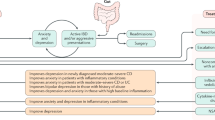
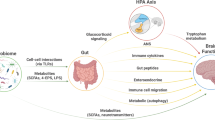
Abstract
Bariatric surgery induces sustained weight loss and metabolic benefits via notable effects on the gut–brain axis that lead to alterations in the neuroendocrine regulation of appetite and glycaemia. However, in a subset of patients, bariatric surgery is associated with adverse effects on mental health, including increased risk of suicide or self-harm as well as the emergence of depression and substance use disorders. The contributing factors behind these adverse effects are not well understood. Accumulating evidence indicates that there are important links between gut-derived hormones, microbial and bile acid profiles, and disorders of mood and substance use, which warrant further exploration in the context of changes in gut–brain signalling after bariatric surgery. Understanding the basis of these adverse effects is essential in order to optimize the health and well-being of people undergoing treatment for obesity.
Key points
-
After bariatric surgery, a subset of patients experience adverse mental health outcomes such as depression, anxiety, and increased use of alcohol and drugs.
-
Preclinical and clinical studies link appetite-related gut peptides, including ghrelin, glucagon-like peptide 1, peptide YY and cholecystokinin, with depression and anxiety.
-
Central ghrelin signalling seems to be involved in driving increases in the rewarding properties of alcohol following bariatric surgery.
-
Gut bacteria might influence mood and behaviour, and exposure to drugs of abuse, such as alcohol and opioids, induces gut microbial dysbiosis.
-
Emerging literature indicates that bile acids can have central effects on mood and behavioural responses induced by cocaine.
-
Taken together, these findings implicate potential mechanisms by which bariatric surgery could influence mood and behaviour; further research is essential to understand and prevent adverse mental health outcomes after bariatric surgery.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Sjostrom, L. et al. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N. Engl. J. Med. 351, 2683–2693 (2004).
Sjostrom, L. et al. Effects of bariatric surgery on mortality in Swedish obese subjects. N. Engl. J. Med. 357, 741–752 (2007).
Evers, S. S., Sandoval, D. A. & Seeley, R. J. The physiology and molecular underpinnings of the effects of bariatric surgery on obesity and diabetes. Annu. Rev. Physiol. 79, 313–334 (2017).
Miras, A. D. & le Roux, C. W. Mechanisms underlying weight loss after bariatric surgery. Nat. Rev. Gastroenterol. Hepatol. 10, 575–584 (2013).
Castaneda, D., Popov, V. B., Wander, P. & Thompson, C. C. Risk of suicide and self-harm is increased after bariatric surgery — a systematic review and meta-analysis. Obes. Surg. 29, 322–333 (2019).
Backman, O., Stockeld, D., Rasmussen, F., Naslund, E. & Marsk, R. Alcohol and substance abuse, depression and suicide attempts after Roux-en-Y gastric bypass surgery. Br. J. Surg. 103, 1336–1342 (2016).
Huang, T. T. et al. Current understanding of gut microbiota in mood disorders: an update of human studies. Front. Genet. 10, 98 (2019).
Ramos, A. et al. Fifth IFSO Global Registry Report. https://www.ifso.com/pdf/5th-ifso-global-registry-report-september-2019.pdf (2019).
Okano-Matsumoto, S., McRoberts, J. A., Tache, Y. & Adelson, D. W. Electrophysiological evidence for distinct vagal pathways mediating CCK-evoked motor effects in the proximal versus distal stomach. J. Physiol. 589, 371–393 (2011).
Meek, C. L., Lewis, H. B., Reimann, F., Gribble, F. M. & Park, A. J. The effect of bariatric surgery on gastrointestinal and pancreatic peptide hormones. Peptides 77, 28–37 (2016).
Morinigo, R. et al. Glucagon-like peptide-1, peptide YY, hunger, and satiety after gastric bypass surgery in morbidly obese subjects. J. Clin. Endocrinol. Metab. 91, 1735–1740 (2006).
