Abstract
Despite the large number of immunomodulatory or immunosuppressive treatments available to treat relapsing–remitting multiple sclerosis (MS), treatment of the progressive phase of the disease has not yet been achieved. This lack of successful treatment approaches is caused by our poor understanding of the mechanisms driving disease progression. Emerging concepts suggest that a combination of persisting focal and diffuse inflammation within the CNS and a gradual failure of compensatory mechanisms, including remyelination, result in disease progression. Therefore, promotion of remyelination presents a promising intervention approach. However, despite our increasing knowledge regarding the cellular and molecular mechanisms regulating remyelination in animal models, therapeutic increases in remyelination remain an unmet need in MS, which suggests that mechanisms of remyelination and remyelination failure differ fundamentally between humans and demyelinating animal models. New and emerging technologies now allow us to investigate the cellular and molecular mechanisms underlying remyelination failure in human tissue samples in an unprecedented way. The aim of this Review is to summarize our current knowledge regarding mechanisms of remyelination and remyelination failure in MS and in animal models of the disease, identify open questions, challenge existing concepts, and discuss strategies to overcome the translational roadblock in the field of remyelination-promoting therapies.
Key points
-
Promotion of remyelination is an unmet therapeutic need in multiple sclerosis.
-
Animal models only partially reflect the human pathology of multiple sclerosis.
-
The relationship between inflammation and remyelination is still unsolved.
-
New models are needed to more precisely mimic remyelination in multiple sclerosis.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Stampanoni Bassi, M., Iezzi, E. & Centonze, D. Multiple sclerosis: inflammation, autoimmunity and plasticity. Handb. Clin. Neurol. 184, 457–470 (2022).
Dobson, R. & Giovannoni, G. Multiple sclerosis — a review. Eur. J. Neurol. 26, 27–40 (2019).
Ontaneda, D., Tallantyre, E., Kalincik, T., Planchon, S. M. & Evangelou, N. Early highly effective versus escalation treatment approaches in relapsing multiple sclerosis. Lancet Neurol. 18, 973–980 (2019).
He, A. et al. Timing of high-efficacy therapy for multiple sclerosis: a retrospective observational cohort study. Lancet Neurol. 19, 307–316 (2020).
Huntemann, N. et al. Failed, interrupted, or inconclusive trials on neuroprotective and neuroregenerative treatment strategies in multiple sclerosis: update 2015–2020. Drugs 81, 1031–1063 (2021).
Klistorner, A. & Barnett, M. Remyelination trials: are we expecting the unexpected? Neurol. Neuroimmunol. Neuroinflamm. 8, e1066 (2021).
Kramer, J. & Wiendl, H. What have failed, interrupted, and withdrawn antibody therapies in multiple sclerosis taught us? Neurotherapeutics 19, 785–807 (2022).
Ploughman, M., Yong, V. W., Spermon, B., Goelz, S. & Giovannoni, G. Remyelination trial failures: repercussions of ignoring neurorehabilitation and exercise in repair. Mult. Scler. Relat. Disord. 58, 103539 (2022).
Perier, O. & Gregoire, A. Electron microscopic features of multiple sclerosis lesions. Brain 88, 937–952 (1965).
Prineas, J. W. & Connell, F. Remyelination in multiple sclerosis. Ann. Neurol. 5, 22–31 (1979).
Patani, R., Balaratnam, M., Vora, A. & Reynolds, R. Remyelination can be extensive in multiple sclerosis despite a long disease course. Neuropathol. Appl. Neurobiol. 33, 277–287 (2007).
Patrikios, P. et al. Remyelination is extensive in a subset of multiple sclerosis patients. Brain 129, 3165–3172 (2006).
Goldschmidt, T., Antel, J., Konig, F. B., Brück, W. & Kuhlmann, T. Remyelination capacity of the MS brain decreases with disease chronicity. Neurology 72, 1914–1921 (2009).
Hess, K. et al. Lesion stage-dependent causes for impaired remyelination in MS. Acta Neuropathol. 140, 359–375 (2020).
Bramow, S. et al. Demyelination versus remyelination in progressive multiple sclerosis. Brain 133, 2983–2998 (2010).
Frischer, J. M. et al. Clinical and pathological insights into the dynamic nature of the white matter multiple sclerosis plaque. Ann. Neurol. 78, 710–721 (2015).
Kuhlmann, T. et al. Gender differences in the histopathology of MS? J. Neurol. Sci. 286, 86–91 (2009).
Albert, M., Antel, J., Bruck, W. & Stadelmann, C. Extensive cortical remyelination in patients with chronic multiple sclerosis. Brain Pathol. 17, 129–138 (2007).
Chang, A. et al. Cortical remyelination: a new target for repair therapies in multiple sclerosis. Ann. Neurol. 72, 918–926 (2012).
Tonietto, M. et al. Periventricular remyelination failure in multiple sclerosis: a substrate for neurodegeneration. Brain 146, 182–194 (2023).
Lazzarotto, A. et al. Clinically relevant profiles of myelin content changes in patients with multiple sclerosis: a multimodal and multicompartment imaging study. Mult. Scler. 28, 1881–1890 (2022).
Trobisch, T. et al. Cross-regional homeostatic and reactive glial signatures in multiple sclerosis. Acta Neuropathol. 144, 987–1003 (2022).
Yaqubi, M. et al. Regional and age-related diversity of human mature oligodendrocytes. Glia 70, 1938–1949 (2022).
Chen, J. T., Collins, D. L., Atkins, H. L., Freedman, M. S. & Arnold, D. L. Magnetization transfer ratio evolution with demyelination and remyelination in multiple sclerosis lesions. Ann. Neurol. 63, 254–262 (2008).
Brown, R. A., Narayanan, S. & Arnold, D. L. Imaging of repeated episodes of demyelination and remyelination in multiple sclerosis. Neuroimage Clin. 6, 20–25 (2014).
Bodini, B. et al. Dynamic imaging of individual remyelination profiles in multiple sclerosis. Ann. Neurol. 79, 726–738 (2016).
Kuhlmann, T. et al. Differentiation block of oligodendroglial progenitor cells as a cause for remyelination failure in chronic multiple sclerosis. Brain 131, 1749–1758 (2008).
Boyd, A., Zhang, H. & Williams, A. Insufficient OPC migration into demyelinated lesions is a cause of poor remyelination in MS and mouse models. Acta Neuropathol. 125, 841–859 (2013).
