Abstract
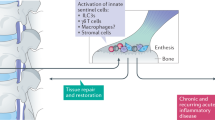
Spondyloarthritis (SpA) is a term that refers to a group of inflammatory diseases that includes psoriatic arthritis, axial SpA and nonradiographic axial SpA, reactive arthritis, enteropathic arthritis and undifferentiated SpA. The disease subtypes share clinical and immunological features, including joint inflammation (peripheral and axial skeleton); skin, gut and eye manifestations; and the absence of diagnostic autoantibodies (seronegative). The diseases also share genetic factors. The aetiology of SpA is still the subject of research by many groups worldwide. Evidence from genetic, experimental and clinical studies has accumulated to indicate a clear role for the IL-17 pathway in the pathogenesis of SpA. The IL-17 family consists of IL-17A, IL-17B, IL-17C, IL-17D, IL-17E and IL-17F, of which IL-17A is the best studied. IL-17A is a pro-inflammatory cytokine that also has the capacity to promote angiogenesis and osteoclastogenesis. Of the six family members, IL-17A has the strongest homology with IL-17F. In this Review, we discuss how IL-17A and IL-17F and their cellular sources might contribute to the immunopathology of SpA.
Key points
-
Genetic and animal model studies indicate that the IL-23–IL-17 axis is involved in the pathogenesis of spondyloarthritis (SpA).
-
IL-17A has been identified directly in the blood and synovial fluid of patients with SpA, with T cells representing a key source of this cytokine.
-
IL-17A and IL-17F act in synergy with other pro-inflammatory mediators to induce pro-inflammatory responses across a range of cell types.
-
IL-23–IL-17-targeted therapies have been shown to be effective in psoriatic arthritis and ankylosing spondylitis.
-
Increased understanding of the pathogenic role of the IL-23–IL-17 axis, the cellular sources of these cytokines and their molecular regulation in SpA is essential to develop novel therapeutic strategies that target this pathway.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Rouvier, E., Luciani, M. F., Mattei, M. G., Denizot, F. & Golstein, P. CTLA-8, cloned from an activated T cell, bearing AU-rich messenger RNA instability sequences, and homologous to a herpesvirus saimiri gene. J. Immunol. 150, 5445–5456 (1993).
Murphy, C. A. et al. Divergent pro-and anti-inflammatory roles for IL-23 and IL-12 in joint autoimmune inflammation. J. Exp. Med. 198, 1951–1958 (2003).
Lubberts, E. The IL-23–IL-17 axis in inflammatory arthritis. Nat. Rev. Rheumatol. 11, 562 (2015).
Korn, T., Bettelli, E., Oukka, M. & Kuchroo, V. K. IL-17 and Th17 Cells. Annu. Rev. Immunol. 27, 485–517 (2009).
Yao, Z. et al. Human IL-17: a novel cytokine derived from T cells. J. Immunol. 155, 5483–5486 (1995).
Kao, C. Y. et al. Up-regulation of CC chemokine ligand 20 expression in human airway epithelium by IL-17 through a JAK-independent but MEK/NF-κB-dependent signaling pathway. J. Immunol. 175, 6676–6685 (2005).
Shahrara, S. et al. IL-17-mediated monocyte migration occurs partially through CC chemokine ligand 2/monocyte chemoattractant protein-1 induction. J. Immunol. 184, 4479–4487 (2010).
Hartupee, J., Liu, C., Novotny, M., Li, X. & Hamilton, T. IL-17 enhances chemokine gene expression through mRNA stabilization. J. Immunol. 179, 4135–4141 (2007).
Schwarzenberger, P. et al. IL-17 stimulates granulopoiesis in mice: use of an alternate, novel gene therapy-derived method for in vivo evaluation of cytokines. J. Immunol. 161, 6383–6389 (1998).
Liang, S. C. et al. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J. Exp. Med. 203, 2271–2279 (2006).
Chabaud, M. et al. Contribution of interleukin 17 to synovium matrix destruction in rheumatoid arthritis. Cytokine 12, 1092–1099 (2000).
Koenders, M. I. et al. Interleukin-17 receptor deficiency results in impaired synovial expression of interleukin-1 and matrix metalloproteinases 3, 9, and 13 and prevents cartilage destruction during chronic reactivated streptococcal cell wall-induced arthritis. Arthritis Rheum. 52, 3239–3247 (2005).
Kotake, S. et al. IL-17 in synovial fluids from patients with rheumatoid arthritis is a potent stimulator of osteoclastogenesis. J. Clin. Invest. 103, 1345–1352 (1999).
Pickens, S. R. et al. IL-17 contributes to angiogenesis in rheumatoid arthritis. J. Immunol. 184, 3233–3241 (2010).
Gullick, N. J. et al. Linking power Doppler ultrasound to the presence of Th17 cells in the rheumatoid arthritis joint. PLOS One 5, e12516 (2010).
Chang, S. H. & Dong, C. A novel heterodimeric cytokine consisting of IL-17 and IL-17F regulates inflammatory responses. Cell Res. 17, 435–440 (2007).
Yang, X. O. et al. Regulation of inflammatory responses by IL-17F. J. Exp. Med. 205, 1063–1075 (2008).
Kawaguchi, M., Adachi, M., Oda, N., Kokubu, F. & Huang, S. K. IL-17 cytokine family. J. Allergy Clin. Immunol. 114, 1265–1273 (2004).
Sorbello, V. et al. Nasal IL-17F is related to bronchial IL-17F/neutrophilia and exacerbations in stable atopic severe asthma. Allergy 70, 236–240 (2015).
Garrod, A. B. The Nature and Treatment of Rheumatic Gout or Chronic Rheumatic Arthritis of all the Joints. (Walton and Maberly, 1857).
Buchanan, W. W. Rheumatoid arthritis: another new world disease? Semin. Arthritis Rheum. 23, 289–294 (1994).
Dörner, T., Egerer, K., Feist, E. & Burmester, G. R. Rheumatoid factor revisited. Curr. Opin. Rheumatol. 16, 246–253 (2004).
Brewerton, D. A. et al. Ankylosing spondylitis and HL-A 27. Lancet 1, 904–907 (1973).
Schlosstein, L., Terasaki, P. I., Bluestone, R. & Pearson, C. M. High association of an HL-A antigen, W27, with ankylosing spondylitis. N. Engl. J. Med. 288, 704–706 (1973).
Moll, J. M. H. & Wright, V. Psoriatic arthritis. Semin. Arthritis Rheum. 3, 55–78 (1973).
Taylor, W. et al. Classification criteria for psoriatic arthritis: development of new criteria from a large international study. Arthritis Rheum. 54, 2665–2673 (2006).
