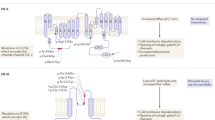
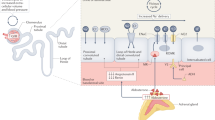
Abstract
Primary aldosteronism is the most common single cause of hypertension and is potentially curable when only one adrenal gland is the culprit. The importance of primary aldosteronism to public health derives from its high prevalence but huge under-diagnosis (estimated to be <1% of all affected individuals), despite the consequences of poor blood pressure control by conventional therapy and enhanced cardiovascular risk. This state of affairs is attributable to the fact that the tools used for diagnosis or treatment are still those that originated in the 1970–1990s. Conversely, molecular discoveries have transformed our understanding of adrenal physiology and pathology. Many molecules and processes associated with constant adrenocortical renewal and interzonal metamorphosis also feature in aldosterone-producing adenomas and aldosterone-producing micronodules. The adrenal gland has one of the most significant rates of non-silent somatic mutations, with frequent selection of those driving autonomous aldosterone production, and distinct clinical presentations and outcomes for most genotypes. The disappearance of aldosterone synthesis and cells from most of the adult human zona glomerulosa is the likely driver of the mutational success that causes aldosterone-producing adenomas, but insights into the pathways that lead to constitutive aldosterone production and cell survival may open up opportunities for novel therapies.
Key points
-
Primary aldosteronism (PA) is a common cause of hypertension; it is potentially curable when caused by a unilateral aldosterone-producing adenoma (APA), but it can lead to resistant hypertension and a high cardiovascular risk in cases in which PA is undiagnosed.
-
Current clinical guidelines and practice centres on risky tests and procedures, such as saline suppression, adrenal sampling and surgery, and poorly tolerated drugs such as spironolactone; these practices have remained largely unchanged over the past 30 years.
-
By contrast, the discovery of somatic mutations over the past decade has identified these as the cause of autonomous aldosterone production in most patients with PA, including 80–90% of APAs; the identified mutations most commonly affect the ion-channel genes KCNJ5 and CACNA1D.
-
Several distinct genotype–phenotype patterns and emerging clinical surrogates for genotype suggest the potential for molecular discovery to predict individual patient outcomes, permitting rational stratification of interventions.
-
In vivo molecular ligands for aldosterone synthase have been validated for non-invasive PET CT detection of APAs; on the left side, ultrasound-guided endoscopic radiofrequency ablation of PET-imaged APAs seems to be a safe alternative to surgery.
-
Genetic deletion models suggest curative potential for the suppression, rather than blockade, of aldosterone synthesis.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Simpson, S. A. et al. [Isolation from the adrenals of a new crystalline hormone with especially high effectiveness on mineral metabolism]. Experientia 9, 333–335 (1953).
Conn, J. W. & Louis, L. H. Primary aldosteronism, a new clinical entity. Ann. Intern. Med. 44, 1–15 (1956).
Andersen, G. S., Toftdahl, D. B., Lund, J. O., Strandgaard, S. & Nielsen, P. E. The incidence rate of phaeochromocytoma and Conn’s syndrome in Denmark, 1977–1981. J. Hum. Hypertens. 2, 187–189 (1988).
Grell, G. A., Hanchard, B., Fletcher, P. R., Clarke, W. F. & Williams, W. Conn’s syndrome. Case report and a review of the syndrome of primary aldosteronism (SOPA). West Indian Med. J. 33, 48–54 (1984).
Simpson, F. & Barnett, A. Primary aldosteronism (Conn’s syndrome): report of a case, and an analysis of published cases. Med. J. Aust. 1, 729–735 (1961).
Merrell, R. C. Aldosterone-producing tumors (Conn’s syndrome). Semin. Surg. Oncol. 6, 66–70 (1990).
Kaplan, N. M. Hypokalemia in the hypertensive patient, with observations on the incidence of primary aldosteronism. Ann. Intern. Med. 66, 1079–1090 (1967).
Conn, J. W., Rovner, D. R., Cohen, E. L. & Nesbit, R. M. Normokalemic primary aldosteronism: its masquerade as essential hypertension. JAMA 195, 21–26 (1966).
Rossi, G. P. et al. A prospective study of the prevalence of primary aldosteronism in 1,125 hypertensive patients. J. Am. Coll. Cardiol. 48, 2293–2300 (2006).
Monticone, S. et al. Prevalence and clinical manifestations of primary aldosteronism encountered in primary care practice. J. Am. Coll. Cardiol. 69, 1811–1820 (2017).
Hundemer, G. L., Curhan, G. C., Yozamp, N., Wang, M. & Vaidya, A. Cardiometabolic outcomes and mortality in medically treated primary aldosteronism: a retrospective cohort study. Lancet Diabetes Endocrinol. 6, 51–59 (2018).
Monticone, S. et al. Cardiovascular events and target organ damage in primary aldosteronism compared with essential hypertension: a systematic review and meta-analysis. Lancet Diabetes Endocrinol. 6, 41–50 (2018).
Williams, B. & Brown, M. J. Investigation of primary aldosteronism in patients with resistant hypertension — Authors’ reply. Lancet Diabetes Endocrinol. 6, 600–601 (2018).
Mulatero, P. et al. Guidelines for primary aldosteronism: uptake by primary care physicians in Europe. J. Hypertens. 34, 2253–2257 (2016).
