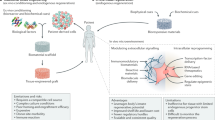
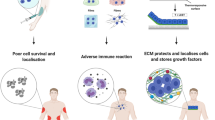
Abstract
Cell transplantation holds immense potential for reversing diseases that are currently incurable and for regenerating tissues. However, poor cell survival, cell aggregation and lack of cell integration into the host tissue constitute major challenges for the clinical translation of cell transplantation approaches. Biomaterials can influence cell behaviour in vitro and in vivo. The mechanical and biochemical properties of biomaterials can be tailored to affect cell survival, differentiation and migration. Therefore, the integration of advanced material design with stem cell biology may hold the key to improving the efficacy of cell transplantation. In this Review, we discuss biomaterial design strategies for their potential to influence the fate of transplanted cells and to manipulate the host microenvironment. We examine how biomaterial properties can be modulated to improve transplanted cell survival, differentiation and cell engraftment and how the host tissue can be manipulated for cell transplantation by inducing plasticity and vascularization. Finally, we emphasize the importance of the host immune cells for tissue repair and cell transplantation and discuss strategies to tune the immune response through modulating the mechanical properties, architecture, chemistry and functionalization of biomaterials.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Godwin, J. The promise of perfect adult tissue repair and regeneration in mammals: learning from regenerative amphibians and fish. Bioessays 36, 861–871 (2014).
Andersson-Rolf, A., Zilbauer, M., Koo, B. K. & Clevers, H. Stem cells in repair of gastrointestinal epithelia. Physiol. (Bethesda) 32, 278–289 (2017).
Gonzalez, G., Sasamoto, Y., Ksander, B. R., Frank, M. H. & Frank, N. Y. Limbal stem cells: identity, developmental origin, and therapeutic potential. Wiley Interdiscip. Rev. Dev. Biol. https://doi.org/10.1002/wdev.303 (2018).
Fuchs, E. Epithelial skin biology: three decades of developmental biology, a hundred questions answered and a thousand new ones to address. Curr. Top. Dev. Biol. 116, 357–374 (2016).
Diehl, A. M. & Chute, J. Underlying potential: cellular and molecular determinants of adult liver repair. J. Clin. Invest. 123, 1858–1860 (2013).
Hatzimichael, E. & Tuthill, M. Hematopoietic stem cell transplantation. Stem Cells Clon. 3, 105–117 (2010).
Cavazzana, M., Six, E., Lagresle-Peyrou, C., Andre-Schmutz, I. & Hacein-Bey-Abina, S. Gene therapy for X-linked severe combined immunodeficiency: where do we stand? Hum. Gene Ther. 27, 108–116 (2016).
Marquardt, L. M. & Heilshorn, S. C. Design of injectable materials to improve stem cell transplantation. Curr. Stem Cell. Rep. 2, 207–220 (2016).
Sortwell, C. E., Pitzer, M. R. & Collier, T. J. Time course of apoptotic cell death within mesencephalic cell suspension grafts: implications for improving grafted dopamine neuron survival. Exp. Neurol. 165, 268–277 (2000).
Muller-Ehmsen, J. et al. Effective engraftment but poor mid-term persistence of mononuclear and mesenchymal bone marrow cells in acute and chronic rat myocardial infarction. J. Mol. Cell. Cardiol. 41, 876–884 (2006).
Richardson, P. M., McGuinness, U. M. & Aguayo, A. J. Axons from CNS neurons regenerate into PNS grafts. Nature 284, 264–265 (1980).
Wictorin, K., Brundin, P., Gustavii, B., Lindvall, O. & Bjorklund, A. Reformation of long axon pathways in adult rat central nervous system by human forebrain neuroblasts. Nature 347, 556–558 (1990).
Shewan, D., Berry, M. & Cohen, J. Extensive regeneration in vitro by early embryonic neurons on immature and adult CNS tissue. J. Neurosci. 15, 2057–2062 (1995).
Peled, A. et al. Dependence of human stem cell engraftment and repopulation of NOD/SCID mice on CXCR4. Science 283, 845–848 (1999).
Voermans, C. et al. In vitro migratory capacity of CD34+ cells is related to hematopoietic recovery after autologous stem cell transplantation. Blood 97, 799–804 (2001).
MacLaren, R. E. et al. Retinal repair by transplantation of photoreceptor precursors. Nature 444, 203–207 (2006).
Payne, S. L. et al. In vitro maturation of human iPSC-derived neuroepithelial cells influences transplant survival in the stroke-injured rat brain. Tissue Eng. Part A. 24, 351–360 (2018).
Bahlmann, L. C., Fokina, A. & Shoichet, M. S. Dynamic bioengineered hydrogels as scaffolds for advanced stem cell and organoid culture. MRS Commun. 7, 472–486 (2017).
Ruprecht, V. et al. How cells respond to environmental cues - insights from bio-functionalized substrates. J. Cell. Sci. 130, 51–61 (2017).
Amer, M. H., White, L. J. & Shakesheff, K. M. The effect of injection using narrow-bore needles on mammalian cells: administration and formulation considerations for cell therapies. J. Pharm. Pharmacol. 67, 640–650 (2015).
Aguado, B. A., Mulyasasmita, W., Su, J., Lampe, K. J. & Heilshorn, S. C. Improving viability of stem cells during syringe needle flow through the design of hydrogel cell carriers. Tissue Eng. Part A. 18, 806–815 (2012).
Agashi, K., Chau, D. Y. & Shakesheff, K. M. The effect of delivery via narrow-bore needles on mesenchymal cells. Regen. Med. 4, 49–64 (2009).
Amer, M. H., Rose, F. R. A. J., Shakesheff, K. M., Modo, M. & White, L. J. Translational considerations in injectable cell-based therapeutics for neurological applications: concepts, progress and challenges. NPJ Regen. Med. 2, 23 (2017).
Rodell, C. B., Kaminski, A. L. & Burdick, J. A. Rational design of network properties in guest-host assembled and shear-thinning hyaluronic acid hydrogels. Biomacromolecules 14, 4125–4134 (2013).
Ballios, B. G. et al. A hyaluronan-based injectable hydrogel improves the survival and integration of stem cell progeny following transplantation. Stem Cell. Rep. 4, 1031–1045 (2015).
Ballios, B. G., Cooke, M. J., van der Kooy, D. & Shoichet, M. S. A hydrogel-based stem cell delivery system to treat retinal degenerative diseases. Biomaterials 31, 2555–2564 (2010).
Roche, E. T. et al. Comparison of biomaterial delivery vehicles for improving acute retention of stem cells in the infarcted heart. Biomaterials 35, 6850–6858 (2014).
Amini, A. R., Laurencin, C. T. & Nukavarapu, S. P. Bone tissue engineering: recent advances and challenges. Crit. Rev. Biomed. Eng. 40, 363–408 (2012).
Fuhrmann, T., Anandakumaran, P. N. & Shoichet, M. S. Combinatorial therapies after spinal cord injury: how can biomaterials help? Adv. Healthc. Mater. https://doi.org/10.1002/adhm.201601130 (2017).
Pan, Z. & Ding, J. Poly(lactide-co-glycolide) porous scaffolds for tissue engineering and regenerative medicine. Interface Focus. 2, 366–377 (2012).
