Abstract
An increasing number of studies are describing potential uses of circulating tumour DNA (ctDNA) in the care of patients with colorectal cancer. Owing to this rapidly developing area of research, the Colon and Rectal–Anal Task Forces of the United States National Cancer Institute convened a panel of multidisciplinary experts to summarize current data on the utility of ctDNA in the management of colorectal cancer and to provide guidance in promoting the efficient development and integration of this technology into clinical care. The panel focused on four key areas in which ctDNA has the potential to change clinical practice, including the detection of minimal residual disease, the management of patients with rectal cancer, monitoring responses to therapy, and tracking clonal dynamics in response to targeted therapies and other systemic treatments. The panel also provides general guidelines with relevance for ctDNA-related research efforts, irrespective of indication.
Similar content being viewed by others
Introduction
Circulating tumour DNA (ctDNA) typically constitutes a small proportion of an individual’s total circulating free DNA (cfDNA); <1% according to some studies1,2,3. However, with improving assay techniques providing greater levels of sensitivity, the analysis of ctDNA is rapidly being accepted as a reliable tool in oncology. In contrast to the analysis of tumour biopsy samples, which are not only invasive to obtain but often also do not fully capture tumour heterogeneity and evolution, the analysis of ctDNA offers a non-invasive method of repeatedly evaluating the genomic profile of a patient’s cancer. Although ctDNA is typically thought to represent DNA isolated from blood, multiple other body fluids, such as cerebrospinal fluid, saliva, pleural effusions, urine and stool samples, can now all be used as sources of tumour DNA4,5,6,7,8. The proportion of patients with colorectal cancer (CRC) in whom ctDNA can be detected depends on the extent of tumour volume and ranges from 50% in those with non-metastatic disease to nearly 90% in patients with metastatic disease9. In those who have undergone curative resection, earlier studies suggest that the postoperative detection of ctDNA ranges from 10–15% of patients with stage II disease to nearly 50% in those with stage IV disease9,10,11,12,13,14,15,16. Building on these studies, data on the potential uses of ctDNA are rapidly accumulating in the continuum of care across multiple cancers, including CRC (Fig. 1). However, most of these research efforts have been heterogeneous and involved disparate settings, assays and end points. Given the rapid pace of developments in this field and the many proposed uses of ctDNA in patients with CRC, an urgent need exists to identify the key opportunities and provide guidance towards accelerating the integration of ctDNA into the routine care of patients with CRC.
Circulating tumour DNA (ctDNA) provides a more sensitive method of detecting malignancies than imaging or other conventional approaches. This sensitivity can be exploited in several ways: early diagnosis of colorectal cancer prior to the emergence of clinical or radiological manifestations and in the detection of minimal residual disease (MRD), defined as the detection of ctDNA with no other clinical evidence of disease recurrence in patients who have completed all potentially curative therapies. In patients with radiographically evident disease, ctDNA also seems to be more sensitive to changes in tumour burden and might assist in tailoring the intensity of therapy in the neoadjuvant setting and in monitoring for tumour response in patients requiring palliative treatment. Furthermore, qualitative assessments of the types of aberrations and their subsequent alterations might assist in assessments of tumour evolution and heterogeneity that lead to the emergence of resistance as well as in selection of the most appropriate therapies.
Acknowledging this heterogeneity in research efforts, the Colon and Rectal–Anal Task Forces of the United States National Cancer Institute (NCI) convened this panel with the tasks of summarizing the current state of the field of ctDNA research in CRC, identifying the opportunities and defining the goals that could be best addressed using ctDNA, and providing guidance towards achieving these goals, considering issues such as feasibility, the challenges that are most likely to be encountered and the potential for clinical trials. As discussed earlier, the panel focused on four key clinical areas in CRC care in which ctDNA could have the greatest potential.