Rudwaleit, M. et al. The Assessment of SpondyloArthritis International Society classification criteria for peripheral spondyloarthritis and for spondyloarthritis in general. Ann. Rheum. Dis. 70, 25–31 (2011).
Baeten, D., Breban, M., Lories, R., Schett, G. & Sieper, J. Are spondylarthritides related but distinct conditions or a single disease with a heterogeneous phenotype? Arthritis Rheum. 65, 12–20 (2013).
Lim, C. S. E., Sengupta, R. & Gaffney, K. The clinical utility of human leucocyte antigen B27 in axial spondyloarthritis. Rheumatology 57, 959–968 (2018).
Cortes, A. et al. Major histocompatibility complex associations of ankylosing spondylitis are complex and involve further epistasis with ERAP1. Nat. Commun. 6, 7146 (2015).
Brewerton, D. A., Caffrey, M., Nicholls, A., Walters, D. & James, D. C. HL-A 27 and arthropathies associated with ulcerative colitis and psoriasis. Lancet 1, 956–958 (1974).
Brown, M. A. et al. HLA class I associations of ankylosing spondylitis in the white population in the United Kingdom. Ann. Rheum. Dis. 55, 268–270 (1996).
Winchester, R. et al. HLA associations reveal genetic heterogeneity in psoriatic arthritis and in the psoriasis phenotype. Arthritis Rheum. 64, 1134–1144 (2012).
Haroon, M., Winchester, R., Giles, J. T., Heffernan, E. & FitzGerald, O. Certain class I HLA alleles and haplotypes implicated in susceptibility play a role in determining specific features of the psoriatic arthritis phenotype. Ann. Rheum. Dis. 75, 155–162 (2016).
Jadon, D. R. et al. Axial Disease in Psoriatic Arthritis study: defining the clinical and radiographic phenotype of psoriatic spondyloarthritis. Ann. Rheum. Dis. 76, 701–707 (2017).
Bowes, J. et al. Cross-phenotype association mapping of the MHC identifies genetic variants that differentiate psoriatic arthritis from psoriasis. Ann. Rheum. Dis. 76, 1774–1779 (2017).
Winchester, R. et al. Implications of the diversity of class I HLA associations in psoriatic arthritis. Clin. Immunol. 172, 29–33 (2016).
Fiorillo, M. T., Maragno, M., Butler, R., Dupuis, M. L. & Sorrentino, R. CD8+ T cell autoreactivity to an HLA-B27-restricted self-epitope correlates with ankylosing spondylitis. J. Clin. Invest. 106, 47–53 (2000).
Allen, R. L., O’Callaghan, C. A., McMichael, A. J. & Bowness, P. Cutting edge: HLA-B27 can form a novel β2-microglobulin-free heavy chain homodimer structure. J. Immunol. 162, 5045–5048 (1999).
DeLay, M. L. et al. HLA-B27 misfolding and the unfolded protein response augment interleukin-23 production and are associated with Th17 activation in transgenic rats. Arthritis Rheum. 60, 2633–2643 (2009).
Colbert, R. A., DeLay, M. L., Klenk, E. I. & Layh-Schmitt, G. From HLA-B27 to spondyloarthritis: a journey through the ER. Immunol. Rev. 233, 181–202 (2010).
Bowness, P. et al. Th17 cells expressing KIR3DL2+ and responsive to HLA-B27 homodimers are increased in ankylosing spondylitis. J. Immunol. 186, 2672–2680 (2011).
Saric, T. et al. An IFN-γ-induced aminopeptidase in the ER, ERAP1, trims precursors to MHC class I-presented peptides. Nat. Immunol. 3, 1169–1176 (2002).
York, I. A. et al. The ER aminopeptidase ERAP1 enhances or limits antigen presentation by trimming epitopes to 8–9 residues. Nat. Immunol. 3, 1177–1184 (2002).
Wellcome Trust Case Control Consortium et al. Association scan of 14,500 nonsynonymous SNPs in four diseases identifies autoimmunity variants. Nat. Genet. 39, 1329–1337 (2007).
Woolf, E. et al. Runx3 and Runx1 are required for CD8 T cell development during thymopoiesis. Proc. Natl Acad. Sci. USA 100, 7731–7736 (2003).
Cruz-Guilloty, F. et al. Runx3 and T-box proteins cooperate to establish the transcriptional program of effector CTLs. J. Exp. Med. 206, 51–59 (2009).
Shan, Q. et al. The transcription factor Runx3 guards cytotoxic CD8+ effector T cells against deviation towards follicular helper T cell lineage. Nat. Immunol. 18, 931 (2017).
Evans, D. M. et al. Interaction between ERAP1 and HLA-B27 in ankylosing spondylitis implicates peptide handling in the mechanism for HLA-B27 in disease susceptibility. Nat. Genet. 43, 761 (2011).
Tsoi, L. C. et al. Identification of 15 new psoriasis susceptibility loci highlights the role of innate immunity. Nat. Genet. 44, 1341–1348 (2012).
Apel, M. et al. Variants in RUNX3 contribute to susceptibility to psoriatic arthritis, exhibiting further common ground with ankylosing spondylitis. Arthritis Rheum. 65, 1224–1231 (2013).
Bowes, J. et al. Dense genotyping of immune-related susceptibility loci reveals new insights into the genetics of psoriatic arthritis. Nat. Commun. 6, 6046 (2015).
Li, Z. et al. Epigenetic and gene expression analysis of ankylosing spondylitis-associated loci implicate immune cells and the gut in the disease pathogenesis. Genes Immun. 18, 135–143 (2017).
Ferreira, M. A. et al. Quantitative trait loci for CD4:CD8 lymphocyte ratio are associated with risk of type 1 diabetes and HIV-1 immune control. Am. J. Hum. Genet. 86, 88–92 (2010).
Vecellio, M. et al. The genetic association of RUNX3 with ankylosing spondylitis can be explained by allele-specific effects on IRF4 recruitment that alter gene expression. Ann. Rheumat. Diseases 75, 1534–1540 (2016).
International Genetics of Ankylosing Spondylitis Consortium et al. Identification of multiple risk variants for ankylosing spondylitis through high-density genotyping of immune-related loci. Nat. Genet. 45, 730 (2013).
Filer, C. et al. Investigation of association of the IL12B and IL23R genes with psoriatic arthritis. Arthritis Rheum. 58, 3705–3709 (2008).
Bowes, J. et al. Confirmation of TNIP1 and IL23A as susceptibility loci for psoriatic arthritis. Ann. Rheumat. Diseases 70, 1641–1644 (2011).
Jostins, L. et al. Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature 491, 119–124 (2012).
