Abstract
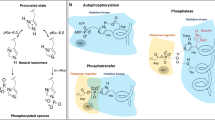
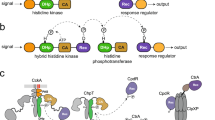
Two-component systems reprogramme diverse aspects of microbial physiology in response to environmental cues. Canonical systems are composed of a transmembrane sensor histidine kinase and its cognate response regulator. They catalyse three reactions: autophosphorylation of the histidine kinase, transfer of the phosphoryl group to the regulator and dephosphorylation of the phosphoregulator. Elucidating signal transduction between sensor and output domains is highly challenging given the size, flexibility and dynamics of histidine kinases. However, recent structural work has provided snapshots of the catalytic mechanisms of the three enzymatic reactions and described the conformation and dynamics of the enzymatic moiety in the kinase-competent and phosphatase-competent states. Insight into signalling mechanisms across the membrane is also starting to emerge from new crystal structures encompassing both sensor and transducer domains of sensor histidine kinases. In this Progress article, we highlight such important advances towards understanding at the molecular level the signal transduction mechanisms mediated by these fascinating molecular machines.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Zschiedrich, C. P., Keidel, V. & Szurmant, H. Molecular mechanisms of two-component signal transduction. J. Mol. Biol. 428, 3752–3775 (2016).
Krell, T. et al. Bacterial sensor-kinases: diversity in the recognition of environmental signals. Annu. Rev. Microbiol. 64, 539–559 (2010).
Mascher, T., Helmann, J. D. & Unden, G. Stimulus perception in bacterial signal-transducing histidine kinases. Microbiol. Mol. Biol. Rev. 70, 910–938 (2006).
Stock, A. M., Robinson, V. L. & Goudreau, P. N. Two-component signal transduction. Annu. Rev. Biochem. 69, 183–215 (2000).
Gushchin, I. & Gordeliy, V. Transmembrane signal transduction in two-component systems: piston, scissoring, or helical rotation? Bioessays 40, 2 (2018).
Parkinson, J. S., Hazelbauer, G. L. & Falke, J. J. Signaling and sensory adaptation in Escherichia coli chemoreceptors: 2015 update. Trends Microbiol. 23, 257–266 (2015).
Gao, R. & Stock, A. M. Biological insights from structures of two-component proteins. Annu. Rev. Microbiol. 63, 133–154 (2009).
Rivera-Cancel, G., Ko, W. H., Tomchick, D. R., Correa, F. & Gardner, K. H. Full-length structure of a monomeric histidine kinase reveals basis for sensory regulation. Proc. Natl Acad. Sci. USA 111, 17839–17844 (2014).
Pfluger, T. et al. Signaling ammonium across membranes through an ammonium sensor histidine kinase. Nat. Commun. 9, 164 (2018).
Ortega, A., Zhulin, I. B. & Krell, T. Sensory repertoire of bacterial chemoreceptors. Microbiol. Mol. Biol. Rev. 81, 28 (2017).
Albanesi, D. et al. Structural plasticity and catalysis regulation of a thermosensor histidine kinase. Proc. Natl Acad. Sci. USA 106, 16185–16190 (2009).
Möglich, A., Ayers, R. A. & Moffat, K. Design and signaling mechanism of light-regulated histidine kinases. J. Mol. Biol. 385, 1433–1444 (2009).
Moukhametzianov, R. et al. Development of the signal in sensory rhodopsin and its transfer to the cognate transducer. Nature 440, 115–119 (2006).
Vogt, S. & Raivio, T. Just scratching the surface: an expanding view of the Cpx envelope stress response. FEMS Microbiol. Lett. 326, 2–11 (2012).
Dupre, E. et al. Virulence regulation with venus flytrap domains: structure and function of the periplasmic moiety of the sensor-kinase BvgS. PLOS Pathog. 11, e1004700 (2015).
Dubrac, S. & Msadek, T. Tearing down the wall: peptidoglycan metabolism and the WalK/WalR (YycG/YycF) essential two-component system. Adv. Exp. Med. Biol. 631, 214–228 (2008).
Leonard, P. G., Golemi-Kotra, D. & Stock, A. M. Phosphorylation-dependent conformational changes and domain rearrangements in Staphylococcus aureus VraR activation. Proc. Natl Acad. Sci. USA 110, 8525–8530 (2013).