Fernandes-Rosa, F. L., Boulkroun, S. & Zennaro, M. C. Somatic and inherited mutations in primary aldosteronism. J. Mol. Endocrinol. 59, R47–R63 (2017).
Nanba, K. et al. Targeted molecular characterization of aldosterone-producing adenomas in white Americans. J. Clin. Endocrinol. Metab. 103, 3869–3876 (2018).
De Sousa, K. et al. Genetic, cellular, and molecular heterogeneity in adrenals with aldosterone-producing adenoma. Hypertension 75, 1034–1044 (2020).
Choi, M. et al. K+ channel mutations in adrenal aldosterone-producing adenomas and hereditary hypertension. Science 331, 768–772 (2011).
Gomez-Sanchez, C. E. et al. Development of monoclonal antibodies against human CYP11B1 and CYP11B2. Mol. Cell Endocrinol. 383, 111–117 (2014).
Wang, T. et al. Gene expression profiles in aldosterone-producing adenomas and adjacent adrenal glands. Eur. J. Endocrinol. 164, 613–619 (2011).
Azizan, E. A. et al. Microarray, qPCR, and KCNJ5 sequencing of aldosterone-producing adenomas reveal differences in genotype and phenotype between zona glomerulosa- and zona fasciculata-like tumors. J. Clin. Endocrinol. Metab. 97, E819–E829 (2012).
Azizan, E. A. et al. Somatic mutations in ATP1A1 and CACNA1D underlie a common subtype of adrenal hypertension. Nat. Genet. 45, 1055–1060 (2013).
Kobuke, K. et al. Calneuron 1 Increased Ca2+ in the endoplasmic reticulum and aldosterone production in aldosterone-producing adenoma. Hypertension 71, 125–133 (2018).
Monticone, S. et al. Immunohistochemical, genetic and clinical characterization of sporadic aldosterone-producing adenomas. Mol. Cell Endocrinol. 411, 146–154 (2015).
Åkerström, T. et al. Novel somatic mutations and distinct molecular signature in aldosterone-producing adenomas. Endocr. Relat. Cancer 22, 735–744 (2015).
Takeda, M., Go, H., Imai, T., Nishiyama, T. & Morishita, H. Laparoscopic adrenalectomy for primary aldosteronism: report of initial ten cases. Surgery 115, 621–625 (1994).
Dunnick, N. R. et al. CT in the diagnosis of primary aldosteronism: sensitivity in 29 patients. AJR Am. J. Roentgenol. 160, 321–324 (1993).
Young, W. F. et al. Role for adrenal venous sampling in primary aldosteronism. Surgery 136, 1227–1235 (2004).
Funder, J. W. et al. The management of primary aldosteronism: case detection, diagnosis, and treatment: an endocrine society clinical practice guideline. J. Clin. Endocrinol. Metab. 101, 1889–1916 (2016).
Hiramatsu, K. et al. A screening test to identify aldosterone-producing adenoma by measuring plasma renin activity. Results in hypertensive patients. Arch. Intern. Med. 141, 1589–1593 (1981).
Gros, R., Ding, Q., Liu, B., Chorazyczewski, J. & Feldman, R. D. Aldosterone mediates its rapid effects in vascular endothelial cells through GPER activation. Am. J. Physiol. Cell Physiol. 304, C532–C540 (2013).
Funder, J. W. Primary aldosteronism and salt. Pflugers Arch. 467, 587–594 (2015).
Funder, J. in Stress: Neuroendocrinology and Neurobiology 221–225 (Academic Press, 2017).
Brown, J. M. et al. The unrecognized prevalence of primary aldosteronism: a cross-sectional study. Ann. Intern. Med. 173, 10–20 (2020).
Drake, W. M. & Brown, M. J. in Oxford Textbook of Endocrinology and Diabetes 3rd edition (eds Wass, J. A. H. et al.) (Oxford University Press, 2022).
Nishimoto, K. et al. Adrenocortical zonation in humans under normal and pathological conditions. J. Clin. Endocrinol. Metab. 95, 2296–2305 (2010).
Gomez-Sanchez, C. E. & Gomez-Sanchez, E. P. Immunohistochemistry of the adrenal in primary aldosteronism. Curr. Opin. Endocrinol. Diabetes Obes. 23, 242–248 (2016).
Omata, K. et al. Cellular and genetic causes of idiopathic hyperaldosteronism. Hypertension 72, 874–880 (2018).
Yizhak, K. et al. RNA sequence analysis reveals macroscopic somatic clonal expansion across normal tissues. Science 364, eaaw0726 (2019).
Lifton, R. P. et al. A chimaeric 11 beta-hydroxylase/aldosterone synthase gene causes glucocorticoid-remediable aldosteronism and human hypertension. Nature 355, 262–265 (1992).
Geller, D. S. et al. A novel form of human mendelian hypertension featuring nonglucocorticoid-remediable aldosteronism. J. Clin. Endocrinol. Metab. 93, 3117–3123 (2008).
Burton, T. J. et al. Evaluation of the sensitivity and specificity of (11)C-metomidate positron emission tomography (PET)-CT for lateralizing aldosterone secretion by Conn’s adenomas. J. Clin. Endocrinol. Metab. 97, 100–109 (2012).