Coffre, M. et al. Combinatorial control of Th17 and Th1 cell functions by genetic variations in genes associated with the interleukin-23 signaling pathway in spondyloarthritis. Arthritis Rheum. 65, 1510–1521 (2013).
McGonagle, D., Aydin, S. Z., Gul, A., Mahr, A. & Direskeneli, H. ‘MHC-I-opathy’-unified concept for spondyloarthritis and Behcet disease. Nat. Rev. Rheumatol 11, 731–740 (2015).
Qian, Y. et al. The adaptor Act1 is required for interleukin 17-dependent signaling associated with autoimmune and inflammatory disease. Nat. Immunol. 8, 247–256 (2007).
Danoy, P. et al. Association of variants at 1q32 and STAT3 with ankylosing spondylitis suggests genetic overlap with Crohn’s disease. PLOS Genet. 6, e1001195 (2010).
Davidson, S. I. et al. Association of STAT3 and TNFRSF1A with ankylosing spondylitis in Han Chinese. Ann. Rheum. Dis. 70, 289–292 (2011).
Cenit, M. C. et al. Influence of the STAT3 genetic variants in the susceptibility to psoriatic arthritis and Behcet’s disease. Hum. Immunol. 74, 230–233 (2013).
Harris, T. J. et al. Cutting edge: an in vivo requirement for STAT3 signaling in TH17 development and TH17-dependent autoimmunity. J. Immunol. 179, 4313–4317 (2007).
de Beaucoudrey, L. et al. Mutations in STAT3 and IL12RB1 impair the development of human IL-17-producing T cells. J. Exp. Med. 205, 1543–1550 (2008).
Nair, R. P. et al. Genome-wide scan reveals association of psoriasis with IL-23 and NF-κB pathways. Nat. Genet. 41, 199–204 (2009).
Ellinghaus, D. et al. Analysis of five chronic inflammatory diseases identifies 27 new associations and highlights disease-specific patterns at shared loci. Nat. Genet. 48, 510–518 (2016).
Garg, A. V., Ahmed, M., Vallejo, A. N., Ma, A. & Gaffen, S. L. The deubiquitinase A20 mediates feedback inhibition of interleukin-17 receptor signaling. Sci. Signal. 6, ra44 (2013).
Ippagunta, S. K. et al. Keratinocytes contribute intrinsically to psoriasis upon loss of Tnip1 function. Proc. Natl Acad. Sci. USA 113, E6162–E6171 (2016).
Billingham, M. E. J. Models of arthritis and the search for anti-arthritic drugs. Pharmacol. Ther. 21, 389–428 (1983).
Bush, K. A., Farmer, K. M., Walker, J. S. & Kirkham, B. W. Reduction of joint inflammation and bone erosion in rat adjuvant arthritis by treatment with interleukin-17 receptor IgG1 Fc fusion protein. Arthritis Rheumatol. 46, 802–805 (2002).
Nakae, S., Nambu, A., Sudo, K. & Iwakura, Y. Suppression of immune induction of collagen-induced arthritis in IL-17-deficient mice. J. Immunol. 171, 6173–6177 (2003).
Corneth, O. B. et al. Absence of interleukin-17 receptor a signaling prevents autoimmune inflammation of the joint and leads to a Th2-like phenotype in collagen-induced arthritis. Arthritis Rheum. 66, 340–349 (2014).
Lubberts, E., Koenders, M. & van den Berg, W. The role of T cell interleukin-17 in conducting destructive arthritis: lessons from animal models. Arthritis Res. Ther. 7, 29–37 (2005).
Lubberts, E. et al. Overexpression of IL-17 in the knee joint of collagen type II immunized mice promotes collagen arthritis and aggravates joint destruction. Inflamm. Res. 51, 102–104 (2002).
Koenders, M. I. et al. Blocking of interleukin-17 during reactivation of experimental arthritis prevents joint inflammation and bone erosion by decreasing RANKL and interleukin-1. Am J. Pathol. 167, 141–149 (2005).
Vieira-Sousa, E., van Duivenvoorde, L. M., Fonseca, J. E., Lories, R. J. & Baeten, D. L. Review: animal models as a tool to dissect pivotal pathways driving spondyloarthritis. Arthritis Rheumatol. 67, 2813–2827 (2015).
Glatigny, S. et al. Proinflammatory Th17 cells are expanded and induced by dendritic cells in spondylarthritis-prone HLA-B27-transgenic rats. Arthritis Rheum. 64, 110–120 (2012).
May, E. et al. CD8 αβ T cells are not essential to the pathogenesis of arthritis or colitis in HLA-B27 transgenic rats. J. Immunol. 170, 1099–1105 (2003).
Taurog, J. D. et al. Spondylarthritis in HLA-B27/human β2-microglobulin-transgenic rats is not prevented by lack of CD8. Arthritis Rheum. 60, 1977–1984 (2009).
Sakaguchi, N. et al. Altered thymic T cell selection due to a mutation of the ZAP-70 gene causes autoimmune arthritis in mice. Nature 426, 454–460 (2003).
Ruutu, M. et al. β-glucan triggers spondylarthritis and Crohn’s disease-like ileitis in SKG mice. Arthritis Rheum. 64, 2211–2222 (2012).
Benham, H. et al. Interleukin-23 mediates the intestinal response to microbial β-1,3-glucan and the development of spondyloarthritis pathology in SKG mice. Arthritis Rheumatol 66, 1755–1767 (2014).
Gillet, P. et al. Studies on type II collagen induced arthritis in rats: an experimental model of peripheral and axial ossifying enthesopathy. J. Rheumatol 16, 721–728 (1989).
Sherlock, J. P. et al. IL-23 induces spondyloarthropathy by acting on ROR-γt+ CD3+CD4–CD8– entheseal resident T cells. Nat. Med. 18, 1069–1076 (2012).
Abe, Y. et al. Ankylosing enthesitis associated with up-regulated IFN-γ and IL-17 production in (BXSB x NZB) F1 male mice: a new mouse model. Mod. Rheumatol 19, 316–322 (2009).
Ebihara, S., Date, F., Dong, Y. & Ono, M. Interleukin-17 is a critical target for the treatment of ankylosing enthesitis and psoriasis-like dermatitis in mice. Autoimmunity 48, 259–266 (2015).
Chabaud, M. et al. Human interleukin-17: a T cell-derived proinflammatory cytokine produced by the rheumatoid synovium. Arthritis Rheum. 42, 963–970 (1999).
Leipe, J. et al. Role of Th17 cells in human autoimmune arthritis. Arthritis Rheum. 62, 2876–2885 (2010).
Chen, D.-Y. et al. Increasing levels of circulating Th17 cells and interleukin-17 in rheumatoid arthritis patients with an inadequate response to anti-TNF-α therapy. Arthritis Res. Ther. 13, R126 (2011).