Jakel, S. et al. Altered human oligodendrocyte heterogeneity in multiple sclerosis. Nature 566, 543–547 (2019).
Macnair, W. et al. Single nuclei RNAseq stratifies multiple sclerosis patients into three distinct white matter glia responses. Preprint at bioRxiv https://doi.org/10.1101/2022.04.06.487263 (2022).
Schirmer, L. et al. Neuronal vulnerability and multilineage diversity in multiple sclerosis. Nature 573, 75–82 (2019).
Jurewicz, A., Biddison, W. E. & Antel, J. P. MHC-Class I-restricted lysis of human oligodendrocytes by myelin basic protein peptide-specific CD8 T lymphocytes. J. Immunol. 160, 3056–3059 (1998).
Petrova, N., Carassiti, D., Altmann, D. R., Baker, D. & Schmierer, K. Axonal loss in the multiple sclerosis spinal cord revisited. Brain Pathol. 28, 334–348 (2018).
Bechler, M. E., Byrne, L. & Ffrench-Constant, C. CNS myelin sheath lengths are an intrinsic property of oligodendrocytes. Curr. Biol. 25, 2411–2416 (2015).
Demerens, C. et al. Induction of myelination in the central nervous system by electrical activity. Proc. Natl Acad. Sci. USA 93, 9887–9892 (1996).
Wake, H. et al. Nonsynaptic junctions on myelinating glia promote preferential myelination of electrically active axons. Nat. Commun. 6, 7844 (2015).
Coman, I., Barbin, G., Charles, P., Zalc, B. & Lubetzki, C. Axonal signals in central nervous system myelination, demyelination and remyelination. J. Neurol. Sci. 233, 67–71 (2005).
Duncan, G. J., Simkins, T. J. & Emery, B. Neuron-oligodendrocyte interactions in the structure and integrity of axons. Front. Cell Dev. Biol. 9, 653101 (2021).
Coman, I. et al. Nodal, paranodal and juxtaparanodal axonal proteins during demyelination and remyelination in multiple sclerosis. Brain 129, 3186–3195 (2006).
Charles, P. et al. Re-expression of PSA-NCAM by demyelinated axons: an inhibitor of remyelination in multiple sclerosis? Brain 125, 1972–1979 (2002).
Kornek, B. et al. Multiple sclerosis and chronic autoimmune encephalomyelitis. A comparative quantitative study of axonal injury in active, inactive, and remyelinated lesions. Am. J. Pathol. 157, 267–276 (2000).
Kuhlmann, T., Lingfeld, G., Bitsch, A., Schuchardt, J. & Brück, W. Acute axonal damage in multiple sclerosis is most extensive in early disease stages and decreases over time. Brain 125, 2202–2212 (2002).
Manrique-Hoyos, N. et al. Late motor decline after accomplished remyelination: impact for progressive multiple sclerosis. Ann. Neurol. 71, 227–244 (2012).
Mews, I., Bergmann, M., Bunkowski, S., Gullotta, F. & Brück, W. Oligodendrocyte and axon pathology in clinically silent multiple sclerosis lesions. Mult. Scler. 4, 55–62 (1998).
Brück, W. et al. Monocyte/macrophage differentiation in early multiple sclerosis lesions. Ann. Neurol. 38, 788–796 (1995).
Boven, L. A. et al. Myelin-laden macrophages are anti-inflammatory, consistent with foam cells in multiple sclerosis. Brain 129, 517–526 (2006).
Healy, L. M. et al. MerTK is a functional regulator of myelin phagocytosis by human myeloid cells. J. Immunol. 196, 3375–3384 (2016).
Bottcher, C. et al. Single-cell mass cytometry reveals complex myeloid cell composition in active lesions of progressive multiple sclerosis. Acta Neuropathol. Commun. 8, 136 (2020).
Zrzavy, T. et al. Loss of ‘homeostatic’ microglia and patterns of their activation in active multiple sclerosis. Brain 140, 1900–1913 (2017).
Masuda, T. et al. Spatial and temporal heterogeneity of mouse and human microglia at single-cell resolution. Nature 566, 388–392 (2019).
Miedema, A. et al. Brain macrophages acquire distinct transcriptomes in multiple sclerosis lesions and normal appearing white matter. Acta Neuropathol. Commun. 10, 8 (2022).
Frischer, J. M. et al. The relation between inflammation and neurodegeneration in multiple sclerosis brains. Brain 132, 1175–1189 (2009).
Fransen, N. L. et al. Tissue-resident memory T cells invade the brain parenchyma in multiple sclerosis white matter lesions. Brain 143, 1714–1730 (2020).
Bitsch, A., Schuchardt, J., Bunkowski, S., Kuhlmann, T. & Brück, W. Acute axonal injury in multiple sclerosis. Correlation with demyelination and inflammation. Brain 123, 1174–1183 (2000).
van Nierop, G. P. et al. Phenotypic and functional characterization of T cells in white matter lesions of multiple sclerosis patients. Acta Neuropathol. 134, 383–401 (2017).
Barnett, M. H. & Prineas, J. W. Relapsing and remitting multiple sclerosis: pathology of the newly forming lesion. Ann. Neurol. 55, 458–468 (2004).
Wheeler, M. A. et al. MAFG-driven astrocytes promote CNS inflammation. Nature 578, 593–599 (2020).
Absinta, M. et al. A lymphocyte-microglia-astrocyte axis in chronic active multiple sclerosis. Nature 597, 709–714 (2021).
Brosnan, C. F. & Raine, C. S. The astrocyte in multiple sclerosis revisited. Glia 61, 453–465 (2013).
Liddelow, S. A. et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature 541, 481–487 (2017).
El Behi, M. et al. Adaptive human immunity drives remyelination in a mouse model of demyelination. Brain 140, 967–980 (2017).
Moore, C. S. et al. Direct and indirect effects of immune and central nervous system-resident cells on human oligodendrocyte progenitor cell differentiation. J. Immunol. 194, 761–772 (2015).
Starost, L. et al. Extrinsic immune cell-derived, but not intrinsic oligodendroglial factors contribute to oligodendroglial differentiation block in multiple sclerosis. Acta Neuropathol. 140, 715–736 (2020).
Larochelle, C. et al. Pro-inflammatory T helper 17 directly harms oligodendrocytes in neuroinflammation. Proc. Natl Acad. Sci. USA 118, e2025813118 (2021).