Laycock, K. et al. ‘Pseudo-failure’ of adrenal vein sampling due to cortisol co-secretion by KCNJ5-mutant adenoma, and prediction of complete clinical success by urine hybrid steroid assay. Endocrine Abstracts https://doi.org/10.1530/endoabs.91.OC1 (2023).
Yamada, M. et al. KCNJ5 mutations in aldosterone- and cortisol-co-secreting adrenal adenomas. Endocr. J. 59, 735–741 (2012).
Gomez-Sanchez, C. E. & Oki, K. Minireview: potassium channels and aldosterone dysregulation: is primary aldosteronism a potassium channelopathy? Endocrinology 155, 47–55 (2014).
Chen, A. X., Nishimoto, K., Nanba, K. & Rainey, W. E. Potassium channels related to primary aldosteronism: expression similarities and differences between human and rat adrenals. Mol. Cell Endocrinol. 417, 141–148 (2015).
Maria, A. G. et al. Mosaicism for KCNJ5 causing early-onset primary aldosteronism due to bilateral adrenocortical hyperplasia. Am. J. Hypertens. 33, 124–130 (2020).
Cheng, C. J. et al. Novel KCNJ5 mutations in sporadic aldosterone-producing adenoma reduce Kir3.4 membrane abundance. J. Clin. Endocrinol. Metab. 100, E155–E163 (2015).
Murthy, M., Azizan, E. A., Brown, M. J. & O’Shaughnessy, K. M. Characterization of a novel somatic KCNJ5 mutation delI157 in an aldosterone-producing adenoma. J. Hypertens. 30, 1827–1833 (2012).
Charmandari, E. et al. A novel point mutation in the KCNJ5 gene causing primary hyperaldosteronism and early-onset autosomal dominant hypertension. J. Clin. Endocrinol. Metab. 97, E1532–E1539 (2012).
Zheng, F. F. et al. A novel somatic mutation 145-147delETEinsK in KCNJ5 increases aldosterone production. J. Hum. Hypertens. 31, 756–759 (2017).
Kuppusamy, M. et al. A novel KCNJ5-insT149 somatic mutation close to, but outside, the selectivity filter causes resistant hypertension by loss of selectivity for potassium. J. Clin. Endocrinol. Metab. 99, E1765–E1773 (2014).
Rege, J., Turcu, A. F. & Rainey, W. E. Primary aldosteronism diagnostics: KCNJ5 mutations and hybrid steroid synthesis in aldosterone-producing adenomas. Gland. Surg. 9, 3–13 (2020).
Scholl, U. I. et al. Hypertension with or without adrenal hyperplasia due to different inherited mutations in the potassium channel KCNJ5. Proc. Natl Acad. Sci. USA 109, 2533–2538 (2012).
Monticone, S. et al. A case of severe hyperaldosteronism caused by a de novo mutation affecting a critical salt bridge Kir3.4 residue. J. Clin. Endocrinol. Metab. 100, E114–E118 (2015).
Mulatero, P. et al. KCNJ5 mutations in European families with nonglucocorticoid remediable familial hyperaldosteronism. Hypertension 59, 235–240 (2012).
Monticone, S. et al. A novel Y152C KCNJ5 mutation responsible for familial hyperaldosteronism type III. J. Clin. Endocrinol. Metab. 98, E1861–E1865 (2013).
Lenzini, L. et al. A meta-analysis of somatic KCNJ5 K+ channel mutations in 1636 patients with an aldosterone-producing adenoma. J. Clin. Endocrinol. Metab. 100, E1089–E1095 (2015).
Wang, B. et al. Prevalence and characterization of somatic mutations in Chinese aldosterone-producing adenoma patients. Medicine 94, e708 (2015).
Nanba, K. et al. Genetic characteristics of aldosterone-producing adenomas in Blacks. Hypertension 73, 885–892 (2019).
Azizan, E. et al. Half of an unselected series of aldosterone-producing adenomas have somatic mutation of the KCNJ5 channel and a zona fasciculata profile. J. Hum. Hypertens. 25, 627 (2011).
Okamura, T. et al. Characteristics of Japanese aldosterone-producing adenomas with KCNJ5 mutations. Endocr. J. 64, 39–47 (2017).
Wu, X. et al. [(11)C]metomidate PET-CT versus adrenal vein sampling for diagnosing surgically curable primary aldosteronism: a prospective, within-patient trial. Nat. Med. 29, 190–202 (2023).
Zhou, J. et al. Transcriptome pathway analysis of pathological and physiological aldosterone-producing human tissues. Hypertension 68, 1424–1431 (2016).
Maniero, C. et al. NEFM (Neurofilament Medium) Polypeptide, a marker for zona glomerulosa cells in human adrenal, inhibits D1R (Dopamine D1 Receptor)-mediated secretion of aldosterone. Hypertension 70, 357–364 (2017).
Maniero, C. A functional study on novel genes involved in regulating aldosterone secretion in normal human zona glomerulosa and in aldosterone-producing adenomas. Doctoral dissertation, University of Cambridge (2017).
Beuschlein, F. et al. Somatic mutations in ATP1A1 and ATP2B3 lead to aldosterone-producing adenomas and secondary hypertension. Nat. Genet. 45, 440–444 (2013). 444e1–2.
Stindl, J. et al. Pathogenesis of adrenal aldosterone-producing adenomas carrying mutations of the Na+/K+-ATPase. Endocrinology 156, 4582–4591 (2015).