Metawi, S., Abbas, D., Kamal, M. & Ibrahim, M. Serum and synovial fluid levels of interleukin-17 in correlation with disease activity in patients with RA. Clin. Rheumatol. 30, 1201–1207 (2011).
Gullick, N. J. et al. Enhanced and persistent levels of IL-17+CD4+ T cells and serum IL-17 in patients with early inflammatory arthritis. Clin. Exp. Immunol. 174, 292–301 (2013).
Raza, K. et al. Early rheumatoid arthritis is characterized by a distinct and transient synovial fluid cytokine profile of T cell and stromal cell origin. Arthritis Res. Ther. 7, 784–795 (2005).
Wendling, D., Cedoz, J.-P., Racadot, E. & Dumoulin, G. Serum IL-17, BMP-7, and bone turnover markers in patients with ankylosing spondylitis. Joint Bone Spine 74, 304–305 (2007).
Romero-Sanchez, C. et al. Association between Th-17 cytokine profile and clinical features in patients with spondyloarthritis. Clin. Exp. Rheumatol. 29, 828–834 (2011).
Chen, W.-S. et al. Association of serum interleukin-17 and interleukin-23 levels with disease activity in Chinese patients with ankylosing spondylitis. J. Chinese Med. Associ. 75, 303–308 (2012).
Xueyi, L. et al. Levels of circulating Th17 cells and regulatory T cells in ankylosing spondylitis patients with an inadequate response to anti-TNF-α therapy. J. Clin. Immunol. 33, 151–161 (2013).
Singh, R., Aggarwal, A. & Misra, R. Th1/Th17 cytokine profiles in patients with reactive arthritis/undifferentiated spondyloarthropathy. J. Rheumatol 34, 2285–2290 (2007).
Raychaudhuri, S. P., Raychaudhuri, S. K. & Genovese, M. C. IL-17 receptor and its functional significance in psoriatic arthritis. Mol. Cell. Biochem. 359, 419–429 (2012).
Ciccia, F. et al. Overexpression of interleukin-23, but not interleukin-17, as an immunologic signature of subclinical intestinal inflammation in ankylosing spondylitis. Arthritis Rheum. 60, 955–965 (2009).
Zrioual, S. et al. Genome-wide comparison between IL-17A- and IL-17F-induced effects in human rheumatoid arthritis synoviocytes. J. Immunol. 182, 3112–3120 (2009).
Jain, M. et al. Increased plasma IL-17F levels in rheumatoid arthritis patients are responsive to methotrexate, anti-TNF, and T cell costimulatory modulation. Inflammation 38, 180–186 (2015).
Sarkar, S. et al. Interleukin (IL)-17A, F and AF in inflammation: a study in collagen-induced arthritis and rheumatoid arthritis. Clin. Exp. Immunol. 177, 652–661 (2014).
van Baarsen, L. et al. Heterogeneous expression pattern of interleukin-17A (IL-17A), IL-17F and their receptors in synovium of rheumatoid arthritis, psoriatic arthritis and osteoarthritis: possible explanation for non-response to anti-IL-17 therapy? Arthritis Res. Ther. 16, 426 (2014).
Glatt, S. et al. Dual IL-17A and IL-17F neutralisation by bimekizumab in psoriatic arthritis: evidence from preclinical experiments and a randomised placebo-controlled clinical trial that IL-17F contributes to human chronic tissue inflammation. Ann. Rheum. Dis. 77, 523–532 (2017).
Fossiez, F. et al. T cell interleukin-17 induces stromal cells to produce proinflammatory and hematopoietic cytokines. J. Exp. Med. 183, 2593–2603 (1996).
Zrioual, S. et al. IL-17RA and IL-17RC receptors are essential for IL-17A-induced ELR+ CXC chemokine expression in synoviocytes and are overexpressed in rheumatoid blood. J. Immunol. 180, 655–663 (2008).
McAllister, F. et al. Role of IL-17A, IL-17F, and the IL-17 receptor in regulating growth-related oncogene-alpha and granulocyte colony-stimulating factor in bronchial epithelium: implications for airway inflammation in cystic fibrosis. J. Immunol. 175, 404–412 (2005).
Iyoda, M. et al. IL-17A and IL-17F stimulate chemokines via MAPK pathways (ERK1/2 and p38 but not JNK) in mouse cultured mesangial cells: synergy with TNF-α and IL-1β. Am. J. Physiol. Renal Physiol. 298, F779–F787 (2010).
Koenders, M. I. et al. TNF / IL-17 interplay induces S100A8, IL-1β, and MMPs, and drives irreversible cartilage destruction in vivo: rationale for combination treatment during arthritis. Arthritis Rheum. 63, 2329–2339 (2011).
van Lent, P. L. et al. Myeloid-related proteins S100A8/S100A9 regulate joint inflammation and cartilage destruction during antigen-induced arthritis. Ann. Rheum. Dis. 67, 1750–1758 (2008).
Hot, A., Zrioual, S., Lenief, V. & Miossec, P. IL-17 and tumour necrosis factor α combination induces a HIF-1α-dependent invasive phenotype in synoviocytes. Ann. Rheum. Dis. 71, 1393–1401 (2012).
Kirkham, B. W. et al. Synovial membrane cytokine expression is predictive of joint damage progression in rheumatoid arthritis: A two-year prospective study (the DAMAGE study cohort). Arthritis Rheum. 54, 1122–1131 (2006).
Chabaud, M. & Miossec, P. The combination of tumor necrosis factor α blockade with interleukin-1 and interleukin-17 blockade is more effective for controlling synovial inflammation and bone resorption in an ex vivo model. Arthritis Rheum. 44, 1293–1303 (2001).
Fischer, J. A. et al. Combined inhibition of tumor necrosis factor α and interleukin-17 as a therapeutic opportunity in rheumatoid arthritis: development and characterization of a novel bispecific antibody. Arthritis Rheumatol. 67, 51–62 (2015).
Magrey, M. N. & Khan, M. A. The paradox of bone formation and bone loss in ankylosing spondylitis: evolving new concepts of bone formation and future trends in management. Curr. Rheumatol. Rep. 19, 17 (2017).
Osta, B., Lavocat, F., Eljaafari, A. & Miossec, P. Effects of interleukin-17A on osteogenic differentiation of isolated human mesenchymal stem cells. Front. Immunol. 5, 425 (2014).
Chabaud, M., Fossiez, F., Taupin, J.-L. & Miossec, P. Enhancing effect of IL-17 on IL-1-induced IL-6 and leukemia inhibitory factor production by rheumatoid arthritis synoviocytes and its regulation by Th2 cytokines. J. Immunol. 161, 409–414 (1998).