Bourret, R. B. & Silversmith, R. E. Two-component signal transduction. Curr. Opin. Microbiol. 13, 113–115 (2010).
Casino, P., Rubio, V. & Marina, A. The mechanism of signal transduction by two-component systems. Curr. Opin. Struct. Biol. 20, 763–771 (2010).
Cheung, J. & Hendrickson, W. A. Sensor domains of two-component regulatory systems. Curr. Opin. Microbiol. 13, 116–123 (2010).
Galperin, M. Y. Diversity of structure and function of response regulator output domains. Curr. Opin. Microbiol. 13, 150–159 (2010).
Gao, R. & Stock, A. M. Molecular strategies for phosphorylation-mediated regulation of response regulator activity. Curr. Opin. Microbiol. 13, 160–167 (2010).
Bhate, M. P., Molnar, K. S., Goulian, M. & DeGrado, W. F. Signal transduction in histidine kinases: insights from new structures. Structure 23, 981–994 (2015).
Casino, P., Rubio, V. & Marina, A. Structural insight into partner specificity and phosphoryl transfer in two-component signal transduction. Cell 139, 325–336 (2009).
Etzkorn, M. et al. Plasticity of the PAS domain and a potential role for signal transduction in the histidine kinase DcuS. Nat. Struct. Mol. Biol. 15, 1031–1039 (2008).
Hulko, M. et al. The HAMP domain structure implies helix rotation in transmembrane signaling. Cell 126, 929–940 (2006).
Molnar, K. S. et al. Cys-scanning disulfide crosslinking and bayesian modeling probe the transmembrane signaling mechanism of the histidine kinase, PhoQ. Structure 22, 1239–1251 (2014).
Paul, R. et al. Allosteric regulation of histidine kinases by their cognate response regulator determines cell fate. Cell 133, 452–461 (2008).
Trajtenberg, F. et al. Allosteric activation of bacterial response regulators: the role of the cognate histidine kinase beyond phosphorylation. mBio 5, e02105 (2014).
Mechaly, A. E. et al. Structural coupling between autokinase and phosphotransferase reactions in a bacterial histidine kinase. Structure 25, 939–944 (2017).
Rinaldi, J. et al. Structural insights into the HWE histidine kinase family: the Brucella blue light-activated histidine kinase domain. J. Mol. Biol. 428, 1165–1179 (2016).
Mechaly, A. E., Sassoon, N., Betton, J. M. & Alzari, P. M. Segmental helical motions and dynamical asymmetry modulate histidine kinase autophosphorylation. PLOS Biol. 12, e1001776 (2014).
Casino, P., Miguel-Romero, L. & Marina, A. Visualizing autophosphorylation in histidine kinases. Nat. Commun. 5, 3258 (2014).
Cai, Y. et al. Conformational dynamics of the essential sensor histidine kinase WalK. Acta Crystallogr. D Struct. Biol. 73, 793–803 (2017).
Ferris, H. U., Coles, M., Lupas, A. N. & Hartmann, M. D. Crystallographic snapshot of the Escherichia coli EnvZ histidine kinase in an active conformation. J. Struct. Biol. 186, 376–379 (2014).
Wang, C. et al. Mechanistic insights revealed by the crystal structure of a histidine kinase with signal transducer and sensor domains. PLOS Biol. 11, e1001493 (2013).
Dago, A. E. et al. Structural basis of histidine kinase autophosphorylation deduced by integrating genomics, molecular dynamics, and mutagenesis. Proc. Natl Acad. Sci. USA 109, E1733–E1742 (2012).
Trajtenberg, F., Grana, M., Ruetalo, N., Botti, H. & Buschiazzo, A. Structural and enzymatic insights into the ATP binding and autophosphorylation mechanism of a sensor histidine kinase. J. Biol. Chem. 285, 24892–24903 (2010).
Ashenberg, O., Keating, A. E. & Laub, M. T. Helix bundle loops determine whether histidine kinases autophosphorylate in cis or in trans. J. Mol. Biol. 425, 1198–1209 (2013).
Marsico, F. et al. Multiscale approach to the activation and phosphotransfer mechanism of CpxA histidine kinase reveals a tight coupling between conformational and chemical steps. Biochem. Biophys. Res. Commun. 498, 305–312 (2017).