Meyer, D. J., Gatto, C. & Artigas, P. On the effect of hyperaldosteronism-inducing mutations in Na/K pumps. J. Gen. Physiol. 149, 1009–1028 (2017).
Schlingmann, K. P. et al. Germline de novo mutations in ATP1A1 cause renal hypomagnesemia, refractory seizures, and intellectual disability. Am. J. Hum. Genet. 103, 808–816 (2018).
Stregapede, F. et al. Hereditary spastic paraplegia is a novel phenotype for germline de novo ATP1A1 mutation. Clin. Genet. 97, 521–526 (2020).
Williams, T. A. et al. Somatic ATP1A1, ATP2B3, and KCNJ5 mutations in aldosterone-producing adenomas. Hypertension 63, 188–195 (2014).
Tauber, P. et al. Cellular pathophysiology of an adrenal adenoma-associated mutant of the plasma membrane Ca2+-ATPase ATP2B3. Endocrinology 157, 2489–2499 (2016).
Zanni, G. et al. Mutation of plasma membrane Ca2+ ATPase isoform 3 in a family with X-linked congenital cerebellar ataxia impairs Ca2+ homeostasis. Proc. Natl Acad. Sci. USA 109, 14514–14519 (2012).
Scholl, U. I. et al. Somatic and germline CACNA1D calcium channel mutations in aldosterone-producing adenomas and primary aldosteronism. Nat. Genet. 45, 1050–1054 (2013).
Catterall, W. A. Signaling complexes of voltage-gated sodium and calcium channels. Neurosci. Lett. 486, 107–116 (2010).
Dutta, R. K., Söderkvist, P. & Gimm, O. Genetics of primary hyperaldosteronism. Endocr. Relat. Cancer 23, R437–R454 (2016).
Azizan, E. A. & Brown, M. J. Novel genetic determinants of adrenal aldosterone regulation. Curr. Opin. Endocrinol. Diabetes Obes. 23, 209–217 (2016).
Korah, H. E. & Scholl, U. I. an update on familial hyperaldosteronism. Horm. Metab. Res. 47, 941–946 (2015).
Tseng, C. S. et al. A novel somatic mutation of CACNA1H p.V1937M in unilateral primary hyperaldosteronism. Front. Endocrinol. 13, 816476 (2022).
Nanba, K. et al. Somatic CACNA1H mutation as a cause of aldosterone-producing adenoma. Hypertension 75, 645–649 (2020).
Scholl, U. I. et al. Recurrent gain of function mutation in calcium channel CACNA1H causes early-onset hypertension with primary aldosteronism. Elife 4, e06315 (2015).
Scholl, U. I. et al. CLCN2 chloride channel mutations in familial hyperaldosteronism type II. Nat. Genet. 50, 349–354 (2018).
Rege, J. et al. Identification of somatic mutations in CLCN2 in aldosterone-producing adenomas. J. Endocr. Soc. 4, bvaa123 (2020).
Fernandes-Rosa, F. L. et al. A gain-of-function mutation in the CLCN2 chloride channel gene causes primary aldosteronism. Nat. Genet. 50, 355–361 (2018).
Dutta, R. K. et al. A somatic mutation in CLCN2 identified in a sporadic aldosterone-producing adenoma. Eur. J. Endocrinol. 181, K37–K41 (2019).
Teo, A. E. et al. Pregnancy, primary aldosteronism, and adrenal CTNNB1 mutations. N. Engl. J. Med. 373, 1429–1436 (2015).
Backman, S. et al. RNA sequencing provides novel insights into the transcriptome of aldosterone producing adenomas. Sci. Rep. 9, 6269 (2019).
Zhou, J. et al. Somatic mutations of GNA11 and GNAQ in CTNNB1-mutant aldosterone-producing adenomas presenting in puberty, pregnancy or menopause. Nat. Genet. 53, 1360–1372 (2021).
Ayturk, U. M. et al. Somatic activating mutations in GNAQ and GNA11 are associated with congenital hemangioma. Am. J. Hum. Genet. 98, 1271 (2016).
Van Raamsdonk, C. D. et al. Mutations in GNA11 in uveal melanoma. N. Engl. J. Med. 363, 2191–2199 (2010).
Shirley, M. D. et al. Sturge-Weber syndrome and port-wine stains caused by somatic mutation in GNAQ. N. Engl. J. Med. 368, 1971–1979 (2013).
Saner-Amigh, K. et al. Elevated expression of luteinizing hormone receptor in aldosterone-producing adenomas. J. Clin. Endocrinol. Metab. 91, 1136–1142 (2006).
Rey, R. A. et al. Unexpected mosaicism of R201H-GNAS1 mutant-bearing cells in the testes underlie macro-orchidism without sexual precocity in McCune-Albright syndrome. Hum. Mol. Genet. 15, 3538–3543 (2006).
Wu, X. et al. Somatic intramembranous mutations of CADM1 in aldosterone-producing adenomas, and gap junction-dependent regulation of aldosterone production. Nat. Genet. (2023).
Fujita, E., Urase, K., Soyama, A., Kouroku, Y. & Momoi, T. Distribution of RA175/TSLC1/SynCAM, a member of the immunoglobulin superfamily, in the developing nervous system. Brain Res. Dev. Brain Res. 154, 199–209 (2005).
Crumbley, C., Wang, Y., Kojetin, D. J. & Burris, T. P. Characterization of the core mammalian clock component, NPAS2, as a REV-ERBα/RORα target gene. J. Biol. Chem. 285, 35386–35392 (2010).