Kawashiri, S. Y. et al. Proinflammatory cytokines synergistically enhance the production of chemokine ligand 20 (CCL20) from rheumatoid fibroblast-like synovial cells in vitro and serum CCL20 is reduced in vivo by biologic disease-modifying antirheumatic drugs. J. Rheumatol 36, 2397–2402 (2009).
Zhang, Y. et al. Synergistic effects of interleukin-1β and interleukin-17A antibodies on collagen-induced arthritis mouse model. Int. Immunopharmacol. 15, 199–205 (2013).
Qi, J. et al. A bispecific antibody against IL-1β and IL-17A is beneficial for experimental rheumatoid arthritis. Int. Immunopharmacol. 14, 770–778 (2012).
Teunissen, M. B. M., Bos, J. D., Koomen, C. W., de Waal Malefyt, R. & Wierenga, E. A. Interleukin-17 and interferon-γ synergize in the enhancement of proinflammatory cytokine production by human keratinocytes. J. Invest. Dermatol. 111, 645–649 (1998).
Wedebye Schmidt, E. G. et al. TH17 cell induction and effects of IL-17A and IL-17F blockade in experimental colitis. Inflamm. Bowel Dis. 19, 1567–1576 (2013).
Henness, S. et al. IL-17A acts via p38 MAPK to increase stability of TNF-α-induced IL-8 mRNA in human ASM. Am. J. Physiol. Lung Cell. Mol. Physiol. 290, L1283–L1290 (2006).
Friday, S. C. & Fox, D. A. Phospholipase D enzymes facilitate IL-17- and TNFα-induced expression of proinflammatory genes in rheumatoid arthritis synovial fibroblasts (RASF). Immunol. Lett. 174, 9–18 (2016).
Srenathan, U., Steel, K. & Taams, L. S. IL-17+ CD8+ T cells: differentiation, phenotype and role in inflammatory disease. Immunol. Lett. 178, 20–26 (2016).
Papotto, P. H., Ribot, J. C. & Silva-Santos, B. IL-17+ γδ T cells as kick-starters of inflammation. Nat. Immunol. 18, 604–611 (2017).
Hazenberg, M. D. & Spits, H. Human innate lymphoid cells. Blood 124, 700–709 (2014).
Aarvak, T., Chabaud, M., Miossec, P. & Natvig, J. B. IL-17 is produced by some proinflammatory Th1/Th0 cells but not by Th2 cells. J. Immunol. 162, 1246–1251 (1999).
Nistala, K. et al. Interleukin-17-producing T cells are enriched in the joints of children with arthritis, but have a reciprocal relationship to regulatory T cell numbers. Arthritis Rheum. 58, 875–887 (2008).
Cosmi, L. et al. Evidence of the transient nature of the Th17 phenotype of CD4+CD161+ T cells in the synovial fluid of patients with juvenile idiopathic arthritis. Arthritis Rheum. 63, 2504–2515 (2011).
Menon, B. et al. IL-17+CD8+ T cells are enriched in the joints of patients with psoriatic arthritis and correlate with disease activity and joint damage progression. Arthritis Rheumatol. 66, 1272–1281 (2014).
Jandus, C. et al. Increased numbers of circulating polyfunctional Th17 memory cells in patients with seronegative spondylarthritides. Arthritis Rheum. 58, 2307–2317 (2008).
Shen, H., Goodall, J. C. & Gaston, J. S. H. Frequency and phenotype of peripheral blood Th17 cells in ankylosing spondylitis and rheumatoid arthritis. Arthritis Rheum. 60, 1647–1656 (2009).
Shen, H., Goodall, J. C. & Gaston, J. S. Frequency and phenotype of T helper 17 cells in peripheral blood and synovial fluid of patients with reactive arthritis. J. Rheumatol. 37, 2096–2099 (2010).
Al-Mossawi, M. H. et al. Unique transcriptome signatures and GM-CSF expression in lymphocytes from patients with spondyloarthritis. Nat. Commun. 8, 1510 (2017).
Benham, H. et al. Th17 and Th22 cells in psoriatic arthritis and psoriasis. Arthritis Res. Ther. 15, R136 (2013).
Zizzo, G. et al. Synovial fluid-derived T helper 17 cells correlate with inflammatory activity in arthritis, irrespectively of diagnosis. Clin. Immunol. 138, 107–116 (2011).
Tzartos, J. S. et al. Interleukin-17 production in central nervous system-infiltrating T cells and glial cells is associated with active disease in multiple sclerosis. Am. J. Pathol. 172, 146–155 (2008).
Ortega, C. et al. IL-17-producing CD8+ T lymphocytes from psoriasis skin plaques are cytotoxic effector cells that secrete Th17-related cytokines. J. Leukocyte Biol. 86, 435–443 (2009).
Res, P. C. M. et al. Overrepresentation of IL-17A and IL-22 producing CD8 T cells in lesional skin suggests their involvement in the pathogenesis of psoriasis. PLOS One 5, e14108 (2010).
Hijnen, D. et al. CD8+ T cells in the lesional skin of atopic dermatitis and psoriasis patients are an important source of IFN-γ, IL-13, IL-17, and IL-22. J. Invest. Dermatol. 133, 973–979 (2013).
Di Meglio, P. et al. Targeting CD8+ T cells prevents psoriasis development. J. Allergy Clin. Immunol. 138, 274–276 (2016).
Wang, C., Liao, Q., Hu, Y. & Zhong, D. T lymphocyte subset imbalances in patients contribute to ankylosing spondylitis. Exp. Ther. Med. 9, 250–256 (2015).
Steel, K. J. et al. Synovial IL-17+CD8+ T cells are a pro-inflammatory tissue resident population enriched in spondyloarthritis [abstract]. Ann. Rheum. Dis. 77 (Suppl. 1), 0016 (2018).
Sathaliyawala, T. et al. Distribution and compartmentalization of human circulating and tissue-resident memory T cell subsets. Immunity 38, 187–197 (2013).
Iijima, N. & Iwasaki, A. Tissue instruction for migration and retention of TRM cells. Trends Immunol. 36, 556–564 (2015).
Masopust, D., Vezys, V., Marzo, A. L. & Lefrancois, L. Preferential localization of effector memory cells in nonlymphoid tissue. Science 291, 2413–2417 (2001).
Watanabe, R. et al. Human skin is protected by four functionally and phenotypically discrete populations of resident and recirculating memory T cells. Sci. Transl Med. 7, 279ra239 (2015).
Cheuk, S. et al. CD49a expression defines tissue-resident CD8+ T cells poised for cytotoxic function in human skin. Immunity 46, 287–300 (2017).