Bozkurt, G. et al. Chitosan channels containing spinal cord-derived stem/progenitor cells for repair of subacute spinal cord injury in the rat. Neurosurgery 67, 1733–1744 (2010).
Frisch, S. M. & Francis, H. Disruption of epithelial cell-matrix interactions induces apoptosis. J. Cell Biol. 124, 619–626 (1994).
Lee, J. W. & Juliano, R. Mitogenic signal transduction by integrin- and growth factor receptor-mediated pathways. Mol. Cells 17, 188–202 (2004).
Vachon, P. H. Integrin signaling, cell survival, and anoikis: distinctions, differences, and differentiation. J. Signal. Transduct 2011, 738137 (2011).
Mitrousis, N., Tam, R. Y., Baker, A. E. G., van der Kooy, D. & Shoichet, M. S. Hyaluronic acid-based hydrogels enable rod photoreceptor survival and maturation in vitro through activation of the mTOR pathway. Adv. Funct. Mater. 26, 1975–1985 (2016).
Tate, C. C. et al. Laminin and fibronectin scaffolds enhance neural stem cell transplantation into the injured brain. J. Tissue Eng. Regen. Med. 3, 208–217 (2009).
Pierschbacher, M. D. & Ruoslahti, E. Cell attachment activity of fibronectin can be duplicated by small synthetic fragments of the molecule. Nature 309, 30–33 (1984).
Plow, E. F., Haas, T. A., Zhang, L., Loftus, J. & Smith, J. W. Ligand binding to integrins. J. Biol. Chem. 275, 21785–21788 (2000).
Hersel, U., Dahmen, C. & Kessler, H. RGD modified polymers: biomaterials for stimulated cell adhesion and beyond. Biomaterials 24, 4385–4415 (2003).
Ho, S. S., Murphy, K. C., Binder, B. Y., Vissers, C. B. & Leach, J. K. Increased survival and function of mesenchymal stem cell spheroids entrapped in instructive alginate hydrogels. Stem Cells Transl Med. 5, 773–781 (2016).
Adil, M. M. et al. Engineered hydrogels increase the post-transplantation survival of encapsulated hESC-derived midbrain dopaminergic neurons. Biomaterials 136, 1–11 (2017).
Fuhrmann, T. et al. Injectable hydrogel promotes early survival of induced pluripotent stem cell-derived oligodendrocytes and attenuates longterm teratoma formation in a spinal cord injury model. Biomaterials 83, 23–36 (2016).
Mhanna, R. et al. GFOGER-modified MMP-sensitive polyethylene glycol hydrogels induce chondrogenic differentiation of human mesenchymal stem cells. Tissue Eng. Part A. 20, 1165–1174 (2014).
Moshayedi, P. et al. Systematic optimization of an engineered hydrogel allows for selective control of human neural stem cell survival and differentiation after transplantation in the stroke brain. Biomaterials 105, 145–155 (2016).
Somaa, F. A. et al. Peptide-based scaffolds support human cortical progenitor graft integration to reduce atrophy and promote functional repair in a model of stroke. Cell. Rep. 20, 1964–1977 (2017).
Tam, R. Y., Fuehrmann, T., Mitrousis, N. & Shoichet, M. S. Regenerative therapies for central nervous system diseases: a biomaterials approach. Neuropsychopharmacology 39, 169–188 (2014).
Wang, C., Liu, Y., Fan, Y. & Li, X. The use of bioactive peptides to modify materials for bone tissue repair. Regen. Biomater. 4, 191–206 (2017).
Lemmon, M. A. & Schlessinger, J. Cell signaling by receptor tyrosine kinases. Cell 141, 1117–1134 (2010).
Murase, K. et al. Developmental changes in nerve growth factor level in rat serum. J. Neurosci. Res. 33, 282–288 (1992).
Zadik, Z., Chalew, S. A., McCarter, R. J. Jr, Meistas, M. & Kowarski, A. A. The influence of age on the 24-hour integrated concentration of growth hormone in normal individuals. J. Clin. Endocrinol. Metab. 60, 513–516 (1985).
Parker, J., Mitrousis, N. & Shoichet, M. S. Hydrogel for simultaneous tunable growth factor delivery and enhanced viability of encapsulated cells in vitro. Biomacromolecules 17, 476–484 (2016).
Hill, E., Boontheekul, T. & Mooney, D. J. Regulating activation of transplanted cells controls tissue regeneration. Proc. Natl Acad. Sci. USA 103, 2494–2499 (2006).
Sandgren, E. P., Luetteke, N. C., Palmiter, R. D., Brinster, R. L. & Lee, D. C. Overexpression of TGF alpha in transgenic mice: induction of epithelial hyperplasia, pancreatic metaplasia, and carcinoma of the breast. Cell 61, 1121–1135 (1990).
Matsui, Y., Halter, S. A., Holt, J. T., Hogan, B. L. & Coffey, R. J. Development of mammary hyperplasia and neoplasia in MMTV-TGF alpha transgenic mice. Cell 61, 1147–1155 (1990).
Jhappan, C. et al. TGF alpha overexpression in transgenic mice induces liver neoplasia and abnormal development of the mammary gland and pancreas. Cell 61, 1137–1146 (1990).
Cahill, K. S., Chi, J. H., Day, A. & Claus, E. B. Prevalence, complications, and hospital charges associated with use of bone-morphogenetic proteins in spinal fusion procedures. JAMA 302, 58–66 (2009).
James, A. W. et al. A review of the clinical side effects of bone morphogenetic protein-2. Tissue Eng. Part B. Rev. 22, 284–297 (2016).
Wang, T. Y. et al. Functionalized composite scaffolds improve the engraftment of transplanted dopaminergic progenitors in a mouse model of Parkinson’s disease. Biomaterials 74, 89–98 (2016).
Wiley, H. S. Trafficking of the ErbB receptors and its influence on signaling. Exp. Cell Res. 284, 78–88 (2003).
Fan, V. H. et al. Tethered epidermal growth factor provides a survival advantage to mesenchymal stem cells. Stem Cells 25, 1241–1251 (2007).
Nuschke, A. et al. Epidermal growth factor tethered to beta-tricalcium phosphate bone scaffolds via a high-affinity binding peptide enhances survival of human mesenchymal stem cells/multipotent stromal cells in an immune-competent parafascial implantation assay in mice. Stem Cells Transl Med. 5, 1580–1586 (2016).
Martino, M. M. et al. Engineering the growth factor microenvironment with fibronectin domains to promote wound and bone tissue healing. Sci. Transl. Med. 3, 100ra89 (2011).
Tam, R. Y., Cooke, M. J. & Shoichet, M. S. A covalently modified hydrogel blend of hyaluronan-methyl cellulose with peptides and growth factors influences neural stem/progenitor cell fate. J. Mater. Chem. 22, 19402–19411 (2012).
Vining, K. H. & Mooney, D. J. Mechanical forces direct stem cell behaviour in development and regeneration. Nat. Rev. Mol. Cell. Biol. 18, 728–742 (2017).
Engler, A. J., Sen, S., Sweeney, H. L. & Discher, D. E. Matrix elasticity directs stem cell lineage specification. Cell 126, 677–689 (2006).
Leipzig, N. D. & Shoichet, M. S. The effect of substrate stiffness on adult neural stem cell behavior. Biomaterials 30, 6867–6878 (2009).