Laposky, A. & Turek, F. in Encyclopedia of Neuroscience 909–914 (Elsevier, 2009).
Upton, T. J. U-RHYTHM microdialysis: towards ambulatory metabolodynamics. Doctoral dissertation, University of Otago (2021).
Rege, J. et al. Zinc transporter somatic gene mutations cause primary aldosteronism. Preprint at bioRxiv https://doi.org/10.1101/2022.07.25.501443 (2022).
Williams, T. A. et al. Visinin-like 1 is upregulated in aldosterone-producing adenomas with KCNJ5 mutations and protects from calcium-induced apoptosis. Hypertension 59, 833–839 (2012).
Felizola, S. J. et al. PCP4: a regulator of aldosterone synthesis in human adrenocortical tissues. J. Mol. Endocrinol. 52, 159–167 (2014).
Trejter, M. et al. Visinin-like peptide 1 in adrenal gland of the rat. Gene expression and its hormonal control. Peptides 63, 22–29 (2015).
Zhou, J. et al. DACH1, a zona glomerulosa selective gene in the human adrenal, activates transforming growth factor-β signaling and suppresses aldosterone secretion. Hypertension 65, 1103–1110 (2015).
Shaikh, L. H. et al. LGR5 activates noncanonical wnt signaling and inhibits aldosterone production in the human adrenal. J. Clin. Endocrinol. Metab. 100, E836–E844 (2015).
Teo, A. E. et al. Physiological and pathological roles in human adrenal of the glomeruli-defining matrix protein NPNT (Nephronectin). Hypertension 69, 1207–1216 (2017).
Nishimoto, K. et al. Aldosterone-stimulating somatic gene mutations are common in normal adrenal glands. Proc. Natl Acad. Sci. USA 112, E4591–E4599 (2015).
Maniero, C. et al. ANO4 (Anoctamin 4) is a novel marker of zona glomerulosa that regulates stimulated aldosterone secretion. Hypertension 74, 1152–1159 (2019).
Barker, N. et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 449, 1003–1007 (2007).
Vidal, V. et al. The adrenal capsule is a signaling center controlling cell renewal and zonation through Rspo3. Genes. Dev. 30, 1389–1394 (2016).
Nanba, K. et al. Age-related autonomous aldosteronism. Circulation 136, 347–355 (2017).
van de Wiel, E. et al. Changes of the CYP11B2 expressing zona glomerulosa in human adrenals from birth to 40 years of age. Hypertension 79, 2565–2572 (2022).
Lucas, C. et al. Loss of LGR4/GPR48 causes severe neonatal salt wasting due to disrupted WNT signaling altering adrenal zonation. J. Clin. Invest. 133, e164915 (2023).
Hayashi, T. et al. Expression of aldosterone synthase CYP11B2 was inversely correlated with longevity. J. Steroid Biochem. Mol. Biol. 191, 105361 (2019).
Boulkroun, S. et al. Adrenal cortex remodeling and functional zona glomerulosa hyperplasia in primary aldosteronism. Hypertension 56, 885–892 (2010).
Williams, T. A. et al. International histopathology consensus for unilateral primary aldosteronism. J. Clin. Endocrinol. Metab. 106, 42–54 (2021).
Sun, N. et al. Mass spectrometry imaging establishes 2 distinct metabolic phenotypes of aldosterone-producing cell clusters in primary aldosteronism. Hypertension 75, 634–644 (2020).
Goodchild, E., Stoetaert, J., Drake, W., Linton, K. & Brown, M. J. OR09-05 expression of SLC35F1 in the plasma membrane of cells of aldosterone producing cell clusters (APCCs) and its possible role in aldosterone synthesis. J. Endocr. Soc. 4, OR09–05 (2020).
Markou, A. et al. Stress-induced aldosterone hyper-secretion in a substantial subset of patients with essential hypertension. J. Clin. Endocrinol. Metab. 100, 2857–2864 (2015).
Bogman, K. et al. Preclinical and early clinical profile of a highly selective and potent oral inhibitor of aldosterone synthase (CYP11B2). Hypertension 69, 189–196 (2017).
Nishimoto, K. et al. Case report: nodule development from subcapsular aldosterone-producing cell clusters causes hyperaldosteronism. J. Clin. Endocrinol. Metab. 101, 6–9 (2016).
Nishimoto, K. et al. Human adrenocortical remodeling leading to aldosterone-producing cell cluster generation. Int. J. Endocrinol. 2016, 7834356 (2016).
Baker, J. E. et al. Targeted RNA sequencing of adrenal zones using immunohistochemistry-guided capture of formalin-fixed paraffin-embedded tissue. Mol. Cell Endocrinol. 530, 111296 (2021).
Gene Expression Omnibus. Series GSE68889. https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE68889 (2023).
Ebermann, I. et al. Two truncating USH3A mutations, including one novel, in a German family with Usher syndrome. Mol. Vis. 13, 1539–1547 (2007).
Khan, M. I. et al. CLRN1 mutations cause nonsyndromic retinitis pigmentosa. Ophthalmology 118, 1444–1448 (2011).
Audo, I. et al. A novel DFNB31 mutation associated with Usher type 2 syndrome showing variable degrees of auditory loss in a consanguineous Portuguese family. Mol. Vis. 17, 1598–1606 (2011).