McCarty, T. R., Jirapinyo, P. & Thompson, C. C. Effect of sleeve gastrectomy on ghrelin, GLP-1, PYY, and GIP gut hormones: a systematic review and meta-analysis. Ann. Surg. 272, 72–80 (2020).
Xu, H.-C. et al. Systematic review and meta-analysis of the change in ghrelin levels after roux-en-Y gastric bypass. Obes. Surg. 29, 1343–1351 (2019).
Chambers, A. P. et al. The effects of vertical sleeve gastrectomy in rodents are ghrelin independent. Gastroenterology 144, 50–52.e5 (2013).
Hunt, K. F. et al. Differences in regional brain responses to food ingestion after roux-en-Y gastric bypass and the role of gut peptides: a neuroimaging study. Diabetes Care 39, 1787–1795 (2016).
le Roux, C. W. et al. Gut hormones as mediators of appetite and weight loss after Roux-en-Y gastric bypass. Ann. Surg. 246, 780–785 (2007). This study evaluates the time course and contribution of gut-derived satiety hormones on appetite regulation after RYGB.
Yoshino, M. et al. Effects of diet versus gastric bypass on metabolic function in diabetes. N. Engl. J. Med. 383, 721–732 (2020).
Dirksen, C. et al. Mechanisms of improved glycaemic control after Roux-en-Y gastric bypass. Diabetologia 55, 1890–1901 (2012).
Qin, J. et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature 464, 59–65 (2010).
Ley, R. E. et al. Obesity alters gut microbial ecology. Proc. Natl Acad. Sci. USA 102, 11070–11075 (2005).
Schwiertz, A. et al. Microbiota and SCFA in lean and overweight healthy subjects. Obesity 18, 190–195 (2010).
Le Chatelier, E. et al. Richness of human gut microbiome correlates with metabolic markers. Nature 500, 541–546 (2013).
Canfora, E. E., Meex, R. C. R., Venema, K. & Blaak, E. E. Gut microbial metabolites in obesity, NAFLD and T2DM. Nat. Rev. Endocrinol. 15, 261–273 (2019).
Tremaroli, V. et al. Roux-en-Y gastric bypass and vertical banded gastroplasty induce long-term changes on the human gut microbiome contributing to fat mass regulation. Cell Metab. 22, 228–238 (2015). This paper shows long-term alterations in gut microbial composition and function after bariatric surgery and their potential to modulate host metabolism.
Liou, A. P. et al. Conserved shifts in the gut microbiota due to gastric bypass reduce host weight and adiposity. Sci. Transl. Med. 5, 178ra141 (2013).
Russell, D. W. The enzymes, regulation, and genetics of bile acid synthesis. Annu. Rev. Biochem. 72, 137–174 (2003).
Staudinger, J. L. et al. The nuclear receptor PXR is a lithocholic acid sensor that protects against liver toxicity. Proc. Natl Acad. Sci. USA 98, 3369–3374 (2001).
Makishima, M. et al. Vitamin D receptor as an intestinal bile acid sensor. Science 296, 1313–1316 (2002).
Steinert, R. E. et al. Bile acids and gut peptide secretion after bariatric surgery: a 1-year prospective randomized pilot trial. Obesity 21, E660–E668 (2013).
Jorgensen, N. B. et al. Improvements in glucose metabolism early after gastric bypass surgery are not explained by increases in total bile acids and fibroblast growth factor 19 concentrations. J. Clin. Endocrinol. Metab. 100, E396–E406 (2015).
Chen, Y., Lu, J., Nemati, R., Plank, L. D. & Murphy, R. Acute changes of bile acids and FGF19 after sleeve gastrectomy and roux-en-Y gastric bypass. Obes. Surg. 29, 3605–3621 (2019).
Albaugh, V. L. et al. Early increases in bile acids post roux-en-Y gastric bypass are driven by insulin-sensitizing, secondary bile acids. J. Clin. Endocrinol. Metab. 100, E1225–E1233 (2015).
Nemati, R. et al. Increased bile acids and FGF19 after sleeve gastrectomy and roux-en-Y gastric bypass correlate with improvement in type 2 diabetes in a randomized trial. Obes. Surg. 28, 2672–2686 (2018).