Pernin, F. et al. Diverse injury responses of human oligodendrocyte to mediators implicated in multiple sclerosis. Brain 145, 4320–4333 (2022).
Crawford, A. H. et al. Pre-existing mature oligodendrocytes do not contribute to remyelination following toxin-induced spinal cord demyelination. Am. J. Pathol. 186, 511–516 (2016).
Franklin, R. J. M., Frisen, J. & Lyons, D. A. Revisiting remyelination: towards a consensus on the regeneration of CNS myelin. Semin. Cell Dev. Biol. 116, 3–9 (2021).
Keirstead, H. S. & Blakemore, W. F. Identification of post-mitotic oligodendrocytes incapable of remyelination within the demyelinated adult spinal cord. J. Neuropathol. Exp. Neurol. 56, 1191–1201 (1997).
Duncan, I. D. et al. The adult oligodendrocyte can participate in remyelination. Proc. Natl Acad. Sci. USA 115, E11807–E11816 (2018).
Bacmeister, C. M. et al. Motor learning promotes remyelination via new and surviving oligodendrocytes. Nat. Neurosci. 23, 819–831 (2020).
Neely, S. A. et al. New oligodendrocytes exhibit more abundant and accurate myelin regeneration than those that survive demyelination. Nat. Neurosci. 25, 415–420 (2022).
Duncan, I. D., Brower, A., Kondo, Y., Curlee, J. F. Jr & Schultz, R. D. Extensive remyelination of the CNS leads to functional recovery. Proc. Natl Acad. Sci. USA 106, 6832–6836 (2009).
Gilson, J. & Blakemore, W. F. Failure of remyelination in areas of demyelination produced in the spinal cord of old rats. Neuropathol. Appl. Neurobiol. 19, 173–181 (1993).
Pfeifenbring, S. et al. Extensive acute axonal damage in pediatric multiple sclerosis lesions. Ann. Neurol. 77, 655–667 (2015).
Ruckh, J. M. et al. Rejuvenation of regeneration in the aging central nervous system. Cell Stem Cell 10, 96–103 (2012).
Neumann, B. et al. Metformin restores CNS remyelination capacity by rejuvenating aged stem cells. Cell Stem Cell 25, 473–485 (2019).
Gingele, S. et al. Delayed demyelination and impaired remyelination in aged mice in the cuprizone model. Cells 9, 945 (2020).
Gibson, E. M. et al. Neuronal activity promotes oligodendrogenesis and adaptive myelination in the mammalian brain. Science 344, 1252304 (2014).
Irvine, K. A. & Blakemore, W. F. Remyelination protects axons from demyelination-associated axon degeneration. Brain 131, 1464–1477 (2008).
Back, S. A. et al. Hyaluronan accumulates in demyelinated lesions and inhibits oligodendrocyte progenitor maturation. Nat. Med. 11, 966–972 (2005).
Pendleton, J. C. et al. Chondroitin sulfate proteoglycans inhibit oligodendrocyte myelination through PTPsigma. Exp. Neurol. 247, 113–121 (2013).
Harlow, D. E. & Macklin, W. B. Inhibitors of myelination: ECM changes, CSPGs and PTPs. Exp. Neurol. 251, 39–46 (2014).
Segel, M. et al. Niche stiffness underlies the ageing of central nervous system progenitor cells. Nature 573, 130–134 (2019).
Dendrou, C. A. & Fugger, L. Immunomodulation in multiple sclerosis: promises and pitfalls. Curr. Opin. Immunol. 49, 37–43 (2017).
Bieber, A. J., Kerr, S. & Rodriguez, M. Efficient central nervous system remyelination requires T cells. Ann. Neurol. 53, 680–684 (2003).
Kirby, L. et al. Oligodendrocyte precursor cells present antigen and are cytotoxic targets in inflammatory demyelination. Nat. Commun. 10, 3887 (2019).
Liu, H. et al. IL-17 inhibits oligodendrocyte progenitor cell proliferation and differentiation by increasing K+ channel Kv1.3. Front. Cell Neurosci. 15, 679413 (2021).
Wang, C. et al. IL-17 induced NOTCH1 activation in oligodendrocyte progenitor cells enhances proliferation and inflammatory gene expression. Nat. Commun. 8, 15508 (2017).
Baxi, E. G. et al. Transfer of myelin-reactive th17 cells impairs endogenous remyelination in the central nervous system of cuprizone-fed mice. J. Neurosci. 35, 8626–8639 (2015).
Dombrowski, Y. et al. Regulatory T cells promote myelin regneration in the central nervous system. Nat. Neurosci. 20, 674–680 (2017).
Dombrowski, Y. et al. Regulatory T cells promote myelin regeneration in the central nervous system. Nat. Neurosci. 20, 674–680 (2017).
McIntyre, L. L. et al. Regulatory T cells promote remyelination in the murine experimental autoimmune encephalomyelitis model of multiple sclerosis following human neural stem cell transplant. Neurobiol. Dis. 140, 104868 (2020).
Dittmer, M. et al. Characterization of a murine mixed neuron-glia model and cellular responses to regulatory T cell-derived factors. Mol. Brain 11, 25 (2018).
Creswell, R. & Dombrowski, Y. Innate and adaptive immune mechanisms regulating central nervous system remyelination. Curr. Opin. Pharmacol. 63, 102175 (2022).
Chou, W. C. et al. AIM2 in regulatory T cells restrains autoimmune diseases. Nature 591, 300–305 (2021).
Skihar, V. et al. Promoting oligodendrogenesis and myelin repair using the multiple sclerosis medication glatiramer acetate. Proc. Natl Acad. Sci. USA 106, 17992–17997 (2009).
Harrington, E. P., Bergles, D. E. & Calabresi, P. A. Immune cell modulation of oligodendrocyte lineage cells. Neurosci. Lett. 715, 134601 (2020).
Falcao, A. M. et al. Disease-specific oligodendrocyte lineage cells arise in multiple sclerosis. Nat. Med. 24, 1837–1844 (2018).
Melzer, N. et al. Excitotoxic neuronal cell death during an oligodendrocyte-directed CD8+ T cell attack in the CNS gray matter. J. Neuroinflamm. 10, 121 (2013).
Lisak, R. P. et al. B cells from patients with multiple sclerosis induce cell death via apoptosis in neurons in vitro. J. Neuroimmunol. 309, 88–99 (2017).