Baker, B. M. & Chen, C. S. Deconstructing the third dimension: how 3D culture microenvironments alter cellular cues. J. Cell. Sci. 125, 3015–3024 (2012).
Caliari, S. R., Vega, S. L., Kwon, M., Soulas, E. M. & Burdick, J. A. Dimensionality and spreading influence MSC YAP/TAZ signaling in hydrogel environments. Biomaterials 103, 314–323 (2016).
Dupont, S. et al. Role of YAP/TAZ in mechanotransduction. Nature 474, 179–183 (2011).
Rammensee, S., Kang, M. S., Georgiou, K., Kumar, S. & Schaffer, D. V. Dynamics of mechanosensitive neural stem cell differentiation. Stem Cells 35, 497–506 (2017).
Tharp, K. M. et al. Actomyosin-mediated tension orchestrates uncoupled respiration in adipose tissues. Cell Metab. 27, 602–615.e4 (2018).
Handorf, A. M., Zhou, Y., Halanski, M. A. & Li, W. J. Tissue stiffness dictates development, homeostasis, and disease progression. Organogenesis 11, 1–15 (2015).
Young, J. L. & Engler, A. J. Hydrogels with time-dependent material properties enhance cardiomyocyte differentiation in vitro. Biomaterials 32, 1002–1009 (2011).
Caliari, S. R. & Burdick, J. A. A practical guide to hydrogels for cell culture. Nat. Methods 13, 405–414 (2016).
Yoon, Y. et al. Photocrosslinkable hydrogel for myocyte cell culture and injection. J. Biomed. Mater. Res. 8B, 312–322 (2007).
Killion, J. A. et al. Modulating the mechanical properties of photopolymerised polyethylene glycol–polypropylene glycol hydrogels for bone regeneration. J. Mater. Sci. 47, 6577–6585 (2012).
Rodell, C. B. et al. Shear-thinning supramolecular hydrogels with secondary autonomous covalent crosslinking to modulate viscoelastic properties in vivo. Adv. Funct. Mater. 25, 636–644 (2015).
Huiyuan, W. et al. Covalently adaptable elastin–like protein–hyaluronic acid (ELP–HA) hybrid hydrogels with secondary thermoresponsive crosslinking for injectable stem cell delivery. Adv. Funct. Mater. 27, 1605609 (2017).
De France, K. J. et al. Injectable anisotropic nanocomposite hydrogels direct in situ growth and alignment of myotubes. Nano Lett. 17, 6487–6495 (2017).
Montgomery, M. et al. Flexible shape-memory scaffold for minimally invasive delivery of functional tissues. Nat. Mater. 16, 1038–1046 (2017).
Fujie, T. et al. Micropatterned polymeric nanosheets for local delivery of an engineered epithelial monolayer. Adv. Mater. 26, 1699–1705 (2014).
Yao, R., Zhang, R., Lin, F. & Luan, J. Injectable cell/hydrogel microspheres induce the formation of fat lobule-like microtissues and vascularized adipose tissue regeneration. Biofabrication 4, 045003 (2012).
Huang, C. C. et al. Injectable PLGA porous beads cellularized by hAFSCs for cellular cardiomyoplasty. Biomaterials 33, 4069–4077 (2012).
Griffin, D. R., Weaver, W. M., Scumpia, P. O., Di Carlo, D. & Segura, T. Accelerated wound healing by injectable microporous gel scaffolds assembled from annealed building blocks. Nat. Mater. 14, 737–744 (2015).
Mealy, J. E. et al. Injectable granular hydrogels with multifunctional properties for biomedical applications. Adv. Mater. 30, e1705912 (2018).
Nih, L. R., Sideris, E., Carmichael, S. T. & Segura, T. Injection of microporous annealing particle (MAP) hydrogels in the stroke cavity reduces gliosis and inflammation and promotes NPC migration to the lesion. https://doi.org/10.1002/adma.201606471 (2017).
Huebsch, N. et al. Matrix elasticity of void-forming hydrogels controls transplanted-stem-cell-mediated bone formation. Nat. Mater. 14, 1269–1277 (2015).
Simmons, C. A., Alsberg, E., Hsiong, S., Kim, W. J. & Mooney, D. J. Dual growth factor delivery and controlled scaffold degradation enhance in vivo bone formation by transplanted bone marrow stromal cells. Bone 35, 562–569 (2004).
Murry, C. E. & Keller, G. Differentiation of embryonic stem cells to clinically relevant populations: lessons from embryonic development. Cell 132, 661–680 (2008).
Beederman, M. et al. BMP signaling in mesenchymal stem cell differentiation and bone formation. J. Biomed. Sci. Eng. 6, 32–52 (2013).
Park, S. H. et al. BMP2-modified injectable hydrogel for osteogenic differentiation of human periodontal ligament stem cells. Sci. Rep. 7, 6603 (2017).
Kim, H., Zahir, T., Tator, C. H. & Shoichet, M. S. Effects of dibutyryl cyclic-AMP on survival and neuronal differentiation of neural stem/progenitor cells transplanted into spinal cord injured rats. PLOS One 6, e21744 (2011).
Han, L. H. et al. Winner of the young investigator award of the society for biomaterials at the 10th world biomaterials congress, may 17–22, 2016, Montreal QC, Canada: microribbon-based hydrogels accelerate stem cell-based bone regeneration in a mouse critical-size cranial defect model. J. Biomed. Mater. Res. A. 104, 1321–1331 (2016).
Bencherif, S. A. et al. Injectable preformed scaffolds with shape-memory properties. Proc. Natl Acad. Sci. USA 109, 19590–19595 (2012).
Cameron, A. R., Frith, J. E. & Cooper-White, J. J. The influence of substrate creep on mesenchymal stem cell behaviour and phenotype. Biomaterials 32, 5979–5993 (2011).
McKinnon, D. D., Domaille, D. W., Cha, J. N. & Anseth, K. S. Biophysically defined and cytocompatible covalently adaptable networks as viscoelastic 3D cell culture systems. Adv. Mater. 26, 865–872 (2014).
Chaudhuri, O. et al. Hydrogels with tunable stress relaxation regulate stem cell fate and activity. Nat. Mater. 15, 326–334 (2016).
Darnell, M. et al. Substrate stress-relaxation regulates scaffold remodeling and bone formation in vivo. Adv. Healthc. Mater. 6, 1601185 (2017).
Barthes, J. et al. Cell microenvironment engineering and monitoring for tissue engineering and regenerative medicine: the recent advances. Biomed. Res. Int. 2014, 921905 (2014).
Jones, D. L. & Wagers, A. J. No place like home: anatomy and function of the stem cell niche. Nat. Rev. Mol. Cell. Biol. 9, 11–21 (2008).
Goodship, A. E. & Kenwright, J. The influence of induced micromovement upon the healing of experimental tibial fractures. J. Bone Joint Surg. Br. 67, 650–655 (1985).
Kenwright, J. & Goodship, A. E. Controlled mechanical stimulation in the treatment of tibial fractures. Clin. Orthop. Relat. Res. 241, 36–47 (1989).
Epari, D. R., Duda, G. N. & Thompson, M. S. Mechanobiology of bone healing and regeneration: in vivo models. Proc. Inst. Mech. Eng. H. 224, 1543–1553 (2010).
