Abstract
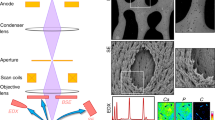
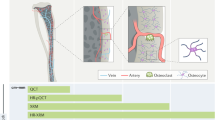
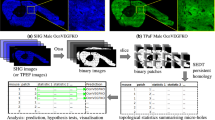
A thorough knowledge of the structures of healthy mineralized tissues, such as bone or cartilage, is key to understanding the pathological changes occurring during disease. Such knowledge enables the underlying mechanisms that are responsible for pathology to be pinpointed. One high-resolution 3D method in particular — focused ion beam-scanning electron microscopy (FIB-SEM) — has fundamentally changed our understanding of healthy vertebrate mineralized tissues. FIB-SEM can be used to study demineralized matrix, the hydrated components of tissue (including cells) using cryo-fixation and even untreated mineralized tissue. The latter requires minimal sample preparation, making it possible to study enough samples to carry out studies capable of detecting statistically significant differences — a pre-requisite for the study of pathological tissues. Here, we present an imaging and characterization strategy for tissue structures at different length scales, describe new insights obtained on healthy mineralized tissues using FIB-SEM, and suggest future research directions for both healthy and diseased mineralized tissues.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Martin, R. B. & Burr, D. B. Structure, Function and Adaptation of Compact Bone (Raven, 1989).
Georgiadis, M., Muller, R. & Schneider, P. Techniques to assess bone ultrastructure organization: orientation and arrangement of mineralized collagen fibrils. J. R. Soc. Interface 13, 20160088 (2017).
Reznikov, N., Shahar, R. & Weiner, S. Bone hierarchical structure in three dimensions. Acta Biomater. 10, 3815–3826 (2014).
Seeman, E. & Delmas, P. D. Bone quality - the material and structural basis of bone strength and fragility. N. Engl. J. Med. 354, 2250–2261 (2006).
O’Sullivan, L. M., Allison, H., Parle, E. E., Schiavi, J. & McNamara, L. M. Secondary alterations in bone mineralisation and trabecular thickening occur after long-term estrogen deficiency in ovariectomised rat tibiae, which do not coincide with initial rapid bone loss. Osteoporos. Int. 31, 587–599 (2020).
Blouin, S. et al. Cortical bone properties in the Brtl/+ mouse model of osteogenesis imperfecta as evidenced by acoustic transmission microscopy. J. Mech. Behav. Biomed. Mat. 90, 125–132 (2019).
Nijhuis, W. H. et al. Current concepts in osteogenesis imperfecta: bone structure, biomechanics and medical management. J. Child. Orthop. 13, 180190 (2019).
Zimmermann, E. A. et al. Modifications to nano- and microstructural quality and the effects on mechanical integrity in Paget’s disease of bone. J. Bone Miner. Res. 30, 264–273 (2015).
Kirsch, T. Determinants of pathological mineralization. Rheumatology 18, 174–180 (2006).
Mebarek, S. et al. Ankylosing spondylitis, late osteoarthritis, vascular calcification, chondrocalcinosis and pseudo gout: toward a possible drug therapy. Curr. Med. Chem. 18, 2196–2203 (2011).
Taylor, J. C. et al. Factors influencing success of clinical genome sequencing across a broad spectrum of disorders. Nat. Genet. 47, 717–726 (2015).
Heaney, R. P. Is the paradigm shifting? Bone 33, 457–465 (2003).
Bousson, V. et al. Volumetric quantitative computed tomography of the proximal femur: relationships linking geometric and densitometric variables to bone strength. Role for compact bone. Osteoporos. Int. 17, 855–864 (2006).
Ascenzi, M.-G., Zonca, A. & Keyak, J. H. Effect of cortical bone micro-structure in fragility fracture patients on lamellar stress. J. Biomech. 100, 109596 (2020).
Lekkala, S., Taylor, E. A., Hunt, H. B. & Donnelly, E. Effects of diabetes on bone material properties. Curr. Osteoporos. Rep. 17, 455–464 (2019).
Sharma, D. et al. The effects of estrogen deficiency on cortical bone microporosity and mineralization. Bone 110, 1–10 (2018).
Weiner, S. & Wagner, H. D. The material bone: structure- mechanical function relations. Ann. Rev. Mat. Sci. 28, 271–298 (1998).