Albaugh, V. L. et al. Role of bile acids and GLP-1 in mediating the metabolic improvements of bariatric surgery. Gastroenterology 156, 1041–1051.e4 (2019).
Kalarchian, M. A. et al. Mental disorders and weight change in a prospective study of bariatric surgery patients: 7 years of follow-up. Surg. Obes. Relat. Dis. 15, 739–748 (2019).
Mitchell, J. E. et al. Course of depressive symptoms and treatment in the longitudinal assessment of bariatric surgery (LABS-2) study. Obesity 22, 1799–1806 (2014).
Mitchell, J. E. et al. Possible risk factors for increased suicide following bariatric surgery. Obesity 21, 665–672 (2013).
Stammers, L. et al. Identifying stress-related eating in behavioural research: a review. Hormones Behav. 124, 104752 (2020).
Lagerros, Y. T., Brandt, L., Hedberg, J., Sundbom, M. & Bodén, R. Suicide, self-harm, and depression after gastric bypass surgery: a nationwide cohort study. Ann. Surg. 265, 235–243 (2017).
Hamad, G. G. et al. The effect of gastric bypass on the pharmacokinetics of serotonin reuptake inhibitors. Am. J. Psychiatry 169, 256–263 (2012).
Neovius, M. et al. Risk of suicide and non-fatal self-harm after bariatric surgery: results from two matched cohort studies. Lancet Diabetes Endocrinol. 6, 197–207 (2018). This article shows an increased risk of suicide and self-harm after bariatric surgery in two separate cohorts, among patients with and without known psychiatric disorders.
Morgan, D. J. R., Ho, K. M. & Platell, C. Incidence and determinants of mental health service use after bariatric surgery. JAMA Psychiatry 77, 60–67 (2020).
Knop, J. & Fischer, A. Duodenal ulcer, suicide, psychopathology and alcoholism. Acta Psychiatr. Scand. 63, 346–355 (1981).
Klarer, M. et al. Gut vagal afferents differentially modulate innate anxiety and learned fear. J. Neurosci. 34, 7067–7076 (2014).
Lutter, M. et al. The orexigenic hormone ghrelin defends against depressive symptoms of chronic stress. Nat. Neurosci. 11, 752–753 (2008). Through several experimental approaches, this paper shows a role of ghrelin in defending against anxiety-like and depression-like behaviour.
Alvarez-Crespo, M. et al. The amygdala as a neurobiological target for ghrelin in rats: neuroanatomical, electrophysiological and behavioral evidence. PLoS ONE 7, e46321 (2012).
Spencer, S. J. et al. Ghrelin regulates the hypothalamic-pituitary-adrenal axis and restricts anxiety after acute stress. Biol. Psychiatry 72, 457–465 (2012).
Asakawa, A. et al. A role of ghrelin in neuroendocrine and behavioral responses to stress in mice. Neuroendocrinology 74, 143–147 (2001).
Currie, P. J. et al. Ghrelin is an orexigenic peptide and elicits anxiety-like behaviors following administration into discrete regions of the hypothalamus. Behav. Brain Res. 226, 96–105 (2012).
Hansson, C. et al. Central administration of ghrelin alters emotional responses in rats: behavioural, electrophysiological and molecular evidence. Neuroscience 180, 201–211 (2011).
Carlini, V. P. et al. Acute ghrelin administration reverses depressive-like behavior induced by bilateral olfactory bulbectomy in mice. Peptides 35, 160–165 (2012).
Huang, H. J. et al. Ghrelin alleviates anxiety- and depression-like behaviors induced by chronic unpredictable mild stress in rodents. Behav. Brain Res. 326, 33–43 (2017).
Schmid, D. A. et al. Ghrelin stimulates appetite, imagination of food, GH, ACTH, and cortisol, but does not affect leptin in normal controls. Neuropsychopharmacology 30, 1187–1192 (2005).