Kumar, B. V. et al. Human tissue-resident memory T cells are defined by core transcriptional and functional signatures in lymphoid and mucosal sites. Cell Rep. 20, 2921–2934 (2017).
Clark, R. A. Resident memory T cells in human health and disease. Sci. Transl Med. 7, 269rv261 (2015).
Milner, J. J. et al. Runx3 programs CD8+ T cell residency in non-lymphoid tissues and tumours. Nature 552, 253 (2017).
Cheuk, S. et al. Epidermal Th22 and Tc17 cells form a localized disease memory in clinically healed psoriasis. J. Immunol. 192, 3111–3120 (2014).
Clark, R. A. et al. The vast majority of CLA+ T cells are resident in normal skin. J. Immunol. 176, 4431–4439 (2006).
Petrelli, A. & van Wijk, F. CD8+ T cells in human autoimmune arthritis: the unusual suspects. Nat. Rev. Rheumatol 12, 421–428 (2016).
Porcelli, S., Yockey, C. E., Brenner, M. B. & Balk, S. P. Analysis of T cell antigen receptor (TCR) expression by human peripheral blood CD4-8- α/β T cells demonstrates preferential use of several V β genes and an invariant TCR α chain. J. Exp. Med. 178, 1–16 (1993).
Martin, E. et al. Stepwise development of MAIT cells in mouse and human. PLOS Biol. 7, e54 (2009).
Le Bourhis, L. et al. Antimicrobial activity of mucosal-associated invariant T cells. Nat. Immunol. 11, 701–708 (2010).
Dusseaux, M. et al. Human MAIT cells are xenobiotic-resistant, tissue-targeted, CD161hi IL-17-secreting T cells. Blood 117, 1250–1259 (2011).
Teunissen, M. B. M. et al. The IL-17A-producing CD8+ T-cell population in psoriatic lesional skin comprises mucosa-associated invariant T cells and conventional T cells. J. Invest. Dermatol. 134, 2898–2907 (2014).
Hayashi, E. et al. Involvement of mucosal-associated invariant T cells in ankylosing spondylitis. J. Rheumatol. 43, 1695–1703 (2016).
Gracey, E. et al. IL-7 primes IL-17 in mucosal-associated invariant T (MAIT) cells, which contribute to the Th17-axis in ankylosing spondylitis. Ann. Rheum. Dis. 75, 2124–2132 (2016).
Yoshiga, Y. et al. Invariant NKT cells produce IL-17 through IL-23-dependent and -independent pathways with potential modulation of Th17 response in collagen-induced arthritis. Int. J. Mol. Med. 22, 369–374 (2008).
Laggner, U. et al. Identification of a novel proinflammatory human skin-homing Vγ9Vδ2 T cell subset with a potential role in psoriasis. J. Immunol. 187, 2783–2793 (2011).
Kenna, T. J. et al. Enrichment of circulating interleukin-17-secreting interleukin-23 receptor-positive γ/δ T cells in patients with active ankylosing spondylitis. Arthritis Rheum. 64, 1420–1429 (2012).
Gaur, P., Misra, R. & Aggarwal, A. Natural killer cell and γδ T cell alterations in enthesitis related arthritis category of juvenile idiopathic arthritis. Clin. Immunol. 161, 163–169 (2015).
Guggino, G. et al. Interleukin (IL)-9/IL-9R axis drives γδ T cells activation in psoriatic arthritis patients. Clin. Exp. Immunol. 186, 277–283 (2016).
Chowdhury, A. C., Chaurasia, S., Mishra, S. K., Aggarwal, A. & Misra, R. IL-17 and IFN-γ producing NK and γδ-T cells are preferentially expanded in synovial fluid of patients with reactive arthritis and undifferentiated spondyloarthritis. Clin. Immunol. 183, 207–212 (2017).
Cai, Y. et al. Pivotal role of dermal IL-17-producing γδ T cells in skin inflammation. Immunity 35, 596–610 (2011).
Gray, E. E., Suzuki, K. & Cyster, J. G. Cutting edge: identification of a motile IL-17-producing γδ T cell population in the dermis. J. Immunol. 186, 6091–6095 (2011).
Campbell, J. J. et al. IL-17-secreting γδ T cells are completely dependent upon CCR6 for homing to inflamed skin. J. Immunol. 199, 3129–3136 (2017).
Reinhardt, A. et al. Interleukin-23-dependent γ/δ T cells produce interleukin-17 and accumulate in the enthesis, aortic valve, and ciliary body in mice. Arthritis Rheumatol. 68, 2476–2486 (2016).
Soare, A. et al. Cutting edge: homeostasis of innate lymphoid cells is imbalanced in psoriatic arthritis. J. Immunol. 200, 1249–1254 (2018).
Leijten, E. F. et al. Brief report: enrichment of activated group 3 innate lymphoid cells in psoriatic arthritis synovial fluid. Arthritis Rheumatol. 67, 2673–2678 (2015).
Noordenbos, T. et al. Interleukin-17–positive mast cells contribute to synovial inflammation in spondylarthritis. Arthritis Rheum. 64, 99–109 (2012).
Noordenbos, T. et al. Human mast cells capture, store, and release bioactive, exogenous IL-17A. J. Leukoc. Biol. 100, 453–462 (2016).
Schett, G., Elewaut, D., McInnes, I. B., Dayer, J.-M. & Neurath, M. F. How cytokine networks fuel inflammation: toward a cytokine-based disease taxonomy. Nat. Med. 19, 822–824 (2013).
Frieder, J., Kivelevitch, D., Haugh, I., Watson, I. & Menter, A. Anti-IL-23 and anti-IL-17 Biologic agents for the treatment of immune-mediated inflammatory conditions. Clin. Pharmacol. Ther. 103, 88–101 (2018).
Griffiths, C. E. et al. Comparison of ustekinumab and etanercept for moderate-to-severe psoriasis. N. Engl. J. Med. 362, 118–128 (2010).
Leonardi, C. L. et al. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 76-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 1). Lancet 371, 1665–1674 (2008).
Papp, K. A. et al. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 52-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 2). Lancet 371, 1675–1684 (2008).
Ritchlin, C. et al. Efficacy and safety of the anti-IL-12/23 p40 monoclonal antibody, ustekinumab, in patients with active psoriatic arthritis despite conventional non-biological and biological anti-tumour necrosis factor therapy: 6-month and 1-year results of the phase 3, multicentre, double-blind, placebo-controlled, randomised PSUMMIT 2 trial. Ann. Rheum. Dis. 73, 990–999 (2014).
McInnes, I. B. et al. Efficacy and safety of ustekinumab in patients with active psoriatic arthritis: 1 year results of the phase 3, multicentre, double-blind, placebo-controlled PSUMMIT 1 trial. Lancet 382, 780–789 (2013).