Goodship, A. E., Cunningham, J. L. & Kenwright, J. Strain rate and timing of stimulation in mechanical modulation of fracture healing. Clin. Orthop. Relat. Res. 355 (Suppl), S105–S115 (1998).
Claes, L. E. & Heigele, C. A. Magnitudes of local stress and strain along bony surfaces predict the course and type of fracture healing. J. Biomech. 32, 255–266 (1999).
Wolf, S. et al. Effects of high-frequency, low-magnitude mechanical stimulus on bone healing. Clin. Orthop. Relat. Res. 385, 192–198 (2001).
Salter, D. M., Wallace, W. H., Robb, J. E., Caldwell, H. & Wright, M. O. Human bone cell hyperpolarization response to cyclical mechanical strain is mediated by an interleukin-1beta autocrine/paracrine loop. J. Bone Miner. Res. 15, 1746–1755 (2000).
Tampieri, A. et al. A conceptually new type of bio-hybrid scaffold for bone regeneration. Nanotechnology 22, 015104 (2011).
Panseri, S. et al. F. J. Biomed. Mater. Res. A. 100, 2278–2286 (2012).
Meng, J. et al. Super-paramagnetic responsive nanofibrous scaffolds under static magnetic field enhance osteogenesis for bone repair in vivo. Sci. Rep. 3, 2655 (2013).
Kotani, H. et al. Strong static magnetic field stimulates bone formation to a definite orientation in vitro and in vivo. J. Bone Miner. Res. 17, 1814–1821 (2002).
Yun, H. M. et al. Magnetic nanocomposite scaffolds combined with static magnetic field in the stimulation of osteoblastic differentiation and bone formation. Biomaterials 85, 88–98 (2016).
Vandenburgh, H. H. & Karlisch, P. Longitudinal growth of skeletal myotubes in vitro in a new horizontal mechanical cell stimulator. In Vitro Cell. Dev. Biol. 25, 607–616 (1989).
Vandenburgh, H. & Kaufman, S. In vitro model for stretch-induced hypertrophy of skeletal muscle. Science 203, 265–268 (1979).
Moon du, G., Christ, G., Stitzel, J. D., Atala, A. & Yoo, J. J. Cyclic mechanical preconditioning improves engineered muscle contraction. Tissue Eng. Part A. 14, 473–482 (2008).
Powell, C. A., Smiley, B. L., Mills, J. & Vandenburgh, H. H. Mechanical stimulation improves tissue-engineered human skeletal muscle. Am. J. Physiol. Cell. Physiol. 283, C1557–C1565 (2002).
Crane, J. D. et al. Massage therapy attenuates inflammatory signaling after exercise-induced muscle damage. Sci. Transl. Med. 4, 119ra13 (2012).
Weerapong, P., Hume, P. A. & Kolt, G. S. The mechanisms of massage and effects on performance, muscle recovery and injury prevention. Sports Med. 35, 235–256 (2005).
Butterfield, T. A., Zhao, Y., Agarwal, S., Haq, F. & Best, T. M. Cyclic compressive loading facilitates recovery after eccentric exercise. Med. Sci. Sports Exerc. 40, 1289–1296 (2008).
Haas, C. et al. Dose-dependency of massage-like compressive loading on recovery of active muscle properties following eccentric exercise: rabbit study with clinical relevance. Br. J. Sports Med. 47, 83–88 (2013).
Haas, C. et al. Massage timing affects postexercise muscle recovery and inflammation in a rabbit model. Med. Sci. Sports Exerc. 45, 1105–1112 (2013).
Cezar, C. A. et al. Biphasic ferrogels for triggered drug and cell delivery. Adv. Healthc. Mater. 3, 1869–1876 (2014).
Cezar, C. A. et al. Biologic-free mechanically induced muscle regeneration. Proc. Natl Acad. Sci. USA 113, 1534–1539 (2016).
Henstock, J. R., Rotherham, M., Rashidi, H., Shakesheff, K. M. & El Haj, A. J. Remotely activated mechanotransduction via magnetic nanoparticles promotes mineralization synergistically with bone morphogenetic protein 2: applications for injectable cell therapy. Stem Cells Transl. Med. 3, 1363–1374 (2014).
Xue, M. & Jackson, C. J. Extracellular matrix reorganization during wound healing and its impact on abnormal scarring. Adv. Wound. Care. (New Rochelle) 4, 119–136 (2015).
Adams, K. L. & Gallo, V. The diversity and disparity of the glial scar. Nat. Neurosci. 21, 9–15 (2018).
McKeon, R. J., Schreiber, R. C., Rudge, J. S. & Silver, J. Reduction of neurite outgrowth in a model of glial scarring following CNS injury is correlated with the expression of inhibitory molecules on reactive astrocytes. J. Neurosci. 11, 3398–3411 (1991).
Fawcett, J. W. The extracellular matrix in plasticity and regeneration after CNS injury and neurodegenerative disease. Prog. Brain Res. 218, 213–226 (2015).
Deepa, S. S. et al. Composition of perineuronal net extracellular matrix in rat brain: a different disaccharide composition for the net-associated proteoglycans. J. Biol. Chem. 281, 17789–17800 (2006).
Bradbury, E. J. et al. Chondroitinase ABC promotes functional recovery after spinal cord injury. Nature 416, 636–640 (2002).
Soleman, S., Yip, P. K., Duricki, D. A. & Moon, L. D. Delayed treatment with chondroitinase ABC promotes sensorimotor recovery and plasticity after stroke in aged rats. Brain 135, 1210–1223 (2012).
Hill, J. J., Jin, K., Mao, X. O., Xie, L. & Greenberg, D. A. Intracerebral chondroitinase ABC and heparan sulfate proteoglycan glypican improve outcome from chronic stroke in rats. Proc. Natl Acad. Sci. USA 109, 9155–9160 (2012).
Karimi-Abdolrezaee, S., Eftekharpour, E., Wang, J., Schut, D. & Fehlings, M. G. Synergistic effects of transplanted adult neural stem/progenitor cells, chondroitinase, and growth factors promote functional repair and plasticity of the chronically injured spinal cord. J. Neurosci. 30, 1657–1676 (2010).
Ikegami, T. et al. Chondroitinase ABC combined with neural stem/progenitor cell transplantation enhances graft cell migration and outgrowth of growth-associated protein-43-positive fibers after rat spinal cord injury. Eur. J. Neurosci. 22, 3036–3046 (2005).
Ma, J., Kabiel, M., Tucker, B. A., Ge, J. & Young, M. J. Combining chondroitinase ABC and growth factors promotes the integration of murine retinal progenitor cells transplanted into Rho(−/−) mice. Mol. Vis. 17, 1759–1770 (2011).
Suzuki, T. et al. Chondroitinase ABC treatment enhances synaptogenesis between transplant and host neurons in model of retinal degeneration. Cell Transplant. 16, 493–503 (2007).
Tester, N. J., Plaas, A. H. & Howland, D. R. Effect of body temperature on chondroitinase ABC’s ability to cleave chondroitin sulfate glycosaminoglycans. J. Neurosci. Res. 85, 1110–1118 (2007).