Hodge, A. J. & Petruska, J. A. in Aspects of Protein Structure (ed. Ramachandran, G. N.) 289–300 (Academic, 1963).
Vidavsky, N. et al. Cryo-FIB-SEM serial milling and block face imaging: Large volume structural analysis of biological tissues preserved close to their native state. J. Struct. Biol. 184, 487–495 (2016).
Schertel, A. et al. Cryo FIB-SEM: volume imaging of cellular ultrastructure in native frozen specimens. J. Struct. Biol. 184, 355–360 (2013).
Stokes, D., Morrissey, F. & Lich, B. A new approach to studying biological and soft materials using focused ion beam scanning electron microscopy (FIB SEM). J. Phys. Conf. Ser. 26, 50 (2006).
Schneider, P., Meier, M., Wepf, R. & Müller, R. Towards quantitative 3D imaging of the osteocyte lacuno-canalicular network. Bone 47, 848–858 (2010).
Schneider, P., Meier, M., Wepf, R. & Müller, R. Serial FIB/SEM imaging for quantitative 3D assessment of the osteocyte lacuno-canalicular network. Bone 49, 304–311 (2011).
Reznikov, N., Almany-Magal, R., Shahar, R. & Weiner, S. Three-dimensional imaging of collagen fibril organization in rat circumferential lamellar bone using a dual beam electron microscope reveals ordered and disordered sub-lamellar structures. Bone 52, 676–683 (2013).
Kanazawa, T. et al. Histomorphometric and ultrastructural analysis of the tendon-bone interface after rotator cuff repair in a rat model. Sci. Rep. 6, 33800 (2016).
Hirashima, S. et al. Three-dimensional ultrastructural analysis of cells in the periodontal ligament using focused ion beam/scanning electron microscope tomography. Sci. Rep. 6, 39435 (2016).
Hashimoto, M. et al. Three-dimensional morphometry of collagen fibrils in membranous bone. Integr. Biol. 9, 868–875 (2017).
Raguin, E., Rechav, K., Brumfeld, V., Shahar, R. & Weiner, S. Unique three-dimensional structure of a fish mandible bone subjected to unusually high mechanical loads. J. Struct. Biol. 211, 107530 (2020).
Zou, Z. et al. Three-dimensional structural interrelations between cells, extracellular matrix and mineral in vertebrate mineralization. Proc. Natl Acad. Sci. USA 117, 14102–14109 (2020).
Gebhardt, W. Ueber funktionell wichtige Anordnungsweisen der eineren und groberen Bauelemente des Wirbeltierknochens.II. Spezieller Teil Der Bau der Haversschen Lamellensysteme und seine funktionelle Bedeutung. Arch. Entwickl. Mech. Org. 20, 187–322 (1905).
Giraud-Guille, M. M. Twisted plywood architecture of collagen fibrils in human compact bone osteons. Calcif. Tissue Int. 42, 167–180 (1988).
Weiner, S., Arad, T., Sabanay, I. & Traub, W. Rotated plywood structure of primary lamellar bone in the rat: orientations of the collagen fibril arrays. Bone 20, 271–298 (1997).
Currey, J. D. Bones: Structure and Mechanics (Princeton Univ. press, 2002).
Reznikov, N., Shahar, R. & Weiner, S. Three-dimensional structure of human lamellar bone: the presence of two different materials and new insights into the hierarchical organization. Bone 59, 93–104 (2014).
Faingold, A., Cohen, S. R., Reznikov, N. & Wagner, H. D. Osteonal lamellae elementary units: lamellar microstructure, curvature and mechanical properties. Acta Biomater. 9, 5956–5962 (2013).
Atkins, A. et al. The three-dimensional structure of anosteocytic lamellated bone of fish. Acta Biomater. 13, 311–323 (2015).
Silvent, J. et al. Zebrafish skeleton development: high resolution micro-CT imaging and FIB-SEM block surface serial imaging for phenotype identification. PLoS ONE 12, e0177731 (2017).
Maria, R. et al. An unusual disordered alveolar bone material in the upper furcation region of minipig mandibles: a 3D hierarchical structural study. J. Struct. Biol. 206, 128–137 (2019).