Methods
A workshop was conducted under the auspices of the Colon and Rectal–Anal Task forces of the US NCI on 3 June 2018 in Chicago, IL, USA, at the ASCO Annual Meeting (co-Chairs A.D., V.M. and S.K.). Members of the Task Forces, along with selected invitees, collectively provided expertise in next-generation sequencing (NGS), ctDNA methodology and other aspects of assay development (S.R.H., M.D.), bioinformatics (M.D.), biostatistics and technical data management (Q.S., G.Y., F.S.O.) as well as in various subdisciplines of clinical care and clinical and translational research, including genomic medicine (S.K., R.B.C.) in CRC and patient advocacy. Additional invitees included select members of the NCI, the NCI’s Cancer Therapy Evaluation Program and the Gastrointestinal Steering Committee. The attendees were split into four Working Groups, each with 2–3 leaders (who prepared literature reviews in advance of the conference): ‘Detection of Minimal Residual Disease’ (M.D., V.M.), ‘Management of Rectal Cancer’ (A.D., Y.N.Y., T.H.), ‘Monitoring Response to Therapy’ (J.S., K.R., R.Y.) and ‘Tracking Clonal Dynamics’ (S.K., R.C., C.M.). The recommendations developed during the thematic Working Groups were then discussed and voted upon individually by all members towards the development of a consensus to recognize the limitations of current knowledge and identify key gaps and to develop recommendations for future research (Box 1; Table 1). A summary of the recommendations in each area is presented in Boxes 2–6. The recommendations of each Working Group were compiled by the group leaders and reviewed by members of that group. A draft of the entire manuscript was subsequently prepared. The draft was circulated amongst all the authors and revised based on their input; an electronic voting system was used to record consensus prior to manuscript submission. The final draft of the manuscript was also reviewed by members of the Cancer Therapy Evaluation Program, whose helpful comments were then incorporated. Of note, the role of ctDNA as a tool for early detection of CRC was not part of the Workshop agenda and is not covered in this manuscript but has been comprehensively reviewed elsewhere17,18.
Technical considerations
Several factors can influence the analytical and clinical validity as well as the clinical utility of ctDNA-based assays. These factors include preanalytical variables, assay characteristics and the bioinformatic analysis of the data provided.
Pre-analytical variables
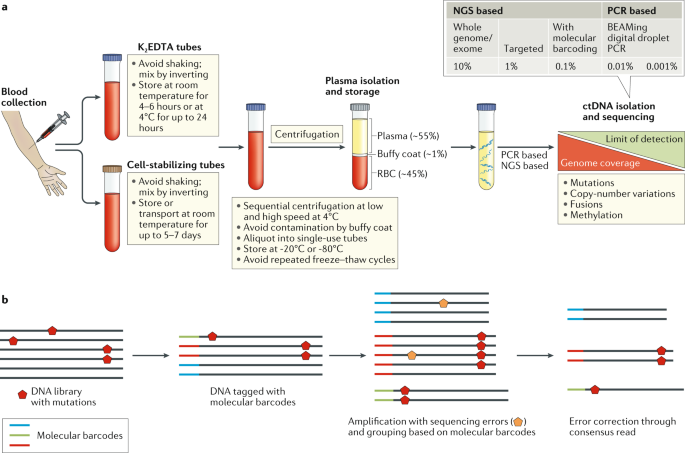
The major challenges at the pre-analytical stage include the typically small proportion of ctDNA relative to total cfDNA, the potential for contamination of samples by DNA released during immune-cell lysis and the labile nature of DNA (and consequent implications for storage and transport)19. Blood samples should be drawn using a large gauge diameter needle (≤21 G) according to established guidelines, and analysis should be performed on ctDNA obtained from plasma rather than on serum as the latter contains greater quantities of DNA released from immune cells during the clotting process20,21,22,23. Blood should be drawn into K2EDTA or cell-stabilizing tubes (such as Streck cfDNA collection tubes). Plasma isolation should be done as soon as possible and no later than 24 hours with K2EDTA (preferably within 4–6 hours) or within 2–7 days if using cell-stabilizing tubes, with interim storage at 4°C for the former and at 10–30°C for the latter20,21,22,23,24,25,26. The volume of blood drawn should be optimized according to the clinical setting (for example, higher volumes of plasma (up to 60 ml) might be required for the detection of minimal residual disease (MRD) compared with attempts to evaluate therapy responses in the metastatic setting, in which 5–10 ml is usually sufficient) and the analytical method used. Blood should be processed for the isolation of plasma using sequential centrifugation at progressively increasing speeds (800–1,600 g) at 4°C or through filtration followed by immediate deep freezing (typically at –80°C)20,21,22. Immune cells obtained from the buffy coat layer can be used as a source of DNA for assessment of mutations as originating from the germ line and/or clonal haematopoiesis of indeterminate potential (CHIP)27,28. Although rapid freezing of plasma samples does not affect subsequent DNA yield, unspun blood must not be frozen (to avoid immune-cell lysis) nor should plasma samples be exposed to multiple freeze–thaw cycles20,21,22,23,24,25,26. The latter issue can easily be avoided by aliquoting plasma into single-use tubes of an appropriate volume. Several methods of DNA extraction and purification from plasma are currently available through ready-to-use commercial kits. However, the DNA purification method needs to be tailored depending on the upstream pre-analytical and downstream analysis methods used20,29 (Fig. 2).