Pakulska, M. M., Vulic, K. & Shoichet, M. S. Affinity-based release of chondroitinase ABC from a modified methylcellulose hydrogel. J. Control. Release 171, 11–16 (2013).
Vulic, K. & Shoichet, M. S. Tunable growth factor delivery from injectable hydrogels for tissue engineering. J. Am. Chem. Soc. 134, 882–885 (2012).
Pakulska, M. M., Tator, C. H. & Shoichet, M. S. Local delivery of chondroitinase ABC with or without stromal cell-derived factor 1alpha promotes functional repair in the injured rat spinal cord. Biomaterials 134, 13–21 (2017).
Fuhrmann, T. et al. Combined delivery of chondroitinase ABC and human induced pluripotent stem cell-derived neuroepithelial cells promote tissue repair in an animal model of spinal cord injury. Biomed. Mater. 13, 024103 (2018).
Lee, H., McKeon, R. J. & Bellamkonda, R. V. Sustained delivery of thermostabilized chABC enhances axonal sprouting and functional recovery after spinal cord injury. Proc. Natl Acad. Sci. USA 107, 3340–3345 (2010).
Begni, V., Riva, M. A. & Cattaneo, A. Cellular and molecular mechanisms of the brain-derived neurotrophic factor in physiological and pathological conditions. Clin. Sci. (Lond.) 131, 123–138 (2017).
Barde, Y. A., Edgar, D. & Thoenen, H. Purification of a new neurotrophic factor from mammalian brain. EMBO J. 1, 549–553 (1982).
Leibrock, J. et al. Molecular cloning and expression of brain-derived neurotrophic factor. Nature 341, 149–152 (1989).
Korte, M. et al. Hippocampal long-term potentiation is impaired in mice lacking brain-derived neurotrophic factor. Proc. Natl Acad. Sci. USA 92, 8856–8860 (1995).
Patterson, S. L. et al. Recombinant BDNF rescues deficits in basal synaptic transmission and hippocampal LTP in BDNF knockout mice. Neuron 16, 1137–1145 (1996).
Bosch, M. & Hayashi, Y. Structural plasticity of dendritic spines. Curr. Opin. Neurobiol. 22, 383–388 (2012).
Mantilla, C. B., Gransee, H. M., Zhan, W. Z. & Sieck, G. C. Motoneuron BDNF/TrkB signaling enhances functional recovery after cervical spinal cord injury. Exp. Neurol. 247, 101–109 (2013).
Ploughman, M. et al. Brain-derived neurotrophic factor contributes to recovery of skilled reaching after focal ischemia in rats. Stroke 40, 1490–1495 (2009).
Bonner, J. F. et al. Grafted neural progenitors integrate and restore synaptic connectivity across the injured spinal cord. J. Neurosci. 31, 4675–4686 (2011).
Tom, V. J. et al. Exogenous BDNF enhances the integration of chronically injured axons that regenerate through a peripheral nerve grafted into a chondroitinase-treated spinal cord injury site. Exp. Neurol. 239, 91–100 (2013).
Seiler, M. J. et al. BDNF-treated retinal progenitor sheets transplanted to degenerate rats: improved restoration of visual function. Exp. Eye Res. 86, 92–104 (2008).
Boyce, V. S., Park, J., Gage, F. H. & Mendell, L. M. Differential effects of brain-derived neurotrophic factor and neurotrophin-3 on hindlimb function in paraplegic rats. Eur. J. Neurosci. 35, 221–232 (2012).
Lu, P. et al. Motor axonal regeneration after partial and complete spinal cord transection. J. Neurosci. 32, 8208–8218 (2012).
Patist, C. M. et al. Freeze-dried poly(D,L-lactic acid) macroporous guidance scaffolds impregnated with brain-derived neurotrophic factor in the transected adult rat thoracic spinal cord. Biomaterials 25, 1569–1582 (2004).
Stokols, S. & Tuszynski, M. H. Freeze-dried agarose scaffolds with uniaxial channels stimulate and guide linear axonal growth following spinal cord injury. Biomaterials 27, 443–451 (2006).
Jain, A., Kim, Y. T., McKeon, R. J. & Bellamkonda, R. V. In situ gelling hydrogels for conformal repair of spinal cord defects, and local delivery of BDNF after spinal cord injury. Biomaterials 27, 497–504 (2006).
Bloch, J., Fine, E. G., Bouche, N., Zurn, A. D. & Aebischer, P. Nerve growth factor- and neurotrophin-3-releasing guidance channels promote regeneration of the transected rat dorsal root. Exp. Neurol. 172, 425–432 (2001).
Cook, D. J. et al. Hydrogel-delivered brain-derived neurotrophic factor promotes tissue repair and recovery after stroke. J. Cereb. Blood Flow Metab. 37, 1030–1045 (2017).
Folkman, J. & Hochberg, M. Self-regulation of growth in three dimensions. J. Exp. Med. 138, 745–753 (1973).
Carmeliet, P. Angiogenesis in health and disease. Nat. Med. 9, 653–660 (2003).
Ferrara, N. Vascular endothelial growth factor. Arterioscler. Thromb. Vasc. Biol. 29, 789–791 (2009).
Miyagi, Y. et al. Biodegradable collagen patch with covalently immobilized VEGF for myocardial repair. Biomaterials 32, 1280–1290 (2011).
Bates, D. O. Vascular endothelial growth factors and vascular permeability. Cardiovasc. Res. 87, 262–271 (2010).
Shen, Y. H., Shoichet, M. S. & Radisic, M. Vascular endothelial growth factor immobilized in collagen scaffold promotes penetration and proliferation of endothelial cells. Acta Biomater. 4, 477–489 (2008).
Chen, T. T. et al. Anchorage of VEGF to the extracellular matrix conveys differential signaling responses to endothelial cells. J. Cell Biol. 188, 595–609 (2010).
Zhu, S., Nih, L., Carmichael, S. T., Lu, Y. & Segura, T. Enzyme-responsive delivery of multiple proteins with spatiotemporal control. Adv. Mater. 27, 3620–3625 (2015).
Li, S. et al. Hydrogels with precisely controlled integrin activation dictate vascular patterning and permeability. Nat. Mater. 16, 953–961 (2017).
Wells, L. A., Valic, M. S., Alexandra, L. & Sefton, M. V. Angiogenic biomaterials to promote tissue vascularization and integration. Isr. J. Chem. 53, 637–645 (2013).
Brauker, J. H. et al. Neovascularization of synthetic membranes directed by membrane microarchitecture. J. Biomed. Mater. Res. 29, 1517–1524 (1995).
Artel, A., Mehdizadeh, H., Chiu, Y. C., Brey, E. M. & Cinar, A. An agent-based model for the investigation of neovascularization within porous scaffolds. Tissue Eng. Part A. 17, 2133–2141 (2011).
Xiao, X. et al. The promotion of angiogenesis induced by three-dimensional porous beta-tricalcium phosphate scaffold with different interconnection sizes via activation of PI3K/Akt pathways. Sci. Rep. 5, 9409 (2015).
Mastrogiacomo, M. et al. Role of scaffold internal structure on in vivo bone formation in macroporous calcium phosphate bioceramics. Biomaterials 27, 3230–3237 (2006).
Bai, F. et al. The correlation between the internal structure and vascularization of controllable porous bioceramic materials in vivo: a quantitative study. Tissue Eng. Part A. 16, 3791–3803 (2010).