Kluge, M. et al. Effects of ghrelin on psychopathology, sleep and secretion of cortisol and growth hormone in patients with major depression. J. Psychiatr. Res. 45, 421–426 (2011).
Lundholm, K. et al. Effects by daily long term provision of ghrelin to unselected weight-losing cancer patients: a randomized double-blind study. Cancer 116, 2044–2052 (2010).
Cain, B. M. et al. Distribution and colocalization of cholecystokinin with the prohormone convertase enzymes PC1, PC2, and PC5 in rat brain. J. Comp. Neurol. 467, 307–325 (2003).
Rezayat, M., Roohbakhsh, A., Zarrindast, M. R., Massoudi, R. & Djahanguiri, B. Cholecystokinin and GABA interaction in the dorsal hippocampus of rats in the elevated plus-maze test of anxiety. Physiol. Behav. 84, 775–782 (2005).
Hernando, F., Fuentes, J. A., Roques, B. P. & Ruiz-Gayo, M. The CCKB receptor antagonist, L-365,260, elicits antidepressant-type effects in the forced-swim test in mice. Eur. J. Pharmacol. 261, 257–263 (1994).
Chen, Q. et al. Bi-directional effect of cholecystokinin receptor-2 overexpression on stress-triggered fear memory and anxiety in the mouse. PLoS ONE 5, e15999 (2010).
Becker, C. et al. Repeated social defeat-induced depression-like behavioral and biological alterations in rats: involvement of cholecystokinin. Mol. Psychiatry 13, 1079–1092 (2008).
Bradwejn, J., Koszycki, D. & Meterissian, G. Cholecystokinin-tetrapeptide induces panic attacks in patients with panic disorder. Can. J. Psychiatry 35, 83–85 (1990).
van Megen, H. J., Westenberg, H. G., den Boer, J. A., Haigh, J. R. & Traub, M. Pentagastrin induced panic attacks: enhanced sensitivity in panic disorder patients. Psychopharmacology 114, 449–455 (1994).
Bradwejn, J. et al. The panicogenic effects of cholecystokinin-tetrapeptide are antagonized by L-365,260, a central cholecystokinin receptor antagonist, in patients with panic disorder. Arch. Gen. Psychiatry 51, 486–493 (1994).
Adams, J. B. et al. A double-blind, placebo-controlled study of a CCK-B receptor antagonist, CI-988, in patients with generalized anxiety disorder. J. Clin. Psychopharmacol. 15, 428–434 (1995).
Kramer, M. S. et al. A placebo-controlled trial of L-365,260, a CCKB antagonist, in panic disorder. Biol. Psychiatry 37, 462–466 (1995).
de Montigny, C. Cholecystokinin tetrapeptide induces panic-like attacks in healthy volunteers. Preliminary findings. Arch. Gen. Psychiatry 46, 511–517 (1989).
Secher, A. et al. The arcuate nucleus mediates GLP-1 receptor agonist liraglutide-dependent weight loss. J. Clin. Invest. 124, 4473–4488 (2014).
Alvarez, E. et al. The expression of GLP-1 receptor mRNA and protein allows the effect of GLP-1 on glucose metabolism in the human hypothalamus and brainstem. J. Neurochem. 92, 798–806 (2005).
Rinaman, L. Interoceptive stress activates glucagon-like peptide-1 neurons that project to the hypothalamus. Am. J. Physiol. 277, R582–R590 (1999).
Kinzig, K. P. et al. CNS glucagon-like peptide-1 receptors mediate endocrine and anxiety responses to interoceptive and psychogenic stressors. J. Neurosci. 23, 6163–6170 (2003).
Isacson, R. et al. The glucagon-like peptide 1 receptor agonist exendin-4 improves reference memory performance and decreases immobility in the forced swim test. Eur. J. Pharmacol. 650, 249–255 (2011).
Anderberg, R. H. et al. GLP-1 is both anxiogenic and antidepressant; divergent effects of acute and chronic GLP-1 on emotionality. Psychoneuroendocrinology 65, 54–66 (2016).