Benjamins, J. A. Direct effects of secretory products of immune cells on neurons and glia. J. Neurol. Sci. 333, 30–36 (2013).
Benjamins, J. A. et al. Exosome-enriched fractions from MS B cells induce oligodendrocyte death. Neurol. Neuroimmunol. Neuroinflamm. 6, e550 (2019).
Calahorra, L., Camacho-Toledano, C., Serrano-Regal, M. P., Ortega, M. C. & Clemente, D. Regulatory cells in multiple sclerosis: from blood to brain. Biomedicines 10, 335 (2022).
Pennati, A., Nylen, E. A., Duncan, I. D. & Galipeau, J. Regulatory B cells normalize CNS myeloid cell content in a mouse model of multiple sclerosis and promote oligodendrogenesis and remyelination. J. Neurosci. 40, 5105–5115 (2020).
Haindl, M. T. et al. Anti-CD20 treatment effectively attenuates cortical pathology in a rat model of widespread cortical demyelination. J. Neuroinflamm. 18, 138 (2021).
Heß, K. et al. Lesion stage-dependent causes for impaired remyelination in MS. Acta Neuropathol. 140, 359–375 (2020).
Miron, V. E. & Franklin, R. J. M. Macrophages and CNS remyelination. J. Neurochem. 130, 165–171 (2014).
Locatelli, G. et al. Mononuclear phagocytes locally specify and adapt their phenotype in a multiple sclerosis model. Nat. Neurosci. 21, 1196–1208 (2018).
Jordao, M. J. C. et al. Single-cell profiling identifies myeloid cell subsets with distinct fates during neuroinflammation. Science 363, eaat7554 (2019).
Kotter, M. R., Li, W. W., Zhao, C. & Franklin, R. J. Myelin impairs CNS remyelination by inhibiting oligodendrocyte precursor cell differentiation. J. Neurosci. 26, 328–332 (2006).
Cantuti-Castelvetri, L. et al. Defective cholesterol clearance limits remyelination in the aged central nervous system. Science 359, 684–688 (2018).
Gouna, G. et al. TREM2-dependent lipid droplet biogenesis in phagocytes is required for remyelination. J. Exp. Med. 218, e20210227 (2021).
Lloyd, A. F. & Miron, V. E. The pro-remyelination properties of microglia in the central nervous system. Nat. Rev. Neurol. 15, 447–458 (2019).
Plemel, J. R., Manesh, S. B., Sparling, J. S. & Tetzlaff, W. Myelin inhibits oligodendroglial maturation and regulates oligodendrocytic transcription factor expression. Glia 61, 1471–1487 (2013).
Berghoff, S. A. et al. Microglia facilitate repair of demyelinated lesions via post-squalene sterol synthesis. Nat. Neurosci. 24, 47–60 (2021).
Bogie, J. F. J. et al. Stearoyl-CoA desaturase-1 impairs the reparative properties of macrophages and microglia in the brain. J. Exp. Med. 217, e20191660 (2020).
Standiford, M. M., Grund, E. M. & Howe, C. L. Citrullinated myelin induces microglial TNFα and inhibits endogenous repair in the cuprizone model of demyelination. J. Neuroinflamm. 18, 305 (2021).
Prineas, J. W. & Parratt, J. D. E. Multiple sclerosis: microglia, monocytes, and macrophage-mediated demyelination. J. Neuropathol. Exp. Neurol. 80, 975–996 (2021).
Larsen, P. H., Wells, J. E., Stallcup, W. B., Opdenakker, G. & Yong, V. W. Matrix metalloproteinase-9 facilitates remyelination in part by processing the inhibitory NG2 proteoglycan. J. Neurosci. 23, 11127–11135 (2003).
Stoffels, J. M. et al. Fibronectin aggregation in multiple sclerosis lesions impairs remyelination. Brain 136, 116–131 (2013).
Miron, V. E. et al. M2 microglia and macrophages drive oligodendrocyte differentiation during CNS remyelination. Nat. Neurosci. 16, 1211–1218 (2013).
Miron, V. E. & Franklin, R. J. Macrophages and CNS remyelination. J. Neurochem. 130, 165–171 (2014).
Mason, J. L., Langaman, C., Morell, P., Suzuki, K. & Matsushima, G. K. Episodic demyelination and subsequent remyelination within the murine central nervous system: changes in axon calibre. Neuropathol. Appl. Neurobiol. 27, 50–58 (2001).
Laflamme, N. et al. mCSF-induced microglial activation prevents myelin loss and promotes its repair in a mouse model of multiple sclerosis. Front. Cell Neurosci. 12, 178 (2018).
Pang, Y. et al. Lipopolysaccharide-activated microglia induce death of oligodendrocyte progenitor cells and impede their development. Neuroscience 166, 464–475 (2010).
Pasquini, L. A. et al. Galectin-3 drives oligodendrocyte differentiation to control myelin integrity and function. Cell Death Differ. 18, 1746–1756 (2011).
Selvaraju, R. et al. Osteopontin is upregulated during in vivo demyelination and remyelination and enhances myelin formation in vitro. Mol. Cell Neurosci. 25, 707–721 (2004).
Zabala, A. et al. P2X4 receptor controls microglia activation and favors remyelination in autoimmune encephalitis. EMBO Mol. Med. 10, e8743 (2018).
Cunha, M. I. et al. Pro-inflammatory activation following demyelination is required for myelin clearance and oligodendrogenesis. J. Exp. Med. 217, e20191390 (2020).
Strachan-Whaley, M., Rivest, S. & Yong, V. W. Interactions between microglia and T cells in multiple sclerosis pathobiology. J. Interferon Cytokine Res. 34, 615–622 (2014).
Cheli, V. T., Correale, J., Paez, P. M. & Pasquini, J. M. Iron metabolism in oligodendrocytes and astrocytes, implications for myelination and remyelination. ASN Neuro 12, 1759091420962681 (2020).
Almolda, B., Gonzalez, B. & Castellano, B. Activated microglial cells acquire an immature dendritic cell phenotype and may terminate the immune response in an acute model of EAE. J. Neuroimmunol. 223, 39–54 (2010).
Brambilla, R. The contribution of astrocytes to the neuroinflammatory response in multiple sclerosis and experimental autoimmune encephalomyelitis. Acta Neuropathol. 137, 757–783 (2019).
Magliozzi, R. et al. Meningeal B-cell follicles in secondary progressive multiple sclerosis associate with early onset of disease and severe cortical patholgoy. Brain 130, 1089–1104 (2007).