Grubich, J. R. Disparity between feeding performance and predicted muscle strength in the pharyngeal musculature of black drum, Pogonias cromis (Sciaenidae). Environ. Biol. Fishes 74, 261–272 (2005).
Shapiro, F. & Wu, J. Y. Woven bone overview: structural classification based on its integral role in developmental, repair and pathological bone formation throughout vertebrate groups. Eur. Cell Mater. 38, 137–167 (2019).
Su, X., Sun, K., Cui, F. Z. & Landis, W. J. Organization of apatite crystals in human woven bone. Bone 32, 150–162 (2003).
Benjamin, M. & Ralphs, J. R. Fibrocartilage in tendons and ligaments - an adaptation to compressive load. J. Anat. 193, 481–494 (1998).
Jing, Y. et al. Chondrogenesis and osteogenesis are one continuous developmental and lineage defined biological process. Sci. Rep. 7, 10020 (2017).
Haimov, H. et al. Mineralization pathways in the active murine epiphyseal growth plate. Bone 130, 115086 (2020).
Lowenstam, H. A. & Weiner, S. On Biomineralization (Oxford Univ. Press, 1989).
Hunziker, E. B. Mechanism of longitudinal bone growth and its regulation by growth plate chondrocytes. Microsc. Res. Tech. 28, 505–519 (1994).
Glenn, D. A. & Denburg, M. R. Bone health in glomerular kidney disease. Curr. Osteoporos. Rep. 17, 570–579 (2019).
Starup-Linde, J., Hygum, K., Harsløf, T. & Langdahl, B. Type 1 diabetes and bone fragility: links and risks. Diabetes Metab. Syndr. Obes. 12, 2539–2547 (2019).
Farlay, D. et al. Bone remodeling and bone matrix quality before and after menopause in healthy women. Bone 128, 115030 (2019).
Xi, L. et al. Bone matrix development in steroid-induced osteoporosis is associated with a consistently reduced fibrillar stiffness linked to altered bone mineral quality. Acta Biomater. 76, 295–307 (2018).
Buss, D. J., Reznikov, N. & McKee, M. D. Crossfibrillar mineral tessellation in normal and Hyp mouse bone as revealed by 3D FIB-SEM microscopy. J. Struct. Biol. 212, 107603 (2020).
Kivirikko, K. I. Collagens and their abnormalities in a wide spectrum of diseases. Ann. Med. 25, 113–126 (1993).
Byers, P. H. & Steiner, R. D. Osteogenesis imperfecta. Annu. Rev. Med. 43, 269–282 (1992).
Prockop, D. J. Mutations that alter the primary structure of type I collagen. The perils of a system for generating large structures by the principle of nucleated growth. J. Biol. Chem. 265, 15349–15352 (1990).
Romagnani, P. et al. Chronic kidney disease. Nat. Rev. Dis. Primers 23, 17088 (2017).
Evenepoel, P., Behets, G. J. S., Laurent, M. R. & D’Haese, P. C. Update on the role of bone biopsy in the management of patients with CKD–MBD. J. Nephrol. 30, 645–652 (2017).
Leonard, M. B. et al. A multi-imaging modality study of bone density, bone structure and the muscle - bone unit in end-stage renal disease. Bone 127, 271–279 (2019).
Choksi, P., Jepsen, K. J. & Clines, G. A. The challenges of diagnosing osteoporosis and the limitations of currently available tools. Clin. Diabetes Endocrinol 4, 12 (2018).
Mussawy, H. et al. Changes in cortical microarchitecture are independent of areal bone mineral density in patients with fragility fractures. Injury 48, 12461–2465 (2017).
Kim, G. J., Yoo, H. S., Lee, K. J., Choi, J. W. & Hee An, J. Image of the micro-computed tomography and atomic-force microscopy of bone in osteoporosis animal model. J. Nanosci. Nanotechnol. 18, 6726–6731 (2018).
Brasileiro, C. B. et al. Use of cone beam computed tomography in identifying postmenopausal women with osteoporosis. Arch. Osteoporos. 12, 26 (2017).
Anderson, P. A., Polly, D. W., Binkley, N. C. & Pickhardt, P. J. Clinical use of opportunistic computed tomography screening for osteoporosis. J. Bone Joint Surg. Am. 100, 2073–2081 (2018).