Strawn, J. R., D’Alessio, D. A., Keck, P. E. Jr. & Seeley, R. J. Failure of glucagon-like peptide-1 to induce panic attacks or anxiety in patients with panic disorder. J. Psychiatr. Res. 42, 787–789 (2008).
van Bloemendaal, L. et al. GLP-1 receptor activation modulates appetite- and reward-related brain areas in humans. Diabetes 63, 4186–4196 (2014).
Bode, B. W. et al. Patient-reported outcomes following treatment with the human GLP-1 analogue liraglutide or glimepiride in monotherapy: results from a randomized controlled trial in patients with type 2 diabetes. Diabetes Obes. Metab. 12, 604–612 (2010).
Kahal, H., Kilpatrick, E., Rigby, A., Coady, A. & Atkin, S. The effects of treatment with liraglutide on quality of life and depression in young obese women with PCOS and controls. Gynecol. Endocrinol. 35, 142–145 (2019).
Grant, P., Lipscomb, D. & Quin, J. Psychological and quality of life changes in patients using GLP-1 analogues. J. Diabetes Complications 25, 244–246 (2011).
Adrian, T. E. et al. Neuropeptide Y distribution in human brain. Nature 306, 584–586 (1983).
Redrobe, J. P., Dumont, Y., Fournier, A. & Quirion, R. The neuropeptide Y (NPY) Y1 receptor subtype mediates NPY-induced antidepressant-like activity in the mouse forced swimming test. Neuropsychopharmacology 26, 615–624 (2002).
Karl, T., Burne, T. H. J. & Herzog, H. Effect of Y1 receptor deficiency on motor activity, exploration, and anxiety. Behav. Brain Res. 167, 87–93 (2006).
Morales-Medina, J. C. et al. Role of neuropeptide Y Y1 and Y2 receptors on behavioral despair in a rat model of depression with co-morbid anxiety. Neuropharmacology 62, 200–208 (2012).
Yamada, C., Mogami, S., Kanno, H. & Hattori, T. Peptide YY causes apathy-like behavior via the dopamine D2 receptor in repeated water-immersed mice. Mol. Neurobiol. 55, 7555–7566 (2018).
Painsipp, E. et al. Reduced anxiety-like and depression-related behavior in neuropeptide Y Y4 receptor knockout mice. Genes Brain Behav. 7, 532–542 (2008).
Asakawa, A. et al. Mouse pancreatic polypeptide modulates food intake, while not influencing anxiety in mice. Peptides 20, 1445–1448 (1999).
Zhou, Z. et al. Genetic variation in human NPY expression affects stress response and emotion. Nature 452, 997–1001 (2008).
Domschke, K. et al. Neuropeptide Y (NPY) gene: Impact on emotional processing and treatment response in anxious depression. Eur. Neuropsychopharmacol. 20, 301–309 (2010).
Kristenssson, E. et al. Acute psychological stress raises plasma ghrelin in the rat. Regul. Pept. 134, 114–117 (2006).
Lambert, E. et al. Ghrelin modulates sympathetic nervous system activity and stress response in lean and overweight men. Hypertension 58, 43–50 (2011).
Langer, F. B. et al. Sleeve gastrectomy and gastric banding: effects on plasma ghrelin levels. Obes. Surg. 15, 1024–1029 (2005).
Diaz Heijtz, R. et al. Normal gut microbiota modulates brain development and behavior. Proc. Natl Acad. Sci. USA 108, 3047–3052 (2011).
Naseribafrouei, A. et al. Correlation between the human fecal microbiota and depression. Neurogastroenterol. Motil. 26, 1155–1162 (2014).
Jiang, H. Y. et al. Altered gut microbiota profile in patients with generalized anxiety disorder. J. Psychiatr. Res. 104, 130–136 (2018).
Zheng, P. et al. Gut microbiome remodeling induces depressive-like behaviors through a pathway mediated by the host’s metabolism. Mol. Psychiatry 21, 786–796 (2016).
Li, J. et al. Short term intrarectal administration of sodium propionate induces antidepressant-like effects in rats exposed to chronic unpredictable mild stress. Front. Psychiatry 9, 454 (2018).