Gorter, R. P. & Baron, W. Recent insights into astrocytes as therapeutic targets for demyelinating diseases. Curr. Opin. Pharmacol. 65, 102261 (2022).
Tognatta, R. et al. Astrocytes are required for oligodendrocyte survival and maintenance of myelin compaction and integrity. Front. Cell Neurosci. 14, 74 (2020).
Madadi, S. et al. Astrocyte ablation induced by La-aminoadipate (L-AAA) potentiates remyelination in a cuprizone demyelinating mouse model. Metab. Brain Dis. 34, 593–603 (2019).
Mayo, L. et al. Regulation of astrocyte activation by glycolipids drives chronic CNS inflammation. Nat. Med. 20, 1147–1156 (2014).
Horng, S. et al. Astrocytic tight junctions control inflammatory CNS lesion pathogenesis. J. Clin. Invest. 127, 3136–3151 (2017).
Nicaise, A. M., Johnson, K. M., Willis, C. M., Guzzo, R. M. & Crocker, S. J. TIMP-1 promotes oligodendrocyte differentiation through receptor-mediated signaling. Mol. Neurobiol. 56, 3380–3392 (2019).
Saitta, K. S. et al. CHPG enhances BDNF and myelination in cuprizone-treated mice through astrocytic metabotropic glutamate receptor 5. Glia 69, 1950–1965 (2021).
Liddelow, S. A. & Barres, B. A. Reactive astrocytes: production, function, and therapeutic potential. Immunity 46, 957–967 (2017).
Ghorbani, S. et al. Versican promotes T helper 17 cytotoxic inflammation and impedes oligodendrocyte precursor cell remyelination. Nat. Commun. 13, 2445 (2022).
Domingues, H. S., Portugal, C. C., Socodato, R. & Relvas, J. B. Oligodendrocyte, astrocyte, and microglia crosstalk in myelin development, damage, and repair. Front. Cell Dev. Biol. 4, 71 (2016).
Clark, I. C. et al. Barcoded viral tracing of single-cell interactions in central nervous system inflammation. Science 372, 6540 (2021).
Lombardi, M. et al. Detrimental and protective action of microglial extracellular vesicles on myelin lesions: astrocyte involvement in remyelination failure. Acta Neuropathol. 138, 987–1012 (2019).
Hofman, M. A. Evolution of the human brain: when bigger is better. Front. Neuroanat. 8, 15 (2014).
Roth, G. & Dicke, U. Evolution of the brain and intelligence. Trends Cogn. Sci. 9, 250–257 (2005).
Craig, A. et al. Quantitative analysis of perinatal rodent oligodendrocyte lineage progression and its correlation with human. Exp. Neurol. 181, 231–240 (2003).
Yeung, M. S. et al. Dynamics of oligodendrocyte generation and myelination in the human brain. Cell 159, 766–774 (2014).
Giedd, J. N. et al. Brain development during childhood and adolescence: a longitudinal MRI study. Nat. Neurosci. 2, 861–863 (1999).
Douvaras, P. et al. Efficient generation of myelinating oligodendrocytes from primary progressive multiple sclerosis patients by induced pluripotent stem cells. Stem Cell Rep. 3, 250–259 (2014).
Wang, S. et al. Human iPSC-derived oligodendrocyte progenitor cells can myelinate and rescue a mouse model of congenital hypomyelination. Cell Stem Cell 12, 252–264 (2013).
Windrem, M. S. et al. Neonatal chimerization with human glial progenitor cells can both remyelinate and rescue the otherwise lethally hypomyelinated shiverer mouse. Cell Stem Cell 2, 553–565 (2008).
Windrem, M. S. et al. A competitive advantage by neonatally engrafted human glial progenitors yields mice whose brains are chimeric for human glia. J. Neurosci. 34, 16153–16161 (2014).
Buchet, D., Garcia, C., Deboux, C., Nait-Oumesmar, B. & Baron-Van, E. A. Human neural progenitors from different foetal forebrain regions remyelinate the adult mouse spinal cord. Brain 134, 1168–1183 (2011).
Chanoumidou, K., Mozafari, S., Baron-Van Evercooren, A. & Kuhlmann, T. Stem cell derived oligodendrocytes to study myelin diseases. Glia 68, 705–720 (2020).
Sim, F. J., Windrem, M. S. & Goldman, S. A. Fate determination of adult human glial progenitor cells. Neuron Glia Biol. 5, 45–55 (2009).
Marques, S. et al. Oligodendrocyte heterogeneity in the mouse juvenile and adult central nervous system. Science 352, 1326–1329 (2016).
Wolswijk, G. Oligodendrocyte precursor cells in the demyelinated spinal cord. Brain 125, 338–349 (2002).
Chang, A., Nishiyama, A., Peterson, J., Prineas, J. & Trapp, B. D. NG2-positive oligodendrocyte progenitor cells in adult human brain and multiple sclerosis lesions. J. Neurosci. 20, 6404–6412 (2000).
Kuhlmann, T., Remington, L., Maruschak, B., Owens, T. & Bruck, W. Nogo-A is a reliable oligodendroglial marker in adult human and mouse CNS and in demyelinated lesions. J. Neuropathol. Exp. Neurol. 66, 238–246 (2007).
Lurbke, A. et al. Limited TCF7L2 expression in MS lesions. PLoS One 8, e72822 (2013).
Fard, M. K. et al. BCAS1 expression defines a population of early myelinating oligodendrocytes in multiple sclerosis lesions. Sci. Transl. Med. 9, eaam7816 (2017).
Staugaitis, S. M. & Trapp, B. D. NG2-positive glia in the human central nervous system. Neuron Glia Biol. 5, 35–44 (2009).
Yeung, M. et al. Dynamics of oligodendrocyte generation in multiple sclerosis. Nature 566, 538–542 (2019).
Xing, Y. L. et al. Adult neural precursor cells from the subventricular zone contribute significantly to oligodendrocyte regeneration and remyelination. J. Neurosci. 34, 14128–14146 (2014).
Nait-Oumesmar, B. et al. Activation of the subventricular zone in multiple sclerosis: evidence for early glial progenitors. Proc. Natl Acad. Sci. USA 104, 4694–4699 (2007).
Tonietto, M. et al. Periventricular remyelination failure in multiple sclerosis: a substrate for neurodegeneration. Brain 146, 182–194 (2022).