a | Circulating cell-free DNA, of which circulating tumour DNA (ctDNA) is a part of, is isolated from plasma samples after serial centrifugation of blood collected in either K2EDTA or cell-stabilizing tubes. ctDNA is subsequently isolated from cell-free DNA using library preparations and analysed for the presence of various genetic aberrations, including mutations, copy-number variations, fusions and/or other alterations such as changes in DNA methylation. b | Molecular barcoding: prior to PCR and sequencing, each DNA fragment can be labelled with unique DNA barcodes; subsequently, reads that share the same barcode (typically in thousands) can be grouped together because they all originate from the same ctDNA fragment. This approach enables sequencing errors (orange pentagon, seen in the minority) to be distinguished from true mutations (red pentagon, seen in the majority). Molecular barcoding also helps correct potential biases in amplification and thus assists in the precise quantification of mutations or amplification frequencies. NGS, next-generation sequencing; RBC, red blood cells.
Assay characteristics
An easy (or perhaps simplistic) method of classifying ctDNA assay techniques would be based on whether they involve PCR or NGS. PCR-based techniques (such as Droplet Digital PCR or beads, emulsion, amplification, magnetics (BEAMing) techniques) rely on the detection of specific known mutations using primers that are complementary to the mutant sequences. This technique offers high levels of sensitivity (variant allele frequency (VAF) for detection of ≤0.01%), although it is also limited to the detection of either a single or a small number of known mutations27. NGS-based techniques theoretically enable sequencing of the entire genome and provide an improved breadth of coverage, although they are typically limited to a panel of genes or hotspots within up to several hundred genes.
Either of the techniques discussed above can be tumour informed, implying that the identification of genomic aberrations in ctDNA can be tailored based on sequencing of the tumour tissue in order to improve sensitivity and reduce the risk of false-positive results related to sequencing errors and also owing to non-tumour-related mutations such as those arising from CHIP. However, this additional level of sensitivity might lead to further expense and delays related to sequencing of the tumour tissue; this latter issue is of particular importance when considering assays for use in the adjuvant setting, in which timely initiation of therapy is crucial30. Furthermore, sequencing of an initial biopsy sample might not necessarily capture the complexities of subsequent treatment-emergent mutations. Sequencing errors might be difficult to distinguish from mutations, especially those of a low VAF (<0.01%). This issue might be addressable using molecular barcoding of the initial DNA molecules with 10–12 random bases, such that the barcode is amplified and sequenced together with the DNA — this would enable the correction of sequencing errors by building a consensus read from all the reads bearing the same barcode20,23,27 (Fig. 2).
Stringent quality control must be maintained at all steps of the pre-analytical and analytical processes, the former being the most common source of errors, accounting for up to two-thirds of all errors made in clinical laboratory medicine20,31. Pre-analytical factors, such as the methodology and types of tubes used for blood draws, plasma isolation, transport and storage must all be carefully documented. Extracted DNA must, at the very least, be evaluated for DNA concentration (typically using either spectrometry, fluorometry or quantitative PCR), for contamination with genomic DNA from lysed immune cells (using electrophoresis or PCR involving amplicons of different lengths building on the longer lengths of cfDNA) and for DNA integrity (by assessing for the level of fragmentation) in order to ensure optimal subsequent sequencing performance20.