Eckhaus, A. A., Fish, J. S., Skarja, G., Semple, J. L. & Sefton, M. V. A preliminary study of the effect of poly(methacrylic acid-co-methyl methacrylate) beads on angiogenesis in rodent skin grafts and the quality of the panniculus carnosus. Plast. Reconstr. Surg. 122, 1361–1370 (2008).
Martin, D. C., Semple, J. L. & Sefton, M. V. Poly(methacrylic acid-co-methyl methacrylate) beads promote vascularization and wound repair in diabetic mice. J. Biomed. Mater. Res. A. 93, 484–492 (2010).
Butler, M. J. & Sefton, M. V. Poly(butyl methacrylate-co-methacrylic acid) tissue engineering scaffold with pro-angiogenic potential in vivo. J. Biomed. Mater. Res. A. 82, 265–273 (2007).
Fitzpatrick, L. E., Lisovsky, A. & Sefton, M. V. The expression of sonic hedgehog in diabetic wounds following treatment with poly(methacrylic acid-co-methyl methacrylate) beads. Biomaterials 33, 5297–5307 (2012).
Lisovsky, A., Zhang, D. K. & Sefton, M. V. Effect of methacrylic acid beads on the sonic hedgehog signaling pathway and macrophage polarization in a subcutaneous injection mouse model. Biomaterials 98, 203–214 (2016).
Wells, L. A., Guo, H., Emili, A. & Sefton, M. V. The profile of adsorbed plasma and serum proteins on methacrylic acid copolymer beads: effect on complement activation. Biomaterials 118, 74–83 (2017).
Chong, M. S., Ng, W. K. & Chan, J. K. Concise review: endothelial progenitor cells in regenerative medicine: applications and challenges. Stem Cells Transl Med. 5, 530–538 (2016).
Patan, S. Vasculogenesis and angiogenesis. Cancer Treat. Res. 117, 3–32 (2004).
Fadini, G. P. et al. Circulating endothelial progenitor cells are reduced in peripheral vascular complications of type 2 diabetes mellitus. J. Am. Coll. Cardiol. 45, 1449–1457 (2005).
Aragona, C. O. et al. Endothelial progenitor cells for diagnosis and prognosis in cardiovascular disease. Stem Cells Int. 2016, 8043792 (2016).
Atluri, P. et al. Tissue-engineered, hydrogel-based endothelial progenitor cell therapy robustly revascularizes ischemic myocardium and preserves ventricular function. J. Thorac. Cardiovasc. Surg. 148, 1090–1097; discussion 1097–1098 (2014).
Hanjaya-Putra, D. et al. Controlled activation of morphogenesis to generate a functional human microvasculature in a synthetic matrix. Blood 118, 804–815 (2011).
Silva, E. A., Kim, E. S., Kong, H. J. & Mooney, D. J. Material-based deployment enhances efficacy of endothelial progenitor cells. Proc. Natl Acad. Sci. USA 105, 14347–14352 (2008).
Chen, X. et al. Prevascularization of a fibrin-based tissue construct accelerates the formation of functional anastomosis with host vasculature. Tissue Eng. Part A. 15, 1363–1371 (2009).
Chen, X. et al. Rapid anastomosis of endothelial progenitor cell-derived vessels with host vasculature is promoted by a high density of cotransplanted fibroblasts. Tissue Eng. Part A. 16, 585–594 (2010).
Wu, X. et al. Tissue-engineered microvessels on three-dimensional biodegradable scaffolds using human endothelial progenitor cells. Am. J. Physiol. Heart Circ. Physiol. 287, H480–H487 (2004).
Baranski, J. D. et al. Geometric control of vascular networks to enhance engineered tissue integration and function. Proc. Natl Acad. Sci. USA 110, 7586–7591 (2013).
Vlahos, A. E., Cober, N. & Sefton, M. V. Modular tissue engineering for the vascularization of subcutaneously transplanted pancreatic islets. Proc. Natl Acad. Sci. USA 114, 9337–9342 (2017).
Sadtler, K. et al. Developing a pro-regenerative biomaterial scaffold microenvironment requires T helper 2 cells. Science 352, 366–370 (2016).
Mase, V. J. Jr et al. Clinical application of an acellular biologic scaffold for surgical repair of a large, traumatic quadriceps femoris muscle defect. Orthopedics 33, 511(2010).
Sicari, B. M. et al. An acellular biologic scaffold promotes skeletal muscle formation in mice and humans with volumetric muscle loss. Sci. Transl. Med. 6, 234ra58 (2014).
Brown, B. N. et al. Macrophage phenotype as a predictor of constructive remodeling following the implantation of biologically derived surgical mesh materials. Acta Biomater. 8, 978–987 (2012).
Mendes Junior, D. et al. Study of mesenchymal stem cells cultured on a poly(lactic-co-glycolic acid) scaffold containing simvastatin for bone healing. J. Appl. Biomater. Funct. Mater. 15, e133–e141 (2017).
Tomita, M. et al. Biodegradable polymer composite grafts promote the survival and differentiation of retinal progenitor cells. Stem Cells 23, 1579–1588 (2005).
Yoshida, M. & Babensee, J. E. Poly(lactic-co-glycolic acid) enhances maturation of human monocyte-derived dendritic cells. J. Biomed. Mater. Res. A. 71, 45–54 (2004).
Yoshida, M., Mata, J. & Babensee, J. E. Effect of poly(lactic-co-glycolic acid) contact on maturation of murine bone marrow-derived dendritic cells. J. Biomed. Mater. Res. A. 80, 7–12 (2007).
Park, J. & Babensee, J. E. Differential functional effects of biomaterials on dendritic cell maturation. Acta Biomater. 8, 3606–3617 (2012).
Park, J., Gerber, M. H. & Babensee, J. E. Phenotype and polarization of autologous T cells by biomaterial-treated dendritic cells. J. Biomed. Mater. Res. A. 103, 170–184 (2015).
Rogers, T. H. & Babensee, J. E. The role of integrins in the recognition and response of dendritic cells to biomaterials. Biomaterials 32, 1270–1279 (2011).
Rogers, T. H. & Babensee, J. E. Altered adherent leukocyte profile on biomaterials in Toll-like receptor 4 deficient mice. Biomaterials 31, 594–601 (2010).
Grandjean-Laquerriere, A. et al. Involvement of toll-like receptor 4 in the inflammatory reaction induced by hydroxyapatite particles. Biomaterials 28, 400–404 (2007).
Misra, S., Hascall, V. C., Markwald, R. R. & Ghatak, S. Interactions between hyaluronan and its receptors (CD44, RHAMM) regulate the activities of inflammation and cancer. Front. Immunol. 6, 201 (2015).
Hu, W. J., Eaton, J. W., Ugarova, T. P. & Tang, L. Molecular basis of biomaterial-mediated foreign body reactions. Blood 98, 1231–1238 (2001).
Ostuni, E., Chapman, R. G., Holmlin, R. E., Takayama, S. & Whitesides, G. M. A. Survey of structure−property relationships of surfaces that resist the adsorption of protein. Langmuir 17, 5605–5620 (2001).
Kou, P. M., Schwartz, Z., Boyan, B. D. & Babensee, J. E. Dendritic cell responses to surface properties of clinical titanium surfaces. Acta Biomater. 7, 1354–1363 (2011).