Sandborn, W. J. et al. Ustekinumab induction and maintenance therapy in refractory Crohn’s disease. N. Engl. J. Med. 367, 1519–1528 (2012).
Hueber, W. et al. Effects of AIN457, a fully human antibody to interleukin-17A, on psoriasis, rheumatoid arthritis, and uveitis. Sci. Transl Med. 2, 52ra72 (2010).
Langley, R. G. et al. Secukinumab in plaque psoriasis—results of two phase 3 trials. N. Engl. J. Med. 371, 326–338 (2014).
Sanford, M. & McKeage, K. Secukinumab: first global approval. Drugs 75, 329–338 (2015).
Griffiths, C. E. M. et al. Comparison of ixekizumab with etanercept or placebo in moderate-to-severe psoriasis (UNCOVER-2 and UNCOVER-3): results from two phase 3 randomised trials. Lancet 386, 541–551 (2015).
Blauvelt, A. et al. Secukinumab is superior to ustekinumab in clearing skin of subjects with moderate-to-severe plaque psoriasis up to 1 year: results from the CLEAR study. J. Am. Acad. Dermatol. 76, 60–69 (2017).
Gordon, K. B. et al. Phase 3 trials of ixekizumab in moderate-to-severe plaque psoriasis. N. Engl. J. Med. 375, 345–356 (2016).
Mease, P. J. et al. Secukinumab inhibition of interleukin-17A in patients with psoriatic arthritis. N. Engl. J. Med. 373, 1329–1339 (2015).
McInnes, I. B. et al. Secukinumab, a human anti-interleukin-17A monoclonal antibody, in patients with psoriatic arthritis (FUTURE 2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 386, 1137–1146 (2015).
Baeten, D. et al. Secukinumab, an interleukin-17A inhibitor, in ankylosing spondylitis. N. Engl. J. Med. 373, 2534–2548 (2015).
van de Kerkhof, P. C. M. et al. Secukinumab long-term safety experience: A pooled analysis of 10 phase II and III clinical studies in patients with moderate to severe plaque psoriasis. J. Am. Acad. Dermatol. 75, 83–98 (2016).
Mease, P. J. et al. Ixekizumab, an interleukin-17A specific monoclonal antibody, for the treatment of biologic-naive patients with active psoriatic arthritis: results from the 24-week randomised, double-blind, placebo-controlled and active (adalimumab)-controlled period of the phase III trial SPIRIT-P1. Ann. Rheum. Dis. 76, 79–87 (2017).
Nash, P. et al. Ixekizumab for the treatment of patients with active psoriatic arthritis and an inadequate response to tumour necrosis factor inhibitors: results from the 24-week randomised, double-blind, placebo-controlled period of the SPIRIT-P2 phase 3 trial. Lancet 389, 2317–2327 (2017).
Dick, A. D. et al. Secukinumab in the treatment of noninfectious uveitis: results of three randomized, controlled clinical trials. Ophthalmology 120, 777–787 (2013).
Hueber, W. et al. Secukinumab, a human anti-IL-17A monoclonal antibody, for moderate to severe Crohn’s disease: unexpected results of a randomised, double-blind placebo-controlled trial. Gut 61, 1693–1700 (2012).
Mease, P. J. et al. Brodalumab, an anti-IL17RA monoclonal antibody, in psoriatic arthritis. N. Engl. J. Med. 370, 2295–2306 (2014).
Papp, K. A. et al. A prospective phase III, randomized, double-blind, placebo-controlled study of brodalumab in patients with moderate-to-severe plaque psoriasis. Br. J. Dermatol. 175, 273–286 (2016).
Lebwohl, M. et al. Phase 3 studies comparing brodalumab with ustekinumab in psoriasis. N. Engl. J. Med. 373, 1318–1328 (2015).
Targan, S. R. et al. A randomized, double-blind, placebo-controlled phase 2 study of brodalumab in patients with moderate-to-severe Crohn’s disease. Am. J. Gastroenterol. 111, 1599–1607 (2016).
Blauvelt, A. et al. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the continuous treatment of patients with moderate to severe psoriasis: results from the phase III, double-blinded, placebo- and active comparator-controlled VOYAGE 1 trial. J. Am. Acad. Dermatol. 76, 405–417 (2017).
Reich, K. et al. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the treatment of patients with moderate to severe psoriasis with randomized withdrawal and retreatment: results from the phase III, double-blind, placebo- and active comparator-controlled VOYAGE 2 trial. J. Am. Acad. Dermatol. 76, 418–431 (2017).
Deodhar, A. et al. OP0218 Efficacy and safety results of guselkumab, an anti-il23 monoclonal antibody, in patients with active psoriatic arthritis over 24 weeks: a phase 2a, randomized, double-blind, placebo-controlled study. Ann. Rheum. Dis. 76, 142–143 (2017).
Smolen, J. S. et al. A randomised phase II study evaluating the efficacy and safety of subcutaneously administered ustekinumab and guselkumab in patients with active rheumatoid arthritis despite treatment with methotrexate. Ann. Rheum. Dis. 76, 831–839 (2017).
Feagan, B. G. et al. Induction therapy with the selective interleukin-23 inhibitor risankizumab in patients with moderate-to-severe Crohn’s disease: a randomised, double-blind, placebo-controlled phase 2 study. Lancet 389, 1699–1709 (2017).
Papp, K. A. et al. Risankizumab versus ustekinumab for moderate-to-severe plaque psoriasis. N. Engl. J. Med. 376, 1551–1560 (2017).
Reich, K. et al. Tildrakizumab versus placebo or etanercept for chronic plaque psoriasis (reSURFACE 1 and reSURFACE 2): results from two randomised controlled, phase 3 trials. Lancet 390, 276–288 (2017).
Belasco, J. et al. Comparative genomic profiling of synovium versus skin lesions in psoriatic arthritis. Arthritis Rheumatol. 67, 934–944 (2015).
Yao, Z. et al. Herpesvirus Saimiri encodes a new cytokine, IL-17, which binds to a novel cytokine receptor. Immunity 3, 811–821 (1995).
Li, H. et al. Cloning and characterization of IL-17B and IL-17C, two new members of the IL-17 cytokine family. Proc. Natl Acad. Sci. USA 97, 773–778 (2000).
Lee, J. et al. IL-17E, a novel proinflammatory ligand for the IL-17 receptor homolog IL-17Rh1. J. Biol. Chem. 276, 1660–1664 M008289200 (2001).
Starnes, T. et al. Cutting edge: IL-17F, a novel cytokine selectively expressed in activated T cells and monocytes, regulates angiogenesis and endothelial cell cytokine production. J. Immunol. 167, 4137–4140 (2001).