Willett, J. W. & Kirby, J. R. Genetic and biochemical dissection of a HisKA domain identifies residues required exclusively for kinase and phosphatase activities. PLOS Genet. 8, e1003084 (2012).
Trajtenberg, F. et al. Regulation of signaling directionality revealed by 3D snapshots of a kinase:regulator complex in action. eLife 5, e21422 (2016).
West, A. H. & Stock, A. M. Histidine kinases and response regulator proteins in two-component signaling systems. Trends Biochem. Sci. 6, 369–376 (2001).
Willett, J. W., Herrou, J., Briegel, A., Rotskoff, G. & Crosson, S. Structural asymmetry in a conserved signaling system that regulates division, replication, and virulence of an intracellular pathogen. Proc. Natl Acad. Sci. USA 112, E3709–3718 (2015).
Zapf, J., Sen, U., Madhusudan, Hoch, J. A. & Varughese, K. I. A transient interaction between two phosphorelay proteins trapped in a crystal lattice reveals the mechanism of molecular recognition and phosphotransfer in signal transduction. Structure 8, 851–862 (2000).
Varughese, K. I., Tsigelny, I. & Zhao, H. The crystal structure of beryllofluoride Spo0F in complex with the phosphotransferase Spo0B represents a phosphotransfer pretransition state. J. Bacteriol. 188, 4970–4977 (2006).
Liu, Y. et al. A pH-gated conformational switch regulates the phosphatase activity of bifunctional HisKA-family histidine kinases. Nat. Commun. 8, 2104 (2017).
Huynh, T. N., Noriega, C. E. & Stewart, V. Conserved mechanism for sensor phosphatase control of two-component signaling revealed in the nitrate sensor NarX. Proc. Natl Acad. Sci. USA 107, 21140–21145 (2010).
Gushchin, I. et al. Mechanism of transmembrane signaling by sensor histidine kinases. Science 356, eaah6345 (2017).
Gordeliy, V. I. et al. Molecular basis of transmembrane signalling by sensory rhodopsin II-transducer complex. Nature 419, 484–487 (2002).
Ishchenko, A. et al. New insights on signal propagation by sensory rhodopsin ii/transducer complex. Sci. Rep. 7, 41811 (2017).
Salvi, M. et al. Sensory domain contraction in histidine kinase CitA triggers transmembrane signaling in the membrane-bound sensor. Proc. Natl Acad. Sci. USA 114, 3115–3120 (2017).
Nan, B. et al. From signal perception to signal transduction: ligand-induced dimeric switch of DctB sensory domain in solution. Mol. Microbiol. 75, 1484–1494 (2010).
Lowe, E. C., Basle, A., Czjzek, M., Firbank, S. J. & Bolam, D. N. A scissor blade-like closing mechanism implicated in transmembrane signaling in a Bacteroides hybrid two-component system. Proc. Natl Acad. Sci. USA 109, 7298–7303 (2012).
Moore, J. O. & Hendrickson, W. A. An asymmetry-to-symmetry switch in signal transmission by the histidine kinase receptor for TMAO. Structure 20, 729–741 (2012).
Zhang, Z. & Hendrickson, W. A. Structural characterization of the predominant family of histidine kinase sensor domains. J. Mol. Biol. 400, 335–353 (2010).
Wang, B., Zhao, A., Novick, R. P. & Muir, T. W. Activation and inhibition of the receptor histidine kinase AgrC occurs through opposite helical transduction motions. Mol. Cell 53, 929–940 (2014).
Monzel, C. & Unden, G. Transmembrane signaling in the sensor kinase DcuS of Escherichia coli: A long-range piston-type displacement of transmembrane helix 2. Proc. Natl Acad. Sci. USA 112, 11042–11047 (2015).
Cheung, J. & Hendrickson, W. A. Structural analysis of ligand stimulation of the histidine kinase NarX. Structure 17, 190–201 (2009).
Neiditch, M. B. et al. Ligand-induced asymmetry in histidine sensor kinase complex regulates quorum sensing. Cell 126, 1095–1108 (2006).
Scheu, P. et al. Polar accumulation of the metabolic sensory histidine kinases DcuS and CitA in Escherichia coli. Microbiology 154, 2463–2472 (2008).