Bioinformatics analysis
This step focuses mainly on variant detection and calling, with a focus on differentiating mutations from background sequencing and/or PCR errors. As discussed above, molecular barcoding addresses this issue to a great extent; care should be taken, however, to ensure that the bioinformatics pipeline is adapted to process molecular tags. Variants might also be evaluated against a background error rate (computed from a reference set or from the sample being analysed). The error rate can be averaged over the entire sequence or calculated per base in addition to the ability to account for the different types of aberrations (such as base substitutions, transitions, transversions, insertions and deletions). The bioinformatics pipeline might also be informed by data from germline and/or tumour sequencing. The eventual bioinformatics pipeline chosen should be dependent upon the characteristics of the library, sequencing methods and proposed clinical application32.
Establishing validity and utility
Obstacles to establishing analytical validity include inter-assay variability in the level of genomic coverage (in terms of both depth and breadth), assay methodology and a lack of an established standard for comparisons. These factors are discussed previously, in addition to inconsistent pre-analytical variables. Although the detection of genomic variants in tumour tissue has often been used to establish the analytical validity of ctDNA-based assays, temporal, inter-tumoural and intra-tumoural heterogeneity limit this approach in several settings. An alternate approach would be to establish samples of reference DNA with prespecified dilutions in an appropriate medium to evaluate ctDNA assays. Establishing guidelines for the desired level of assay coverage and/or performance for each clinical setting, in addition to conducting studies with rigorous cross-assay comparisons by independent groups to enable assays to be used interchangeably, is also imperative.
The methodology for establishing clinical validity and, more importantly, utility is dependent on the clinical scenario. In the detection of MRD, because the outcomes are binary (ctDNA detection and disease recurrence), clinical validity will likely be demonstrated by data from prospective interventional and observational studies correlating ctDNA detection with disease-free survival (DFS), which is a well-established surrogate for overall survival (OS). Equally crucially, these studies will also interrogate the clinical validity of ctDNA clearance in patients receiving adjuvant therapy, which is a more immediate end point, as a surrogate for DFS. Clinical utility for MRD will therefore likely be in the form of de-escalation of therapy and surveillance in patients with ctDNA-negative disease and the converse in those with ctDNA-positive disease; this approach is being explored in ongoing interventional studies. In the metastatic setting, for patients receiving targeted agents based on the presence of established predictive biomarkers (such as RAS, BRAFV600E, HER2 and/or microsatellite instability (MSI)), determining the extent to which ctDNA variations correlate with tissue findings might suffice in establishing clinical validity and utility. However, establishing the clinical validity and utility of dynamic changes in ctDNA levels as predictors of response to therapy is much more difficult. Current data largely originate from small cohort studies using either VAF or cfDNA levels to reflect tumour response. However, as reported in patients with non-small-cell lung cancer in 2019, such studies are affected by substantial intra-patient variations in levels of cfDNA even after accounting for pre-analytical and/or inter-assay variables33. These variations likely reflect a multitude of patient-related biological and demographic factors. Studies might take advantage of the short half-life of cfDNA that enables controlling for certain transient variables, such as fasting, physical exercise, surgery or exposure to radiation, by allowing adequate time after exposure to such factors before blood sampling. Other intransient variables, such as comorbidities or demographic factors, must be carefully documented and adjusted for when interpreting results. Furthermore, no data or consensus are available regarding the most clinically relevant thresholds and cut-offs for the categorization of continuous data (ctDNA levels). The most appropriate method of quantifying ctDNA and whether a multidimensional approach to integrating data on other features, such as fragment size, methylation or other circulating elements, might provide useful additional information will likely evolve with continued research and practice and will likely be unique to each clinical scenario (Box 2).
Management of MRD
The presence of ctDNA-defined MRD, likely reflecting the existence of micrometastases following definitive surgical resection, might serve as a harbinger for persistent and/or recurrent malignancy well before becoming clinically evident. For example, among 231 patients with resected stage II colon cancer, the presence of ctDNA in postoperative plasma samples was strongly associated with recurrence in those who did not receive adjuvant chemotherapy (14/178 patients had detectable ctDNA after surgery, of whom 11 (79%) had disease recurrence at a median follow-up duration of 27 months (HR 18, 95% CI 7.9–40; P < 0.001)) as well as in those who received adjuvant chemotherapy (3/44 patients had detectable ctDNA on completion of adjuvant chemotherapy, all of whom had disease recurrence within 11 months of completion of chemotherapy (HR 11, 95% CI 1.8–68; P = 0.001)), regardless of the ‘low-risk’ or ‘high-risk’ stratification based on clinical features12,34. Similar prognostic implications of MRD have also been reported from multiple other studies involving patients across all stages of CRC12,35,36,37,38,39.