Rayahin, J. E., Buhrman, J. S., Zhang, Y., Koh, T. J. & Gemeinhart, R. A. High and low molecular weight hyaluronic acid differentially influence macrophage activation. ACS Biomater. Sci. Eng. 1, 481–493 (2015).
Siiskonen, H., Oikari, S., Pasonen-Seppanen, S. & Rilla, K. Hyaluronan synthase 1: a mysterious enzyme with unexpected functions. Front. Immunol. 6, 43 (2015).
Mack, J. A. et al. Enhanced inflammation and accelerated wound closure following tetraphorbol ester application or full-thickness wounding in mice lacking hyaluronan synthases Has1 and Has3. J. Invest. Dermatol. 132, 198–207 (2012).
Camenisch, T. D. et al. Disruption of hyaluronan synthase-2 abrogates normal cardiac morphogenesis and hyaluronan-mediated transformation of epithelium to mesenchyme. J. Clin. Invest. 106, 349–360 (2000).
Bauer, C. et al. Chondroprotective effect of high-molecular-weight hyaluronic acid on osteoarthritic chondrocytes in a co-cultivation inflammation model with M1 macrophages. J. Inflamm. (Lond.) 13, 31 (2016).
Suzuki, Y. & Yamaguchi, T. Effects of hyaluronic acid on macrophage phagocytosis and active oxygen release. Agents Act. 38, 32–37 (1993).
Babensee, J. E. & Paranjpe, A. Differential levels of dendritic cell maturation on different biomaterials used in combination products. J. Biomed. Mater. Res. A. 74, 503–510 (2005).
Bollyky, P. L. et al. Intact extracellular matrix and the maintenance of immune tolerance: high molecular weight hyaluronan promotes persistence of induced CD4+CD25+ regulatory T cells. J. Leukoc. Biol. 86, 567–572 (2009).
Jiang, D., Liang, J. & Noble, P. W. Hyaluronan in tissue injury and repair. Annu. Rev. Cell Dev. Biol. 23, 435–461 (2007).
McKee, C. M. et al. Hyaluronan (HA) fragments induce chemokine gene expression in alveolar macrophages. The role of HA size and CD44. J. Clin. Invest. 98, 2403–2413 (1996).
Rizzo, M. et al. Low molecular weight hyaluronan-pulsed human dendritic cells showed increased migration capacity and induced resistance to tumor chemoattraction. PLOS One 9, e107944 (2014).
Yang, C. et al. The high and low molecular weight forms of hyaluronan have distinct effects on CD44 clustering. J. Biol. Chem. 287, 43094–43107 (2012).
Lesley, J., Hascall, V. C., Tammi, M. & Hyman, R. Hyaluronan binding by cell surface CD44. J. Biol. Chem. 275, 26967–26975 (2000).
Wolny, P. M. et al. Analysis of CD44-hyaluronan interactions in an artificial membrane system: insights into the distinct binding properties of high and low molecular weight hyaluronan. J. Biol. Chem. 285, 30170–30180 (2010).
Jiang, D. et al. Regulation of lung injury and repair by Toll-like receptors and hyaluronan. Nat. Med. 11, 1173–1179 (2005).
Tesar, B. M. et al. The role of hyaluronan degradation products as innate alloimmune agonists. Am. J. Transplant. 6, 2622–2635 (2006).
Thevenot, P., Hu, W. & Tang, L. Surface chemistry influences implant biocompatibility. Curr. Top. Med. Chem. 8, 270–280 (2008).
Keselowsky, B. G., Collard, D. M. & Garcia, A. J. Surface chemistry modulates focal adhesion composition and signaling through changes in integrin binding. Biomaterials 25, 5947–5954 (2004).
Grafahrend, D. et al. Degradable polyester scaffolds with controlled surface chemistry combining minimal protein adsorption with specific bioactivation. Nat. Mater. 10, 67–73 (2011).
Kingshott, P. & Griesser, H. J. Surfaces that resist bioadhesion. Curr. Opin. Solid State Mater. Sci. 4, 403–412 (1999).
Lynn, A. D., Kyriakides, T. R. & Bryant, S. J. Characterization of the in vitro macrophage response and in vivo host response to poly(ethylene glycol)-based hydrogels. J. Biomed. Mater. Res. A. 93, 941–953 (2010).
Lynn, A. D., Blakney, A. K., Kyriakides, T. R. & Bryant, S. J. Temporal progression of the host response to implanted poly(ethylene glycol)-based hydrogels. J. Biomed. Mater. Res. A. 96, 621–631 (2011).
Li, W. A. et al. The effect of surface modification of mesoporous silica micro-rod scaffold on immune cell activation and infiltration. Biomaterials 83, 249–256 (2016).
Zhang, L. et al. Zwitterionic hydrogels implanted in mice resist the foreign-body reaction. Nat. Biotechnol. 31, 553–556 (2013).
Jiang, S. & Cao, Z. Ultralow-fouling, functionalizable, and hydrolyzable zwitterionic materials and their derivatives for biological applications. Adv. Mater. 22, 920–932 (2010).
Chen, S., Zheng, J., Li, L. & Jiang, S. Strong resistance of phosphorylcholine self-assembled monolayers to protein adsorption: insights into nonfouling properties of zwitterionic materials. J. Am. Chem. Soc. 127, 14473–14478 (2005).
Schlenoff, J. B. Zwitteration: coating surfaces with zwitterionic functionality to reduce nonspecific adsorption. Langmuir 30, 9625–9636 (2014).
Clarke, M. L., Wang, J. & Chen, Z. Conformational changes of fibrinogen after adsorption. J. Phys. Chem. B 109, 22027–22035 (2005).
Grunkemeier, J., Wan, C. & Horbett, T. Changes in binding affinity of a monoclonal antibody to a platelet binding domain of fibrinogen adsorbed to biomaterials. J. Biomater. Sci. Polym. Ed. 8, 189–209 (1996).
Chiumiento, A., Lamponi, S. & Barbucci, R. Role of fibrinogen conformation in platelet activation. Biomacromolecules 8, 523–531 (2007).
Kao, W. J., Lee, D., Schense, J. C. & Hubbell, J. A. Fibronectin modulates macrophage adhesion and FBGC formation: the role of RGD, PHSRN, and PRRARV domains. J. Biomed. Mater. Res. 55, 79–88 (2001).
Swartzlander, M. D. et al. Linking the foreign body response and protein adsorption to PEG-based hydrogels using proteomics. Biomaterials 41, 26–36 (2015).
Tang, D. et al. Regulation of macrophage polarization and promotion of endothelialization by NO generating and PEG-YIGSR modified vascular graft. Mater. Sci. Eng. C. Mater. Biol. Appl. 84, 1–11 (2018).
Matlaga, B. F., Yasenchak, L. P. & Salthouse, T. N. Tissue response to implanted polymers: the significance of sample shape. J. Biomed. Mater. Res. 10, 391–397 (1976).
Taylor, S. R. & Gibbons, D. F. Effect of surface texture on the soft tissue response to polymer implants. J. Biomed. Mater. Res. 17, 205–227 (1983).