Kelly, J. R. et al. Transferring the blues: depression-associated gut microbiota induces neurobehavioural changes in the rat. J. Psychiatr. Res. 82, 109–118 (2016).
Kelly, J. R. et al. Lost in translation? The potential psychobiotic Lactobacillus rhamnosus (JB-1) fails to modulate stress or cognitive performance in healthy male subjects. Brain Behav. Immun. 61, 50–59 (2017).
Messaoudi, M. et al. Assessment of psychotropic-like properties of a probiotic formulation (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in rats and human subjects. Br. J. Nutr. 105, 755–764 (2011).
Pinto-Sanchez, M. I. et al. Probiotic bifidobacterium longum NCC3001 reduces depression scores and alters brain activity: a pilot study in patients with irritable bowel syndrome. Gastroenterology 153, 448–459.e8 (2017).
Valles-Colomer, M. et al. The neuroactive potential of the human gut microbiota in quality of life and depression. Nat. Microbiol. 4, 623–632 (2019).
Yamawaki, Y. et al. Antidepressant-like effect of sodium butyrate (HDAC inhibitor) and its molecular mechanism of action in the rat hippocampus. World J. Biol. Psychiatry 13, 458–467 (2012).
Sun, J. et al. Antidepressant-like effects of sodium butyrate and its possible mechanisms of action in mice exposed to chronic unpredictable mild stress. Neurosci. Lett. 618, 159–166 (2016).
Huang, C. et al. Identification of functional farnesoid X receptors in brain neurons. FEBS Lett. 590, 3233–3242 (2016).
Huang, F. et al. Deletion of mouse FXR gene disturbs multiple neurotransmitter systems and alters neurobehavior. Front. Behav. Neurosci. 9, 70 (2015).
Chen, W. G., Zheng, J. X., Xu, X., Hu, Y. M. & Ma, Y. M. Hippocampal FXR plays a role in the pathogenesis of depression: a preliminary study based on lentiviral gene modulation. Psychiatry Res. 264, 374–379 (2018).
Lu, X. et al. Tauroursodeoxycholic acid produces antidepressant-like effects in a chronic unpredictable stress model of depression via attenuation of neuroinflammation, oxido-nitrosative stress, and endoplasmic reticulum stress. Fundam. Clin. Pharmacol. 32, 363–377 (2018).
Şen, O., Ünübol, H., Türkçapar, A. G. & Yerdel, M. A. Risk of alcohol use disorder after sleeve gastrectomy. J. Laparoendosc. Adv. Surg. Tech. A 31, 24–28 (2020).
Li, L. & Wu, L. T. Substance use after bariatric surgery: a review. J. Psychiatr. Res. 76, 16–29 (2016).
Raebel, M. A. et al. Chronic use of opioid medications before and after bariatric surgery. JAMA 310, 1369–1376 (2013). A retrospective cohort study of 11,719 individuals that shows increased opioid use following bariatric surgery.
Bak, M., Seibold-Simpson, S. M. & Darling, R. The potential for cross-addiction in post-bariatric surgery patients: considerations for primary care nurse practitioners. J. Am. Assoc. Nurse Pract. 28, 675–682 (2016).
McFadden, K. M. Cross-addiction: from morbid obesity to substance abuse. Bariatr. Nurs. Surg. Patient Care 5, 145–178 (2010).
King, W. C. et al. Prevalence of alcohol use disorders before and after bariatric surgery. JAMA 307, 2516–2525 (2012). The first large multicentre observational study showing increased alcohol use and incidence of AUD in the second year following bariatric surgery as compared with the year before or 1 year after.
Hajnal, A. et al. Alcohol reward is increased after Roux-en-Y gastric bypass in dietary obese rats with differential effects following ghrelin antagonism. PLoS ONE 7, e49121 (2012).
Sirohi, S., Richardson, B. D., Lugo, J. M., Rossi, D. J. & Davis, J. F. Impact of Roux-en-Y gastric bypass surgery on appetite, alcohol intake behaviors, and midbrain ghrelin signaling in the rat. Obesity 25, 1228–1236 (2017).