Lemmin, T., Soto, C. S., Clinthorne, G., DeGrado, W. F. & Dal Peraro, M. Assembly of the transmembrane domain of E. coli PhoQ histidine kinase: implications for signal transduction from molecular simulations. PLOS Comput. Biol. 9, e1002878 (2013).
Goldberg, S. D., Clinthorne, G. D., Goulian, M. & DeGrado, W. F. Transmembrane polar interactions are required for signaling in the Escherichia coli sensor kinase PhoQ. Proc. Natl Acad. Sci. USA 107, 8141–8146 (2010).
Abriata, L. A., Albanesi, D., Dal Peraro, M. & de Mendoza, D. Signal sensing and transduction by histidine kinases as unveiled through studies on a temperature sensor. Acc. Chem. Res. 50, 1359–1366 (2017).
Aravind, L. & Ponting, C. P. The cytoplasmic helical linker domain of receptor histidine kinase and methyl-accepting proteins is common to many prokaryotic signalling proteins. FEMS Microbiol. Lett. 176, 111–116 (1999).
Ferris, H. U. et al. Mechanism of regulation of receptor histidine kinases. Structure 20, 56–66 (2012).
Ferris, H. U. et al. The mechanisms of HAMP-mediated signaling in transmembrane receptors. Structure 19, 378–385 (2011).
Airola, M. V., Watts, K. J., Bilwes, A. M. & Crane, B. R. Structure of concatenated HAMP domains provides a mechanism for signal transduction. Structure 18, 436–448 (2010).
Swain, K. E., Gonzalez, M. A. & Falke, J. J. Engineered socket study of signaling through a four-helix bundle: evidence for a yin-yang mechanism in the kinase control module of the aspartate receptor. Biochemistry 48, 9266–9277 (2009).
Zhu, L., Bolhuis, P. G. & Vreede, J. The HAMP signal relay domain adopts multiple conformational states through collective piston and tilt motions. PLOS Comput. Biol. 9, e1002913 (2013).
Parkinson, J. S. Signaling mechanisms of HAMP domains in chemoreceptors and sensor kinases. Annu. Rev. Microbiol. 64, 101–122 (2010).
Sukomon, N., Widom, J., Borbat, P. P., Freed, J. H. & Crane, B. R. Stability and conformation of a chemoreceptor HAMP domain chimera correlates with signaling properties. Biophys. J. 112, 1383–1395 (2017).
Dunin-Horkawicz, S. & Lupas, A. N. Comprehensive analysis of HAMP domains: implications for transmembrane signal transduction. J. Mol. Biol. 397, 1156–1174 (2010).
Matamouros, S., Hager, K. R. & Miller, S. I. HAMP domain rotation and tilting movements associated with signal transduction in the PhoQ sensor kinase. mBio 6, e00616–00615 (2015).
Anantharaman, V., Balaji, S. & Aravind, L. The signaling helix: a common functional theme in diverse signaling proteins. Biol. Direct 1, 25 (2006).
Stewart, V. & Chen, L. L. The S helix mediates signal transmission as a HAMP domain coiled-coil extension in the NarX nitrate sensor from Escherichia coli K-12. J. Bacteriol. 192, 734–745 (2010).
Saita, E. et al. A coiled coil switch mediates cold sensing by the thermosensory protein DesK. Mol. Microbiol. 98, 258–271 (2015).
Lesne, E. et al. Balance between coiled-coil stability and dynamics regulates activity of BvgS sensor kinase in Bordetella. mBio 7, e02089 (2016).
Lesne, E. et al. Coiled coils antagonisms regulate activity of Venus flytrap-domain-containing sensor-kinases of the BvgS family. mBio 9, e02052–17 (2018).
Schmidt, N. W., Grigoryan, G. & DeGrado, W. F. The accommodation index measures the perturbation associated with insertions and deletions in coiled-coils. Application to understand signaling in histidine kinases. Protein Sci. 26, 414–435 (2016).
Kitanovic, S., Ames, P. & Parkinson, J. S. A. Trigger residue for transmembrane signaling in the Escherichia coli serine chemoreceptor. J. Bacteriol. 197, 2568–2579 (2015).