Kersten, F. F. et al. Association of whirlin with Cav1.3 (α1D) channels in photoreceptors, defining a novel member of the usher protein network. Invest. Ophthalmol. Vis. Sci. 51, 2338–2346 (2010).
Platzer, J. et al. Congenital deafness and sinoatrial node dysfunction in mice lacking class D L-type Ca2+ channels. Cell 102, 89–97 (2000).
Bai, Y. et al. Primary cilium in kidney development, function and disease. Front. Endocrinol. 13, 952055 (2022).
Mackenzie, I. S. & Brown, M. J. Molecular and clinical investigations in patients with low-renin hypertension. Clin. Exp. Nephrol. 13, 1–8 (2009).
Arnaldi, G. et al. ACTH receptor mRNA in human adrenocortical tumors: overexpression in aldosteronomas. Endocr. Res. 24, 845–849 (1998).
Assié, G. et al. Steroidogenesis in aldosterone-producing adenoma revisited by transcriptome analysis. J. Clin. Endocrinol. Metab. 90, 6638–6649 (2005).
Bassett, M. H. et al. Expression profiles for steroidogenic enzymes in adrenocortical disease. J. Clin. Endocrinol. Metab. 90, 5446–5455 (2005).
Fallo, F. et al. Quantitative assessment of CYP11B1 and CYP11B2 expression in aldosterone-producing adenomas. Eur. J. Endocrinol. 147, 795–802 (2002).
Schubert, B. et al. Angiotensin II type 1 receptor and ACTH receptor expression in human adrenocortical neoplasms. Clin. Endocrinol. 54, 627–632 (2001).
Williams, T. A. et al. Teratocarcinoma-derived growth factor-1 is upregulated in aldosterone-producing adenomas and increases aldosterone secretion and inhibits apoptosis in vitro. Hypertension 55, 1468–1475 (2010).
Ye, P., Mariniello, B., Mantero, F., Shibata, H. & Rainey, W. E. G-protein-coupled receptors in aldosterone-producing adenomas: a potential cause of hyperaldosteronism. J. Endocrinol. 195, 39–48 (2007).
Yang, Y. et al. BEX1 is differentially expressed in aldosterone-producing adenomas and protects human adrenocortical cells from ferroptosis. Hypertension 77, 1647–1658 (2021).
GTEx Portal. GTEx Analysis Release V8 (dbGaP Accession phs000424.v8.p2. https://gtexportal.org/home/gene/KCNJ5 (2023).
Papp, F. et al. TMEM266 is a functional voltage sensor regulated by extracellular Zn(2). Elife 8 https://doi.org/10.7554/elife.42372 (2019).
Kawai, T. et al. Insight into the function of a unique voltage-sensor protein (TMEM266) and its short form in mouse cerebellum. Biochem. J. 479, 1127–1145 (2022).
Guagliardo, N. A. et al. TASK-3 channel deletion in mice recapitulates low-renin essential hypertension. Hypertension 59, 999–1005 (2012).
Barrett, P. Q. et al. Role of voltage-gated calcium channels in the regulation of aldosterone production from zona glomerulosa cells of the adrenal cortex. J. Physiol. 594, 5851–5860 (2016).
Le Floch, E. et al. Identification of risk loci for primary aldosteronism in genome-wide association studies. Nat. Commun. 13, 5198 (2022).
Spät, A., Rohács, T., Horváth, A., Szabadkai, G. & Enyedi, P. The role of voltage-dependent calcium channels in angiotensin-stimulated glomerulosa cells. Endocr. Res. 22, 569–576 (1996).
Davies, L. A. et al. TASK channel deletion in mice causes primary hyperaldosteronism. Proc. Natl Acad. Sci. USA 105, 2203–2208 (2008).
Wannachalee, T., Lieberman, L. & Turcu, A. F. High prevalence of autonomous aldosterone production in hypertension: how to identify and treat it. Curr. Hypertens. Rep. 24, 123–132 (2022).
Silins, I. et al. First-in-human evaluation of [18F]CETO: a novel tracer for adrenocortical tumours. Eur. J. Nucl. Med Mol. Imaging 50, 398–409 (2023).
Goodchild, E. et al. in Endocrine Abstracts Vol. 86 (Bioscientifica, 2022).
Sander, K. et al. Development of [18F]aldoview as the first highly selective aldosterone synthase pet tracer for imaging of primary hyperaldosteronism. J. Med. Chem. 64, 9321–9329 (2021).
Hu, J. et al. Accuracy of gallium-68 pentixafor positron emission tomography-computed tomography for subtyping diagnosis of primary aldosteronism. JAMA Netw. Open. 6, e2255609 (2023).
Martín-de-Saavedra, M. D., Santos, M. D. & Penzes, P. Intercellular signaling by ectodomain shedding at the synapse. Trends Neurosci. 45, 483–498 (2022).
Guo, R. Q., Li, Y. M. & Li, X. G. Comparison of the radiofrequency ablation versus laparoscopic adrenalectomy for aldosterone-producing adenoma: a meta-analysis of perioperative outcomes and safety. Updates Surg. 73, 1477–1485 (2021).
Fintelmann, F. J. et al. Catecholamine surge during image-guided ablation of adrenal gland metastases: predictors, consequences, and recommendations for management. J. Vasc. Interv. Radiol. 27, 395–402 (2016).