Bogie, J. F., Stinissen, P. & Hendriks, J. J. Macrophage subsets and microglia in multiple sclerosis. Acta Neuropathol. 128, 191–213 (2014).
Wolswijk, G. Oligodendrocyte survival, loss and birth in lesions of chronic-stage multiple sclerosis. Brain 123, 105–115 (2000).
Lloyd, A. F., Davies, C. L. & Miron, V. E. Microglia: origins, homeostasis, and roles in myelin repair. Curr. Opin. Neurobiol. 47, 113–120 (2017).
Hauser, S. L. et al. Ocrelizumab versus interferon beta-1a in relapsing multiple sclerosis. N. Engl. J. Med. 376, 221–234 (2017).
Montalban, X. et al. Ocrelizumab versus placebo in primary progressive multiple sclerosis. N. Engl. J. Med. 376, 209–220 (2017).
Bhargava, P., Hartung, H. P. & Calabresi, P. A. Contribution of B cells to cortical damage in multiple sclerosis. Brain 145, 3363–3373 (2022).
Riedhammer, C. & Weissert, R. Antigen presentation, autoantigens, and immune regulation in multiple sclerosis and other autoimmune diseases. Front. Immunol. 6, 322 (2015).
Goebels, N. et al. Repertoire dynamics of autoreactive T cells in multiple sclerosis patients and healthy subjects: epitope spreading versus clonal persistence. Brain 123, 508–518 (2000).
Mentis, A. A., Dardiotis, E., Grigoriadis, N., Petinaki, E. & Hadjigeorgiou, G. M. Viruses and multiple sclerosis: from mechanisms and pathways to translational research opportunities. Mol. Neurobiol. 54, 3911–3923 (2017).
Covre, L. P., De Maeyer, R. P. H., Gomes, D. C. O. & Akbar, A. N. The role of senescent T cells in immunopathology. Aging Cell 19, e13272 (2020).
Mogilenko, D. A., Shchukina, I. & Artyomov, M. N. Immune ageing at single-cell resolution. Nat. Rev. Immunol. 22, 484–498 (2022).
Dutta, S. & Sengupta, P. Men and mice: relating their ages. Life Sci. 152, 244–248 (2016).
Pfeifenbring, S., Nessler, S., Wegner, C., Stadelmann, C. & Bruck, W. Remyelination after cuprizone-induced demyelination is accelerated in Juvenile Mice. J. Neuropathol. Exp. Neurol. 74, 756–766 (2015).
Brown, R. A., Narayanan, S., Banwell, B. & Arnold, D. L., Canadian Pediatric Demyelinating Disease Network. Magnetization transfer ratio recovery in new lesions decreases during adolescence in pediatric-onset multiple sclerosis patients. Neuroimage Clin. 6, 237–242 (2014).
Luo, J. X. X. et al. Human oligodendrocyte myelination potential; relation to age and differentiation. Ann. Neurol. 91, 178–191 (2022).
Dubois-Dalcq, M. et al. From fish to man: understanding endogenous remyelination in central nervous system demyelinating diseases. Brain 131, 1686–1700 (2008).
Flurkey, K., Currer, J.M. & Harrison, D.E. In The Mouse in Biomedical Research 2nd edn Vol. 3 Ch. 20 (eds Fox, J. G. et al.) 637–672 (Academic Press, 2007).
Zhange, Y. et al. Inhibition of LINGO-1 promotes functional recovery after experimental spinal cord demyelination. Exp. Neurol. 266, 68–73 (2015).
Yamazaki, R. et al. Pharmacological treatment promoting remyelination enhances motor function after internal capsule demyelination in mice. Neurochem. Int. 164, 105505 (2023).
Liu, L. et al. Ginsendoside Rg1 promotes remyelination and functional recovery in demyelinating disease by enhancing oligodendrocyte precursor cells-mediated myelin repair. Phytomedicine 106, 154309 (2022).
Lubetzki, C., Zalc, B., Williams, A., Stadelmann, C. & Stankoff, B. Remyelination in multiple sclerosis: from basic science to clinical translation. Lancet Neurol. 19, 678–688 (2020).
Stangel, M., Kuhlmann, T., Matthews, P. M. & Kilpatrick, T. J. Achievements and obstacles of remyelinating therapies in multiple sclerosis. Nat. Rev. Neurol. 13, 742–754 (2017).
Cree, B. A. C., Hartung, H. P. & Barnett, M. New drugs for multiple sclerosis: new treatment algorithms. Curr. Opin. Neurol. 35, 262–270 (2022).
Oh, J. & Bar-Or, A. Emerging therapies to target CNS pathophysiology in multiple sclerosis. Nat. Rev. Neurol. 18, 466–475 (2022).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT04002934 (2022).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03536559 (2022).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT05122559 (2022).
Kaufmann, M. et al. Identification of early neurodegenerative pathways in progressive multiple sclerosis. Nat. Neurosci. 25, 944–955 (2022).
Walker, J. M. et al. Differential protein expression in the hippocampi of resilient individuals identified by digital spatial profiling. Acta Neuropathol. Commun. 10, 23 (2022).
Kennedy-Darling, J. et al. Highly multiplexed tissue imaging using repeated oligonucleotide exchange reaction. Eur. J. Immunol. 51, 1262–1277 (2021).
Kinkhabwala, A. et al. MACSima imaging cyclic staining (MICS) technology reveals combinatorial target pairs for CAR T cell treatment of solid tumors. Sci. Rep. 12, 1911 (2022).
Mei, F. et al. Micropillar arrays as a high-throughput screening platform for therapeutics in multiple sclerosis. Nat. Med. 20, 954–960 (2014).
Najm, F. J. et al. Drug-based modulation of endogenous stem cells promotes functional remyelination in vivo. Nature 522, 216–220 (2015).
Deshmukh, V. A. et al. A regenerative approach to the treatment of multiple sclerosis. Nature 502, 327–332 (2013).
Himmelstein, D. S. et al. Systematic integration of biomedical knowledge prioritizes drugs for repurposing. eLlife 6, e26726 (2017).
Baranzini, S. E. et al. A biomedical open knowledge network harnesses the power of AI to understand deep human biology. AI Mag. 43, 46–58 (2022).
Mozafari, S. et al. Multiple sclerosis iPS-derived oligodendroglia conserve their properties to functionally interact with axons and glia in vivo. Sci. Adv. 6, eabc6983 (2020).