King, W. C. et al. Alcohol and other substance use after bariatric surgery: prospective evidence from a U.S. multicenter cohort study. Surg. Obes. Relat. Dis. 13, 1392–1402 (2017).
Orellana, E. R., Jamis, C., Horvath, N. & Hajnal, A. Effect of vertical sleeve gastrectomy on alcohol consumption and preferences in dietary obese rats and mice: a plausible role for altered ghrelin signaling. Brain Res. Bull. 138, 26–36 (2018).
Saules, K. K. et al. Bariatric surgery history among substance abuse treatment patients: prevalence and associated features. Surg. Obes. Relat. Dis. 6, 615–621 (2010).
Biegler, J. M., Freet, C. S., Horvath, N., Rogers, A. M. & Hajnal, A. Increased intravenous morphine self-administration following Roux-en-Y gastric bypass in dietary obese rats. Brain Res. Bull. 123, 47–52 (2016).
Wiedemann, A. A., Saules, K. K. & Ivezaj, V. Emergence of new onset substance use disorders among post-weight loss surgery patients. Clin. Obes. 3, 194–201 (2013).
Ivezaj, V., Saules, K. K. & Wiedemann, A. A. “I didn’t see this coming”: why are postbariatric patients in substance abuse treatment? Patients’ perceptions of etiology and future recommendations. Obes. Surg. 22, 1308–1314 (2012).
Sketriene, D., Ch’ng, S. S. & Brown, R. M. in Anti-Obesity Drug Discovery and Development Vol. 5 (eds Atta-ur-Rahman & Choudhary, M. I.) Ch. 1, 1–57 (Bentham Science Publishers, 2020).
Brown, R. M. et al. Addiction-like synaptic impairments in diet-induced obesity. Biol. Psychiatry 81, 797–806 (2017).
Yoder, R., MacNeela, P., Conway, R. & Heary, C. How do individuals develop alcohol use disorder after bariatric surgery? A grounded theory exploration. Obes. Surg. 28, 717–724 (2018).
Hardman, C. A. & Christiansen, P. Psychological issues and alcohol misuse following bariatric surgery. Nat. Rev. Endocrinol. 14, 377–378 (2018).
Acevedo, M. B. et al. Sleeve gastrectomy surgery: when 2 alcoholic drinks are converted to 4. Surg. Obes. Relat. Dis. 14, 277–283 (2018).
Hagedorn, J. C., Encarnacion, B., Brat, G. A. & Morton, J. M. Does gastric bypass alter alcohol metabolism? Surg. Obes. Relat. Dis. 3, 543–548 (2007).
Lloret-Linares, C. et al. Effect of a Roux-en-Y gastric bypass on the pharmacokinetics of oral morphine using a population approach. Clin. Pharmacokinet. 53, 919–930 (2014).
Strommen, M., Helland, A., Kulseng, B. & Spigset, O. Bioavailability of methadone after sleeve gastrectomy: a planned case observation. Clin. Ther. 38, 1532–1536 (2016).
Whitfield, J. B. et al. Variation in alcohol pharmacokinetics as a risk factor for alcohol dependence. Alcohol. Clin. Exp. Res. 25, 1257–1263 (2001).
Polston, J. E. et al. Roux-en-Y gastric bypass increases intravenous ethanol self-administration in dietary obese rats. PLoS ONE 8, e83741 (2013).
Davis, J. F. et al. Roux en Y gastric bypass increases ethanol intake in the rat. Obes. Surg. 23, 920–930 (2013).
Thiele, T. E., Sparta, D. R., Hayes, D. M. & Fee, J. R. A role for neuropeptide Y in neurobiological responses to ethanol and drugs of abuse. Neuropeptides 38, 235–243 (2004).
Davis, J. F. et al. Gastric bypass surgery attenuates ethanol consumption in ethanol-preferring rats. Biol. Psychiatry 72, 354–360 (2012).
Skibicka, K. P., Hansson, C., Alvarez-Crespo, M., Friberg, P. A. & Dickson, S. L. Ghrelin directly targets the ventral tegmental area to increase food motivation. Neuroscience 180, 129–137 (2011).