Liu, J. et al. Mutational analysis of dimeric linkers in peri- and cytoplasmic domains of histidine kinase DctB reveals their functional roles in signal transduction. Open Biol. 4, 140023 (2014).
Zhou, Q., Ames, P. & Parkinson, J. S. Mutational analyses of HAMP helices suggest a dynamic bundle model of input-output signalling in chemoreceptors. Mol. Microbiol. 73, 801–814 (2009).
Möglich, A., Ayers, R. A. & Moffat, K. Structure and signaling mechanism of Per-ARNT-Sim domains. Structure 17, 1282–1294 (2009).
Ayers, R. A. & Moffat, K. Changes in quaternary structure in the signaling mechanisms of PAS domains. Biochemistry 47, 12078–12086 (2008).
Diensthuber, R. P., Bommer, M., Gleichmann, T. & Möglich, A. Full-length structure of a sensor histidine kinase pinpoints coaxial coiled coils as signal transducers and modulators. Structure 21, 1127–1136 (2013).
Berntsson, O. et al. Time-resolved x-ray solution scattering reveals the structural photoactivation of a light-oxygen-voltage photoreceptor. Structure 25, 933–938 (2017).
Berntsson, O. et al. Sequential conformational transitions and α-helical supercoiling regulate a sensor histidine kinase. Nat. Commun. 8, 284 (2017).
Yamada, S. et al. Structure of PAS-linked histidine kinase and the response regulator complex. Structure 17, 1333–1344 (2009).
Garcia, D., Watts, K. J., Johnson, M. S. & Taylor, B. L. Delineating PAS-HAMP interaction surfaces and signalling-associated changes in the aerotaxis receptor Aer. Mol. Microbiol. 100, 156–172 (2016).
Bem, A. E. et al. Bacterial histidine kinases as novel antibacterial drug targets. ACS Chem. Biol. 10, 213–224 (2015).
Tiwari, S. et al. Two-component signal transduction systems of pathogenic bacteria as targets for antimicrobial therapy: an overview. Front. Microbiol. 8, 1878 (2017).
Velikova, N. et al. Putative histidine kinase inhibitors with antibacterial effect against multi-drug resistant clinical isolates identified by in vitro and in silico screens. Sci. Rep. 6, 26085 (2016).
Acknowledgements
The authors thank E. Pradel for carefully reading the manuscript. They also thank A. Buschiazzo, F. Trajtenberg and J. Imelio (Institut Pasteur of Montevideo) for kindly sharing the coordinates file of the DesK–DesR complex model shown in Fig. 2a. The work in F.J.-D.’s group was supported by the Agence Nationale de la Recherche (grant ANR-10-BLAN-1306). The authors apologize to all colleagues whose excellent work on two-component systems could not be cited because of space limitations.
Reviewer information
Nature Reviews Microbiology thanks V. Gordeliy and A. Möglich and the other anonymous reviewer(s) for their contribution to the peer review of this work.
Author information
Authors and Affiliations
Contributions
F.J.-D., A.M., J.-M.B. and R.A. researched data for the article and made substantial contributions to discussions of the content. F.J.-D., J.-M.B. and A.M. wrote the article. F.J.-D., A.M. and R.A. reviewed and/or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jacob-Dubuisson, F., Mechaly, A., Betton, JM. et al. Structural insights into the signalling mechanisms of two-component systems. Nat Rev Microbiol 16, 585–593 (2018). https://doi.org/10.1038/s41579-018-0055-7
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41579-018-0055-7
This article is cited by
-
TcrXY is an acid-sensing two-component transcriptional regulator of Mycobacterium tuberculosis required for persistent infection
Nature Communications (2024)
-
Bacterial capsules: Occurrence, mechanism, and function
npj Biofilms and Microbiomes (2024)
-
Structure–function analysis of PorXFj, the PorX homolog from Flavobacterium johnsioniae, suggests a role of the CheY-like domain in type IX secretion motor activity
Scientific Reports (2024)
-
Navigating the signaling landscape of Ralstonia solanacearum: a study of bacterial two-component systems
World Journal of Microbiology and Biotechnology (2024)
-
Sequestration of histidine kinases by non-cognate response regulators establishes a threshold level of stimulation for bacterial two-component signaling
Nature Communications (2023)