Oguro, S. et al. Safety and feasibility of radiofrequency ablation using bipolar electrodes for aldosterone-producing adenoma: a multicentric prospective clinical study. Sci. Rep. 12, 14090 (2022).
Zhao, Z. et al. Catheter-based adrenal ablation remits primary aldosteronism: a randomized medication-controlled trial. Circulation 144, 580–582 (2021).
Argentesi, G. et al. in Endocrine Abstracts Vol. 86 (Bioscientifica, 2022).
US National Library of Medicine. ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT05405101 (2023).
Brown, M. J. & Hopper, R. V. Calcium-channel blockade can mask the diagnosis of Conn’s syndrome. Postgrad. Med. J. 75, 235–236 (1999).
Lee, G. et al. Homeostatic responses in the adrenal cortex to the absence of aldosterone in mice. Endocrinology 146, 2650–2656 (2005).
Kang, S. et al. CaV1.3-selective L-type calcium channel antagonists as potential new therapeutics for Parkinson’s disease. Nat. Commun. 3, 1146 (2012).
Xie, C. B. et al. Regulation of aldosterone secretion by Cav1.3. Sci. Rep. 6, 24697 (2016).
Scholl, U. I. et al. Macrolides selectively inhibit mutant KCNJ5 potassium channels that cause aldosterone-producing adenoma. J. Clin. Invest. 127, 2739–2750 (2017).
Cheng, K. et al. The developmental origin and the specification of the adrenal cortex in humans and cynomolgus monkeys. Sci. Adv. 8, eabn8485 (2022).
Freedman, B. D. et al. Adrenocortical zonation results from lineage conversion of differentiated zona glomerulosa cells. Dev. Cell 26, 666–673 (2013).
Ludwig, L. S. et al. Lineage tracing in humans enabled by mitochondrial mutations and single-cell genomics. Cell 176, 1325–1339.e22 (2019).
N, M. P. et al. Improved SNV discovery in barcode-stratified scRNA-seq alignments. Genes 12, 1558 (2021).
Bechmann, N., Berger, I., Bornstein, S. R. & Steenblock, C. Adrenal medulla development and medullary-cortical interactions. Mol. Cell Endocrinol. 528, 111258 (2021).
Bornstein, S. R., Ehrhart-Bornstein, M. & Scherbaum, W. A. Morphological and functional studies of the paracrine interaction between cortex and medulla in the adrenal gland. Microsc. Res. Tech. 36, 520–533 (1997).
Brittain, J. M. et al. An atypical role for collapsin response mediator protein 2 (CRMP-2) in neurotransmitter release via interaction with presynaptic voltage-gated calcium channels. J. Biol. Chem. 284, 31375–31390 (2009).
Simms, B. A. & Zamponi, G. W. Trafficking and stability of voltage-gated calcium channels. Cell Mol. Life Sci. 69, 843–856 (2012).
Di Mattia, T. et al. FFAT motif phosphorylation controls formation and lipid transfer function of inter-organelle contacts. EMBO J. 39, e104369 (2020).
Gu, S. et al. Hair cell α9α10 nicotinic acetylcholine receptor functional expression regulated by ligand binding and deafness gene products. Proc. Natl Acad. Sci. USA 117, 24534–24544 (2020).
Freeman, M. W. et al. Phase 2 trial of baxdrostat for treatment-resistant hypertension. N. Engl. J. Med 388, 395–405 (2022).
Yao, X., Gao, S. & Yan, N. Structural basis for pore blockade of human voltage-gated calcium channel Ca(v)1.3 by motion sickness drug cinnarizine. Cell Res. 32, 946–948 (2022).
Felizola, S. J. et al. Pre-B lymphocyte protein 3 (VPREB3) expression in the adrenal cortex: precedent for non-immunological roles in normal and neoplastic human tissues. Endocr. Pathol. 26, 119–128 (2015).
Guiraldelli, M. F. et al. SHOC1 is a ERCC4-(HhH)2-like protein, integral to the formation of crossover recombination intermediates during mammalian meiosis. PLoS Genet. 14, e1007381 (2018).
Iwamori, T., Iwamori, N., Matsumoto, M., Ono, E. & Matzuk, M. M. Identification of KIAA1210 as a novel X-chromosome-linked protein that localizes to the acrosome and associates with the ectoplasmic specialization in testes. Biol. Reprod. 96, 469–477 (2017).
Iwamori, T., Iwamori, N., Matsumoto, M., Imai, H. & Ono, E. Novel localizations and interactions of intercellular bridge proteins revealed by proteomic profiling†. Biol. Reprod. 102, 1134–1144 (2020).
Ogle, B. M., Cascalho, M. & Platt, J. L. Biological implications of cell fusion. Nat. Rev. Mol. Cell Biol. 6, 567–575 (2005).
Sottile, F., Aulicino, F., Theka, I. & Cosma, M. P. Mesenchymal stem cells generate distinct functional hybrids in vitro via cell fusion or entosis. Sci. Rep. 6, 36863 (2016).
Jacobsen, B. M. et al. Spontaneous fusion with, and transformation of mouse stroma by, malignant human breast cancer epithelium. Cancer Res. 66, 8274–8279 (2006).
Mortensen, K., Lichtenberg, J., Thomsen, P. D. & Larsson, L. I. Spontaneous fusion between cancer cells and endothelial cells. Cell Mol. Life Sci. 61, 2125–2131 (2004).