Palermo, A. et al. BMI and BMD: the potential interplay between obesity and bone fragility. Int. J. Environ. Res. Public Health 13, 544 (2016).
Azetsu, Y. et al. Treatment with synthetic glucocorticoid impairs bone metabolism, as revealed by in vivo imaging of osteoblasts and osteoclasts in medaka fish. Biomed. Pharmacother. 118, 109101 (2019).
Burke, M. et al. The impact of metastasis on the mineral phase of vertebral bone tissue. J. Mech. Behav. Biomed. Mat. 69, 75–84 (2017).
Bailey, S., Hackney, D., Vashishth, D. & Alkalay, R. N. The effects of metastatic lesion on the structural determinants of bone: Current clinical and experimental approaches. Bone 138, 115159 (2020).
Shah, F. A., Thomsen, P. & Palmquist, A. Osseointegration and current interpretations of the bone-implant interface. Acta Biomater. 84, 1–15 (2019).
Reznikov, N., Buss, D. J., Provencher, B., McKee, M. & Piché, N. Deep learning and 3D imaging in structural biology. J. Struct. Biol. 212, 107598 (2020).
Bouxsein, M. L. et al. Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. J. Bone Miner. Res. 25, 1468–1486 (2010).
Riggs, C. M., Vaughan, L. C., Evans, G. P., Lanyon, L. E. & Boyde, A. Mechanical inplications of collagen fibre orientation in cortical bone of the equine radius. Anat. Embryol. 187, 239–248 (1993).
Spehner, D. et al. Cryo-FIB-SEM as a promising tool for localizing proteins in 3D. J. Struct. Biol. 211, 107528 (2020).
Morris, M. D. & Mandair, G. S. Raman assessment of bone quality. Clin. Orthop. Relat. Res. 469, 2160–2169 (2011).
Akiva, A. et al. Intercellular pathways from the vasculature to the forming bone in the zebrafish larval caudal fin: possible role in bone formation. J. Struct. Biol. 206, 139–148 (2019).
Naveh, G., Brumfeld, V., Dean, M., Shahar, R. & Weiner, S. Direct MicroCT imaging of non-mineralized connective tissues at high resolution. Connect. Tissue Res. 55, 52–60 (2014).
Blouin, S. et al. Confocal laser scanning microscopy — a powerful tool in bone research. Wien. Med. Wochenschr. 168, 314–321 (2018).
Reid, S. A. & Boyde, A. Changes in the mineral density distribution in human bone with age: image analysis using backscattered electrons in the SEM. J. Bone Miner. Res. 2, 13–22 (1987).
Loveridge, N., Power, J., Reeve, J. & Boyde, A. Bone mineralization density and femoral neck fragility. Bone 35, 929–941 (2004).
Bennet, M. et al. Simultaneous Raman microspectroscopy and fluorescence imaging of bone mineralization in living zebrafish larvae. Biophys. J. 106, L17–L19 (2014).
Nyman, J. S. et al. Measuring differences in compositional properties of bone tissue by confocal Raman spectroscopy. Calcif. Tissue Int. 89, 111–122 (2011).
Kourkoumelis, N., Zhang, X., Lin, Z. & Wang, J. Fourier transform infrared spectroscopy of bone tissue: bone quality assessment in preclinical and clinical applications of osteoporosis and fragility fracture. Clin. Rev. Bone Miner. Metab. 17, 24–39 (2019).
Wittig, N. K. et al. Bone biomineral properties vary across human osteonal bone. ACS Nano 13, 12949–12956 (2019).
Acknowledgements
S.W. acknowledges the support of the Israel Science Foundation (grant No. 875/15). R.S. acknowledges the support of the Israel Science Foundation (grant No. 700/16) and the support of the Deutsche Forschungsgemeinschaft (grant No. ZA 557/5-1). The Menarini Foundation (Fondazione Internazionale Menarini) supported the Symposium Biomineralization in Health and Disease in Florence, Italy, which served as impetus and inspiration for this article.
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information
Nature Reviews Endocrinology thanks P. Thurner and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Weiner, S., Raguin, E. & Shahar, R. High resolution 3D structures of mineralized tissues in health and disease. Nat Rev Endocrinol 17, 307–316 (2021). https://doi.org/10.1038/s41574-021-00479-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41574-021-00479-0