Osipovitch, M. et al. Human ESC-derived chimeric mouse models of Huntington’s disease reveal cell-intrinsic defects in glial progenitor cell differentiation. Cell Stem Cell 24, 107–122 (2019).
Wang, C. T., Barnett, M. & Barnett, Y. Imaging the multiple sclerosis lesion: insights into pathogenesis, progression and repair. Curr. Opin. Neurol. 32, 338–345 (2019).
Heidari, M. et al. Evoked potentials as a biomarker of remyelination. Proc. Natl Acad. Sci. USA 116, 27074–27083 (2019).
Green, A. J. et al. Clemastine fumarate as a remyelinating therapy for multiple sclerosis (ReBUILD): a randomised, controlled, double-blind, crossover trial. Lancet 390, 2481–2489 (2017).
Huang, J. K. et al. Retinoid X receptor gamma signaling accelerates CNS remyelination. Nat. Neurosci. 14, 45–53 (2011).
Brown, J. W. L. et al. Safety and efficacy of bexarotene in patients with relapsing-remitting multiple sclerosis (CCMR One): a randomised, double-blind, placebo-controlled, parallel-group, phase 2a study. Lancet Neurol. 20, 709–720 (2021).
McMurran, C. E. et al. Remyelination in humans due to a retinoid-X receptor agonist is age-dependent. Ann. Clin. Transl. Neurol. 9, 1090–1094 (2022).
Demicheva, E. et al. Targeting repulsive guidance molecule A to promote regeneration and neuroprotection in multiple sclerosis. Cell Rep. 10, 1887–1898 (2015).
Tanabe, S., Fujita, Y., Ikuma, K. & Yamashita, T. Inhibiting repulsive guidance molecule-a suppresses secondary progression in mouse models of multiple sclerosis. Cell Death Dis. 9, 1061 (2018).
Kalluri, H. V. et al. Phase 1 evaluation of elezanumab (anti-RGMa mAb) in healthy and MS participants. Ann. Neurol. 93, 285–296 (2022).
Cho, Y. K. et al. Erythropoietin promotes oligodendrogenesis and myelin repair following lysolecithin-induced injury in spinal cord slice culture. Biochem. Biophys. Res. Commun. 417, 753–759 (2012).
Mirzaie, J., Raoofi, A., Jamalpoor, Z., Nezhadi, A. & Golmohammadi, R. Protective impacts of erythropoietin on myelinization of oligodendrocytes and Schwann cells in CNS and PNS following cuprizone-induced multiple sclerosis- histology, molecular, and functional studies. J. Chem. Neuroanat. 104, 101750 (2020).
Schreiber, K. et al. High-dose erythropoietin in patients with progressive multiple sclerosis: A randomized, placebo-controlled, phase 2 trial. Mult. Scler. 23, 675–685 (2017).
Lagrèze, W. A. et al. Safety and efficacy of erythropoietin for the treatment of patients with optic neuritis (TONE): a randomized, double-blind, multicentre, placebo-controlled study. Lancet Neurol. 20, 991–1000 (2021).
Filipi, M. & Jack, S. Interferons in the treatment of multiple sclerosis: a clinical efficacy, safety and tolerability update. Int. J. MS Care 22, 165–172 (2020).
Chen, Y. et al. Histamine receptor 3 negatively regulates oligodendrocyte differentiation and remyelination. PLoS One 12, e0189380 (2017).
Schwartzbach, C. J. et al. Lesion remyelinating activity of GSK239512 versus placebo in patients with relapsing-remitting multiple sclerosis: a randomised, single-blind, phase II study. J. Neurol. 264, 304–315 (2017).
Knott, E. P., Assi, M., Rao, S. N., Ghosh, M. & Pearse, D. D. Phosphodiesterase inhibitors as a therapeutic approach to neuroprotection and repair. Int. J. Mol. Sci. 18, 696 (2017).
Fox, R. J. et al. Phase 2 trial of ibudilast in progressive multiple sclerosis. N. Engl. J. Med. 379, 846–855 (2018).
Naismith, R. T. et al. Effects of Ibudilast on MRI measures in the phase 2 SPRINT-MS study. Neurology 96, e491–e500 (2021).
Cunniffe, N. et al. Systematic approach to selecting licensed drugs for repurposing in the treatment of progressive multiple sclerosis. J. Neurol. Neurosurg. Psychiatry 92, 295–302 (2021).
Negrotto, L., Farez, M. F. & Correale, J. Immunologic effects of metformin and pioglitazone treatment on metabolic syndrome and multiple sclerosis. JAMA Neurol. 73, 520–528 (2016).
Mi, S. et al. LINGO-1 negatively regulates myelination by oligodendrocytes. Nat. Neurosci. 8, 745–751 (2005).
Mi, S. et al. LINGO-1 antagonist promotes spinal cord remyelination and axonal integrity in MOG-induced experimental autoimmune encephalomyelitis. Nat. Med. 13, 1228–1233 (2007).
Cadavid, D. et al. Safety and efficacy of opicinumab in acute optic neuritis (RENEW): a randomised, placebo-controlled, phase 2 trial. Lancet Neurol. 16, 189–199 (2017).
Cadavid, D. et al. Safety and efficacy of opicinumab in patients with relapsing multiple sclerosis (SYNERGY): a randomised, placebo-controlled, phase 2 trial. Lancet Neurol. 18, 845–856 (2019).
Mannioui, A. et al. The Xenopus tadpole: an in vivo model to screen drugs favoring remyelination. Mult. Scler. 24, 1421–1432 (2017).
Dietrich, M. et al. Increased remyelination and proregenerative microglia under siponimod therapy in mechanistic models. Neurol. Neuroimmunol. Neuroinflamm. 9, e1161 (2022).
Kappos, L. et al. Siponimod versus placebo in secondary progressive multiple sclerosis (EXPAND): a double-blind, randomised, phase 3 study. Lancet 391, 1263–1273 (2018).
Arnold, D. L. et al. Effect of siponimod on magnetic resonance imaging measures of neurodegeneration and myelination in secondary progressive multiple sclerosis: Gray matter atrophy and magnetization transfer ratio analyses from the EXPAND phase 3 trial. Mult. Scler. 28, 1526–1540 (2022).
Lublin, F. et al. Oral fingolimod in primary progressive multiple sclerosis (INFORMS): a phase 3, randomised, double-blind, placebo-controlled trial. Lancet 387, 1075–1084 (2016).