Veiseh, O. et al. Size- and shape-dependent foreign body immune response to materials implanted in rodents and non-human primates. Nat. Mater. 14, 643–651 (2015).
Kuai, R., Ochyl, L. J., Bahjat, K. S., Schwendeman, A. & Moon, J. J. Designer vaccine nanodiscs for personalized cancer immunotherapy. Nat. Mater. 16, 489–496 (2017).
Cheung, A. S., Zhang, D. K. Y., Koshy, S. T. & Mooney, D. J. Scaffolds that mimic antigen-presenting cells enable ex vivo expansion of primary T cells. Nat. Biotechnol. 36, 160–169 (2018).
Hachim, D., LoPresti, S. T., Yates, C. C. & Brown, B. N. Shifts in macrophage phenotype at the biomaterial interface via IL-4 eluting coatings are associated with improved implant integration. Biomaterials 112, 95–107 (2017).
Mokarram, N., Merchant, A., Mukhatyar, V., Patel, G. & Bellamkonda, R. V. Effect of modulating macrophage phenotype on peripheral nerve repair. Biomaterials 33, 8793–8801 (2012).
Reeves, A. R., Spiller, K. L., Freytes, D. O., Vunjak-Novakovic, G. & Kaplan, D. L. Controlled release of cytokines using silk-biomaterials for macrophage polarization. Biomaterials 73, 272–283 (2015).
Browne, S. & Pandit, A. Biomaterial-mediated modification of the local inflammatory environment. Front. Bioeng. Biotechnol. 3, 67 (2015).
Nih, L. R., Gojgini, S., Carmichael, S. T. & Segura, T. Dual-function injectable angiogenic biomaterial for the repair of brain tissue following stroke. Nat. Mater. 17, 642–651 (2018).
Tuladhar, A. & Shoichet, M. S. Biomaterials driving repair after stroke. Nat. Mater. 17, 573–574 (2018).
Olson, T. S. et al. Megakaryocytes promote murine osteoblastic HSC niche expansion and stem cell engraftment after radioablative conditioning. Blood 121, 5238–5249 (2013).
Caselli, A. et al. IGF-1-mediated osteoblastic niche expansion enhances long-term hematopoietic stem cell engraftment after murine bone marrow transplantation. Stem Cells 31, 2193–2204 (2013).
Calvi, L. M. et al. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature 425, 841–846 (2003).
Adams, G. B. et al. Therapeutic targeting of a stem cell niche. Nat. Biotechnol. 25, 238–243 (2007).
Greenbaum, A. et al. CXCL12 in early mesenchymal progenitors is required for haematopoietic stem-cell maintenance. Nature 495, 227–230 (2013).
Talcott, K. E. et al. Longitudinal study of cone photoreceptors during retinal degeneration and in response to ciliary neurotrophic factor treatment. Invest. Ophthalmol. Vis. Sci. 52, 2219–2226 (2011).
Kauper, K. et al. Two-year intraocular delivery of ciliary neurotrophic factor by encapsulated cell technology implants in patients with chronic retinal degenerative diseases. Invest. Ophthalmol. Vis. Sci. 53, 7484–7491 (2012).
Rhee, K. D. et al. CNTF-mediated protection of photoreceptors requires initial activation of the cytokine receptor gp130 in Muller glial cells. Proc. Natl Acad. Sci. USA 110, E4520–E4529 (2013).
Mosmann, T. R., Cherwinski, H., Bond, M. W., Giedlin, M. A. & Coffman, R. L. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J. Immunol. 136, 2348–2357 (1986).
Berger, A. Th1 and Th2 responses: what are they? BMJ 321, 424 (2000).
Romagnani, S. T cell subsets (Th1 versus Th2). Ann. Allergy Asthma Immunol. 85, 9–18 (2000).
Allen, J. E. & Wynn, T. A. Evolution of Th2 immunity: a rapid repair response to tissue destructive pathogens. PLOS Pathog. 7, e1002003 (2011).
Hirahara, K. & Nakayama, T. CD4+ T cell subsets in inflammatory diseases: beyond the Th1/Th2 paradigm. Int. Immunol. 28, 163–171 (2016).
Arango Duque, G. & Descoteaux, A. Macrophage cytokines: involvement in immunity and infectious diseases. Front. Immunol. 5, 491 (2014).
Stein, M., Keshav, S., Harris, N. & Gordon, S. Interleukin 4 potently enhances murine macrophage mannose receptor activity: a marker of alternative immunologic macrophage activation. J. Exp. Med. 176, 287–292 (1992).
Muraille, E., Leo, O. & Moser, M. TH1/TH2 paradigm extended: macrophage polarization as an unappreciated pathogen-driven escape mechanism? Front. Immunol. 5, 603 (2014).
Sica, A. & Mantovani, A. Macrophage plasticity and polarization: in vivo veritas. J. Clin. Invest. 122, 787–795 (2012).
Sefcik, L. S., Petrie Aronin, C. E., Wieghaus, K. A. & Botchwey, E. A. Sustained release of sphingosine 1-phosphate for therapeutic arteriogenesis and bone tissue engineering. Biomaterials 29, 2869–2877 (2008).
Chow, L. W. et al. A bioactive self-assembled membrane to promote angiogenesis. Biomaterials 32, 1574–1582 (2011).
Binder, B. Y., Sondergaard, C. S., Nolta, J. A. & Leach, J. K. Lysophosphatidic acid enhances stromal cell-directed angiogenesis. PLOS One 8, e82134 (2013).
Anderson, E. M. et al. VEGF and IGF delivered from alginate hydrogels promote stable perfusion recovery in ischemic hind limbs of aged mice and young rabbits. J. Vasc. Res. 54, 288–298 (2017).
Acknowledgements
The authors are grateful for funding from the Natural Sciences and Engineering Research Council of Canada (Discovery Grant to M.S.S., NSERC CREATE in M3 scholarship to N.M.), the Canadian Institutes of Health Research (Foundation Grant to M.S.S.), the Canada First Research Excellence Fund for Medicine by Design at the University of Toronto (to M.S.S.) and the Tier 1 Canada Research Chair (to M.S.S.). The authors thank members of the Shoichet laboratory for thoughtful review of this manuscript.
Author information
Authors and Affiliations
Contributions
N.M., A.F. and M.S.S. conceived the manuscript; N.M. and A.F. wrote the manuscript; A.F. designed the figures; N.M., A.F. and M.S.S. edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests but acknowledge a composition of matter patent on HAMC cell delivery.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mitrousis, N., Fokina, A. & Shoichet, M.S. Biomaterials for cell transplantation. Nat Rev Mater 3, 441–456 (2018). https://doi.org/10.1038/s41578-018-0057-0
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41578-018-0057-0
This article is cited by
-
Hydrogel oxygen reservoirs increase functional integration of neural stem cell grafts by meeting metabolic demands
Nature Communications (2023)
-
Biomimetic hydrogel blanket for conserving and recovering intrinsic cell properties
Biomaterials Research (2022)
-
Engineering the next generation of cell-based therapeutics
Nature Reviews Drug Discovery (2022)
-
Combination Therapy of Stem Cell-derived Exosomes and Biomaterials in the Wound Healing
Stem Cell Reviews and Reports (2022)
-
Alginate-Based Smart Materials and Their Application: Recent Advances and Perspectives
Topics in Current Chemistry (2022)