W. F. et al. Microfabricated tuneable and transferable porous PDMS membranes for organs-on-chips. Sci. Rep. 8, 13524 (2018).
Becker, H. Mind the gap! Lab. Chip 10, 271–273 (2010).
Berthier, E., Young, E. W. & Beebe, D. Engineers are from PDMS-land, biologists are from polystyrenia. Lab. Chip 12, 1224–1237 (2012).
van Meer, B. J. et al. Small molecule absorption by PDMS in the context of drug response bioassays. Biochem. Biophys. Res. Commun. 482, 323–328 (2017).
Ramadan, Q. & Zourob, M. Organ-on-a-chip engineering: toward bridging the gap between lab and industry. Biomicrofluidics 14, 041501 (2020).
Allwardt, V. et al. Translational roadmap for the organs-on-a-chip industry toward broad adoption. Bioengineering 7, 112 (2020).
Ma, C., Peng, Y., Li, H. & Chen, W. Organ-on-a-chip: a new paradigm for drug development. Trends Pharmacol. Sci. 42, 119–133 (2021).
Zhou, T. et al. Generation of induced pluripotent stem cells from urine. J. Am. Soc. Nephrol. 22, 1221–1228 (2011).
Lozito, T. P. et al. Three-dimensional osteochondral microtissue to model pathogenesis of osteoarthritis. Stem Cell Res. Ther. 4, S6 (2013).
Acknowledgements
The authors acknowledge financial support from the Dutch Arthritis Association (ReumaNetherlands grant LLP-25).
Author information
Authors and Affiliations
Contributions
C.A.P., S.L.G. and M.K. researched data for the article and contributed substantially to discussion of the content. All authors wrote the article, reviewed and/or edited the manuscript before submission and agree on the content of the submitted article.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information
Nature Reviews Rheumatology thanks M. Goldring, A. Mainardi, who co-reviewed with I. Martin, and P. Ertl, for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Mechanical actuation module
-
The part of a microfluidic device that enables repeated (cyclic) application of mechanical loading on the cell-laden 3D hydrogel.
- Microfluidic device
-
Module or system used to precisely control and manipulate fluids in micrometre-sized structures. Microfluidics is at the crossroad of different fields such as engineering, physics, chemistry, nano- and micro-biotechnology.
- Uniaxial loading
-
Mechanical stimulation of the joint in one direction only (for example, compression or stretching). Referred to as uniaxial mechanical actuation when a tissue (cell-laden hydrogel or 3D cell construct) is stimulated in vitro.
- Microfluidic chamber
-
Chamber of a microfluidic device of miniaturized dimensions in the micrometre range that is typically filled with a fluid (liquid or air) or a hydrogel material supplemented with cells.
- Microfluidic motherboard
-
Module for controlling nutrient supply to single-tissue units, which can include analytical modalities, to characterize tissue communication and integrated sensors for real-time monitoring. Individual tissue units could be connected to the motherboard, which thereby provides a standardized connection between units.
- Pumping module
-
In organ-on-chip, a module for pressure or flow control that allows application of constant or cyclic pressure for regulating fluid flow in the nutrient compartment and mechanical actuation.
- Plug-and-play solution
-
System that allows easy addition or removal of a single organ-on-chip unit from the overall joint-on-chip device.
- Sensing units
-
Devices and/or modules for detecting events or changes in the physical environment, such as pressure, temperature, oxygen or biomolecules.
- Microfluidic circuitry
-
Micrometre-sized tubing or channels connecting a series of microfluidic and/or organ-on-chip platforms with each other, and possibly incorporating devices for molecular analysis and biochemical sensing.
- Peltier element
-
A thermoelectric component capable of a temperature shift from one side of the system to the other using electrical energy.
- Electrochemical microsensors
-
Micromachined, micrometer-sized (10−3–10−5 m) sensing structures for detecting and quantifying specific chemical and biochemical substances in fluids, through application of a potential to induce an oxidation or reduction reaction, and recording of a current. Typically fabricated from metal materials.
- Non-fouling coatings
-
Chemical coatings that stop the interactions of molecules in solution with surfaces to notably prevent their non-desired adsorption on the surface.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Paggi, C.A., Teixeira, L.M., Le Gac, S. et al. Joint-on-chip platforms: entering a new era of in vitro models for arthritis. Nat Rev Rheumatol 18, 217–231 (2022). https://doi.org/10.1038/s41584-021-00736-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41584-021-00736-6
This article is cited by
-
Bone marrow lesions: plugging the holes in our knowledge using animal models
Nature Reviews Rheumatology (2023)
-
Progress toward ‘bone-on-a-chip’
Nature Reviews Rheumatology (2023)