Rolland, A. et al. The envelope protein of a human endogenous retrovirus-W family activates innate immunity through CD14/TLR4 and promotes Th1-like responses. J. Immunol. 176, 7636–7644 (2006).
Hartung, H. P. et al. Efficacy and safety of temelimab in multiple sclerosis: Results of a randomized phase 2b and extension study. Mult. Scler. 28, 429–440 (2022).
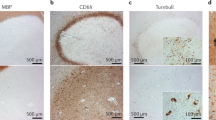
Kuhlmann, T. et al. An updated histological classification system for multiple sclerosis lesions. Acta Neuropathol. 133, 13–24 (2017).
Vallejo, A. et al. snPATHO-seq: unlocking the FFPE archives for single nucleus RNA profiling. Preprint at bioRxiv https://doi.org/10.1101/2022.08.23.505054 (2022).
Chung, H. et al. SnFFPE-Seq: towards scalable single nucleus RNA-Seq of formalin-fixed paraffin-embedded (FFPE) tissue. Preprint at bioRxiv https://doi.org/10.1101/2022.08.25.505257 (2022).
Roberts, K. A. et al. Transcriptome-wide spatial RNA profiling maps the cellular architecture of the developing human neocortex. Preprint at bioRxiv https://doi.org/10.1101/2021.03.20.436265 (2021).
Kular, L. et al. DNA methylation changes in glial cells of the normal-appearing white matter in multiple sclerosis patients. Epigenetics 17, 1311–1330 (2022).
Fernandes, S. J. et al. Deep characterization of paired chromatin and transcriptomes in four immune cell types from multiple sclerosis patients. Epigenomics 13, 1607–1618 (2021).
Mecha, M., Carrillo-Salinas, F. J., Mestre, L., Feliu, A. & Guaza, C. Viral models of multiple sclerosis: neurodegeneration and demyelination in mice infected with Theiler’s virus. Prog. Neurobiol. 101-102, 46–64 (2013).
Psenicka, M. W., Smith, B. C., Tinkey, R. A. & Williams, J. L. Connecting neuroinflammation and neurodegeneration in multiple sclerosis: are oligodendrocyte precursor cells a nexus of disease? Front. Cell Neurosci. 15, 654284 (2021).
Karttunen, M. J. & Lyons, D. A. A drug-inducible transgenic zebrafish model for myelinating glial cell ablation. Methods Mol. Biol. 1936, 227–238 (2019).
Kim, S. et al. Promotion of remyelination by sulfasalazine in a transgenic zebrafish model of demyelination. Mol. Cell 38, 1013–1021 (2015).
Radcliff, A. B., Heidari, M., Field, A. S. & Duncan, I. D. Feline irradiated diet-induced demyelination; a model of the neuropathology of sub-acute combined degeneration? PLoS One 15, e0228109 (2020).
Cassidy, J. P. et al. Leukoencephalomyelopathy in specific pathogen-free cats. Vet. Pathol. 44, 912–916 (2007).
Lee, N. J. et al. Potential role of iron in repair of inflammatory demyelinating lesions. J. Clin. Invest. 129, 4365–4376 (2019).
Acknowledgements
The study was supported by the German Research Foundation (SFB128-B07 and KU1477/11-1 to T.K.; SFB128-A08 and SFB1009 A03 to L.K.) and the Interdisciplinary Center for Clinical Research Münster (KuT3/007/20 to T.K.).
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
L.K. received compensation for serving on Scientific Advisory Boards for Alexion, Biogen, Bristol-Myers Squibb, Genzyme, Horizon, Janssen, Merck Serono, Novartis, Roche and Viatris. She received speaker honoraria and travel support from Bayer, Biogen, Bristol-Myers Squibb, Genzyme, Grifols, Merck Serono, Novartis, Roche, Santhera and Teva. She receives research support from the German Research Foundation, the Interdisciplinary Center for Clinical Research (IZKF) Münster, IMF Münster, Biogen, Immunic AG, Novartis and Merck Serono. J.A. has no competing interests. T.K. received research funding from the German Research Foundation, IZKF Münster, National MS Society, Progressive MS Alliance, European Commission (H2020-MSCA-ITN-2018) and Novartis. She received compensation for serving on scientific advisory boards (Novartis) and speaker honoraria from Biogen, Novartis and Roche.
Peer review
Peer review information
Nature Reviews Neurology thanks Catherine Lubetzki, Richard Reynolds and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Inflammaging
-
Chronic, sterile, low-grade inflammation involving the innate immune system, which accelerates the physiological ageing process.
- M1-polarized myeloid cells
-
Macrophages and microglial cells activated by LPS and cytokines such as IFNγ that contribute to inflammation, host defence and tumour cell elimination.
- M2-polarized myeloid cells
-
Macrophages and microglial cells activated by cytokines, such as IL-4 and IL-13, that contribute to tissue repair, angiogenesis and immune modulation.
- Magnetization transfer ratio
-
Magnetic imaging technique used for the evaluation of myelin content in the CNS.
- Major histocompatibility complex (MHC) class I molecules
-
Molecules expressed on all nucleated cells that present peptides derived from cytosolic proteins to elicit an immune response via cytotoxic CD8+ T cells.
- MHC class II molecules
-
Molecules expressed on professional antigen-presenting cells that present extracellular peptides to elicit an immune response via activation of CD4+ T helper cells.
- Micropillar array
-
High-throughput screening platform where small, standardized pillars serve as ‘axon-like’ structures to enable concentric wrapping by myelin sheaths.
- Normal-appearing white matter
-
Brain areas without focal demyelination.
- Shadow plaques
-
Completely remyelinated multiple sclerosis lesions.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Klotz, L., Antel, J. & Kuhlmann, T. Inflammation in multiple sclerosis: consequences for remyelination and disease progression. Nat Rev Neurol 19, 305–320 (2023). https://doi.org/10.1038/s41582-023-00801-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41582-023-00801-6
This article is cited by
-
Immunological aspects of central neurodegeneration
Cell Discovery (2024)
-
Physiological aging and inflammation-induced cellular senescence may contribute to oligodendroglial dysfunction in MS
Acta Neuropathologica (2024)
-
Association of Mediterranean diet adherence with disease progression, quality of life and physical activity, sociodemographic and anthropometric parameters, and serum biomarkers in community-dwelling older adults with multiple sclerosis: a cross-sectional study
Aging Clinical and Experimental Research (2024)