Pfannkuche, K. Preface. in Cell Fusion: Overviews and Methods https://doi.org/10.1007/978-1-4939-2703-6 (Humana, 2015).
Dörnen, J., Sieler, M., Weiler, J., Keil, S. & Dittmar, T. Cell fusion-mediated tissue regeneration as an inducer of polyploidy and aneuploidy. Int. J. Mol. Sci. 21, 1811 (2020).
Bornstein, S. R., Ehrhart-Bornstein, M. & Scherbaum, W. A. Ultrastructural evidence for cortico-chromaffin hybrid cells in rat adrenals? Endocrinology 129, 1113–1115 (1991).
Hanukoglu, I. Antioxidant protective mechanisms against reactive oxygen species (ROS) generated by mitochondrial P450 systems in steroidogenic cells. Drug. Metab. Rev. 38, 171–196 (2006).
Ziegler, G. A., Vonrhein, C., Hanukoglu, I. & Schulz, G. E. The structure of adrenodoxin reductase of mitochondrial P450 systems: electron transfer for steroid biosynthesis. J. Mol. Biol. 289, 981–990 (1999).
Tadjine, M., Lampron, A., Ouadi, L. & Bourdeau, I. Frequent mutations of beta-catenin gene in sporadic secreting adrenocortical adenomas. Clin. Endocrinol. 68, 264–270 (2008).
Hundemer, G. L., Curhan, G. C., Yozamp, N., Wang, M. & Vaidya, A. Incidence of atrial fibrillation and mineralocorticoid receptor activity in patients with medically and surgically treated primary aldosteronism. JAMA Cardiol. 3, 768–774 (2018).
GTEx Portal. GTEx analysis release V8 (dbGaP accession phs000424.v8.p2). https://gtexportal.org/home/gene/KCNJ5 (accessed 10 April 2023).
RCSB Protein Data Bank. Crystal structure of the G protein-gated inward rectifier K+ channel GIRK2 (Kir3.2) in complex with sodium and PIP2. https://www.rcsb.org/structure/3SYA (2023).
Ortner, N. J. et al. De novo CACAN1D Ca2+ channelopathies: clinical phenotypes and molecular mechanism. Pflugers Arch. 472, 755–773 (2020).
The Human Protein Atlas. SLC35F1. https://www.proteinatlas.org/ENSG00000196376-SLC35F1/tissue/adrenal+gland#img (2023).
Uhlén, M. et al. Tissue-based map of the human proteome. Science 347, 1260419 (2015).
Williams, T. A. et al. Genotype-specific steroid profiles associated with aldosterone-producing adenomas. Hypertension 67, 139–145 (2016).
Seccia, T. M., Caroccia, B., Gomez-Sanchez, E. P., Gomez-Sanchez, C. E. & Rossi, G. P. The biology of normal zona glomerulosa and aldosterone-producing adenoma: pathological implications. Endocr. Rev. 39, 1029–1056 (2018).
Lenders, J. W. M., Eisenhofer, G. & Reincke, M. Subtyping of patients with primary aldosteronism: an update. Horm. Metab. Res. 49, 922–928 (2017).
Eisenhofer, G. et al. Mass spectrometry-based adrenal and peripheral venous steroid profiling for subtyping primary aldosteronism. Clin. Chem. 62, 514–524 (2016).
Satoh, F. et al. Measurement of peripheral plasma 18-oxocortisol can discriminate unilateral adenoma from bilateral diseases in patients with primary aldosteronism. Hypertension 65, 1096–1102 (2015).
Vilela, L. A. P. et al. KCNJ5 somatic mutation is a predictor of hypertension remission after adrenalectomy for unilateral primary aldosteronism. J. Clin. Endocrinol. Metab. 104, 4695–4702 (2019).
Acknowledgements
E.A.B.A.’s work is supported by the Ministry of Higher Education (MOHE) Malaysia, a Long-term Research Grant Scheme-Jejak Sarjana Ulung (LRGS-JSU), LRGS/1/2021/SKK15/UKM/02/2 (mentored by M.J.B); the Royal Society-Newton Advanced Fellowship, NA170257/FF-2018–033 (co-applicant with M.J.B.); and the UK-MY Joint Partnership on Noncommunicable Diseases 2019 program, NEWTON-MRC/2020/002 (co-applicant with M.J.B.). W.M.D.’s and M.J.B.’s work is supported by the National Institute for Health Research (NIHR, Efficacy and Mechanisms Evaluation project 14/145/09), Barts Charity (Project MGU0360), NIHR Integrated Academic Training Clinical Lectureship, the Medical Research Council (MR/S006869/1) and the British Heart Foundation (PG/16/40/32137). M.J.B. is also supported by the British Heart Foundation (Project MCPG1P3R and PhD studentship FS/14/75/31134) and NIHR Barts Biomedical Research Centre (BRC-1215–20022).
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Nephrology thanks Celso Gomez-Sanchez and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
GTEx portal: https://gtexportal.org/home/
RCSB Protein Data Bank: https://www.rcsb.org/
The Human Protein Atlas: www.proteinatlas.org
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Azizan, E.A.B., Drake, W.M. & Brown, M.J. Primary aldosteronism: molecular medicine meets public health. Nat Rev Nephrol 19, 788–806 (2023). https://doi.org/10.1038/s41581-023-00753-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41581-023-00753-6