Jerlhag, E. et al. Requirement of central ghrelin signaling for alcohol reward. Proc. Natl Acad. Sci. USA 106, 11318–11323 (2009). This article shows the involvement of central ghrelin signalling in the rewarding effects of alcohol.
Jerlhag, E., Ivanoff, L., Vater, A. & Engel, J. A. Peripherally circulating ghrelin does not mediate alcohol-induced reward and alcohol intake in rodents. Alcohol. Clin. Exp. Res. 38, 959–968 (2014).
Wee, C. C. et al. High-risk alcohol use after weight loss surgery. Surg. Obes. Relat. Dis. 10, 508–513 (2014).
Shirazi, R. H., Dickson, S. L. & Skibicka, K. P. Gut peptide GLP-1 and its analogue, exendin-4, decrease alcohol intake and reward. PLoS ONE 8, e61965 (2013).
Meckel, K. R. & Kiraly, D. D. A potential role for the gut microbiome in substance use disorders. Psychopharmacology 236, 1513–1530 (2019).
Jadhav, K. S. et al. Gut microbiome correlates with altered striatal dopamine receptor expression in a model of compulsive alcohol seeking. Neuropharmacology 141, 249–259 (2018).
Wang, F. et al. Morphine induces changes in the gut microbiome and metabolome in a morphine dependence model. Sci. Rep. 8, 3596 (2018).
Reddy, I. A. et al. Bile diversion, a bariatric surgery, and bile acid signaling reduce central cocaine reward. PLoS Biol. 16, e2006682 (2018).
Docherty, N. G. & le Roux, C. W. Bariatric surgery for the treatment of chronic kidney disease in obesity and type 2 diabetes mellitus. Nat. Rev. Nephrol. 16, 709–720 (2020).
Acknowledgements
This manuscript received no external funding. R.M.B. acknowledges the support of an ARC DECRA Fellowship (DE190101244). P.S. acknowledges the support of a National Health and Medical Research Council Investigator Grant (1178482). We acknowledge the numerous researchers whose work we were unable to cite due to space limitations.
Author information
Authors and Affiliations
Contributions
P.S., R.M.B. and E.G.H. researched data for the article and wrote the manuscript. All authors substantially contributed to the discussion of content and reviewed and/or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
C.W.l.R. reports grants from Science Foundation Ireland and grants from Health Research Board during the conduct of the study; other from Novo Nordisk and GI Dynamics, personal fees from Eli Lilly, grants and personal fees from Johnson and Johnson, personal fees from Sanofi Aventis, Astra Zeneca, Janssen, Bristol-Myers Squibb and Boehringer-Ingelheim outside the submitted work. P.S. reports personal fees from Novo Nordisk outside the submitted work. The other authors declare no competing interests.
Additional information
Peer review information
Nature Reviews Endocrinology thanks G. Mingrone, who co-reviewed with L. Gissey, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Brown, R.M., Guerrero-Hreins, E., Brown, W.A. et al. Potential gut–brain mechanisms behind adverse mental health outcomes of bariatric surgery. Nat Rev Endocrinol 17, 549–559 (2021). https://doi.org/10.1038/s41574-021-00520-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41574-021-00520-2
This article is cited by
-
Microbiota–gut–brain axis and its therapeutic applications in neurodegenerative diseases
Signal Transduction and Targeted Therapy (2024)
-
Bariatric Surgery and New-Onset Substance Use Disorders: A Systematic review and Meta-analysis
Obesity Surgery (2024)
-
Long-term weight loss outcomes after bariatric surgery: a propensity score study among patients with psychiatric disorders
Surgical Endoscopy (2023)
-
Brazilian guide to nutrition in bariatric and metabolic surgery
Langenbeck's Archives of Surgery (2023)
-
The influence of the subcortex and brain stem on overeating: How advances in functional neuroimaging can be applied to expand neurobiological models to beyond the cortex
Reviews in Endocrine and Metabolic Disorders (2022)