Wright, J. F. et al. The human IL-17F/IL-17A heterodimeric cytokine signals through the IL-17RA/IL-17RC receptor complex. J. Immunol. 181, 2799–2805 (2008).
Langrish, C. L. et al. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J. Exp. Med. 201, 233–240 (2005).
Harrington, L. E. et al. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat. Immunol. 6, 1123–1132 (2005).
Veldhoen, M., Hocking, R. J., Atkins, C. J., Locksley, R. M. & Stockinger, B. TGFβ in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity 24, 179–189 (2006).
Bettelli, E. et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature 441, 235–238 (2006).
Mangan, P. R. et al. Transforming growth factor-β induces development of the TH17 lineage. Nature 441, 231–234 (2006).
Zhou, L. et al. IL-6 programs TH-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat. Immunol. 8, 967–974 (2007).
Manel, N., Unutmaz, D. & Littman, D. R. The differentiation of human Th17 cells requires transforming growth factor-β and induction of the nuclear receptor RORγt. Nat. Immunol. 9, 641–649 (2008).
Acosta-Rodriguez, E. V., Napolitani, G., Lanzavecchia, A. & Sallusto, F. Interleukins 1β and 6 but not transforming growth factor-β are essential for the differentiation of interleukin 17-producing human T helper cells. Nat. Immunol. 8, 942–949 (2007).
Ghoreschi, K. et al. Generation of pathogenic TH17 cells in the absence of TGF-β signalling. Nature 467, 967–971 (2010).
Ivanov, I. I. et al. The orphan nuclear receptor RORγt directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell 126, 1121–1133 (2006).
Yang, X. O. et al. T helper 17 lineage differentiation is programmed by orphan nuclear receptors RORα and RORγ. Immunity 28, 29–39 (2008).
Wei, L., Laurence, A., Elias, K. M. & O’Shea, J. J. IL-21 is produced by Th17 cells and drives IL-17 production in a STAT3-dependent manner. J. Biol. Chem. 282, 34605–34610 (2007).
Brustle, A. et al. The development of inflammatory TH-17 cells requires interferon-regulatory factor 4. Nat. Immunol. 8, 958–966 (2007).
Veldhoen, M. et al. The aryl hydrocarbon receptor links TH17-cell-mediated autoimmunity to environmental toxins. Nature (2008).
Zielinski, C. E. et al. Pathogen-induced human TH17 cells produce IFN-γ or IL-10 and are regulated by IL-1β. Nature 484, 514–518 (2012).
Annunziato, F., Cosmi, L., Liotta, F., Maggi, E. & Romagnani, S. Defining the human T helper 17 cell phenotype. Trends Immunol. 33, 505–512 (2012).
Sallusto, F., Zielinski, C. E. & Lanzavecchia, A. Human Th17 subsets. Eur. J. Immunol. 42, 2215–2220 (2012).
Evans, H. G. et al. TNF-α blockade induces IL-10 expression in human CD4+ T cells. Nat. Commun. 5, 3199 (2014).
Roberts, C. A., Durham, L. E., Fleskens, V., Evans, H. G. & Taams, L. S. TNF blockade maintains an IL-10+ phenotype in human effector CD4+ and CD8+ T cells. Front. Immunol. 8, 157 (2017).
Lee, Y. et al. Induction and molecular signature of pathogenic Th17 cells. Nat. Immunol. 13, 991–999 (2012).
Toy, D. et al. Cutting edge: interleukin 17 signals through a heteromeric receptor complex. J. Immunol. 177, 36–39 (2006).
Gaffen, S. L. Structure and signalling in the IL-17 receptor family. Nat. Rev. Immunol. 9, 556–567 (2009).
Hymowitz, S. G. et al. IL-17s adopt a cystine knot fold: structure and activity of a novel cytokine, IL-17F, and implications for receptor binding. EMBO J. 20, 5332–5341 (2001).
Gaffen, S. L., Jain, R., Garg, A. V. & Cua, D. J. The IL-23-IL-17 immune axis: from mechanisms to therapeutic testing. Nat. Rev. Immunol. 14, 585–600 (2014).
Gu, C., Wu, L. & Li, X. IL-17 family: cytokines, receptors and signaling. Cytokine 64, 477–485 (2013).
Acknowledgements
The authors acknowledge support from the King’s Health Partners Research and Development challenge fund (R140808) (to L.S.T., B.W.K and K.J.A.S), a King’s Health Schools PhD studentship funded by a Medical Research Council doctoral training grant (to L.S.T. and U.S.), a Biotechnology and Biological Sciences Research Council (BBSRC) Collaborative Awards in Science and Engineering (CASE) PhD studentship (BB/M503289/1) (to L.S.T. and L.A.B.) and support from the National Institute for Health Research (NIHR) Biomedical Research Centre based at Guy’s and St Thomas’ NHS Foundation Trust and King’s College London (to L.S.T., B.W.K. and K.J.A.S.). The views expressed are those of the authors and not necessarily those of the National Health Service, the NIHR or the Department of Health.
Reviewer information
Nature Reviews Rheumatology thanks P. Bowness, F. van Wijk and other anonymous reviewer(s) for their contribution to the peer review of this work.
Author information
Authors and Affiliations
Contributions
All authors contributed to researching data, discussion of content, writing, and reviewing and/or editing the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
B.W.K. has received research grants from AbbVie, Eli Lilly & Co, Novartis, Roche and UCB and has been a speaker and adviser for Eli Lilly & Co, Janssen and Novartis. L.S.T. has received research support and speaker fees from GlaxoSmithKline, Novartis, Novo Nordisk A/S and UCB.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Taams, L.S., Steel, K.J.A., Srenathan, U. et al. IL-17 in the immunopathogenesis of spondyloarthritis. Nat Rev Rheumatol 14, 453–466 (2018). https://doi.org/10.1038/s41584-018-0044-2
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41584-018-0044-2
This article is cited by
-
The Role of H3K27me3-Mediated Th17 Differentiation in Ankylosing Spondylitis
Inflammation (2024)
-
The Role of Early Treatment in the Management of Axial Spondyloarthritis: Challenges and Opportunities
Rheumatology and Therapy (2024)
-
Lactococcus lactis as an Interleukin Delivery System for Prophylaxis and Treatment of Inflammatory and Autoimmune Diseases
Probiotics and Antimicrobial Proteins (2024)
-
A new and spontaneous animal model for ankylosing spondylitis is found in cynomolgus monkeys
Arthritis Research & Therapy (2022)
-
Immune-related adverse events (irAEs) in ankylosing spondylitis (AS) patients treated with interleukin (IL)-17 inhibitors: a systematic review and meta-analysis
Inflammopharmacology (2022)