Despite these overwhelming prognostic implications, whether or not ctDNA can be cleared by adjuvant chemotherapy remains uncertain. In a cohort of patients with resected stage III colon cancer, ctDNA became undetectable in 9 out of 18 patients after completion of adjuvant chemotherapy and was associated with improved relapse-free survival relative to those who retained detectable ctDNA (HR 5.1; P = 0.02)13,37. Although limited by small numbers, these data suggest that patients with detectable ctDNA after surgery could benefit from a duration of adjuvant chemotherapy corresponding to ctDNA clearance. The current paradigm tested in most trials in the adjuvant setting is to treat all patients with adjuvant therapy with the primary end point of DFS. However, this approach requires the follow-up monitoring of large numbers of patients over long periods of time in order to provide definitive results. By contrast, conducting these trials in patients who have detectable ctDNA at enrolment with the primary end point of ctDNA clearance would be vastly more efficient by reducing both the number of patients needed to treat and the duration of follow-up monitoring. However, the establishment of ctDNA loss as a valid surrogate end point requires additional, larger-scale observational data evaluating the kinetics of ctDNA during adjuvant therapy. The Working Group provides guidelines recommending the timepoints for standardized sample collection for future protocols (Table 1). We also emphasize the need to establish high-level consensus regarding methods, clinical data collection and data sharing towards enabling pooled analyses of data from several studies in the future. Efforts to improve the sensitivity of ctDNA assays and thereby capture all patients with MRD should continue. Factors affecting the sensitivity of ctDNA assays include the analytical limit of detection, sampling volume, number of input molecules and tumour burden. A typical 10 ml volume of blood yields an average of 4 ml of plasma containing approximately 12 × 103 DNA molecules (6,000 diploid genomes), which theoretically provides a sensitivity limit of 0.01% (1 in 12,000 copies). In terms of MRD detection, in which both the tumour burden and number of input molecules is low, comprehensive NGS panels that enable testing for a large number of genomic and epigenetic alternations might improve assay sensitivity. As discussed elsewhere30, the use of NGS panels might also avoid additional expenses and delays related to sequencing of the tumour tissue, the latter issue being of particular importance in the adjuvant setting, in which timely initiation of therapy is crucial. Current consensus guidelines recommend the initiation of adjuvant therapy as soon as patients are medically able to receive it, ideally no later than 6–8 weeks after surgery. The stochastic distribution of ctDNA molecules in patients with MRD might also be mitigated to a certain extent by increasing the sample volume. This volume (and thus the number of input molecules) can be increased either by drawing a larger volume of blood at a single time point (but might be constrained by the need to draw blood for other purposes) or through serial monitoring; the latter approach is also aided by increasing ctDNA levels reflecting similar changes in tumour volume. These factors might reduce the risk of false-negative results by optimizing the detection of ctDNA that has been shed into the plasma, although they might not account for false negatives related to biological variables. For example, patients with metastatic spread to the peritoneum and brain might not have detectable ctDNA in blood; similarly, a small but clinically relevant proportion of patients with intact primary tumours might have undetectable ctDNA. The underlying reasons for this are unknown but could be related to mechanisms of ctDNA production and release that remain to be elucidated40. Until these factors have been delineated, investigators running clinical trials involving adjuvant therapy with ctDNA-based monitoring for MRD should consider pre-screening patients for ctDNA prior to surgical resection of the primary tumour, with patients who have undetectable ctDNA being excluded from further participation.
Data from ongoing observational trials with large cohorts, such as TRACC (n = 1,000; NCT04050345) and ADNCirc (n = 473; NCT02813928), will help to establish much needed reference benchmarks for ctDNA as a marker for MRD and, collectively with other data, might help to establish ctDNA clearance as a surrogate marker for survival. In other trials, such as IMPROVE-IT2 (n = 254; NCT04084249), investigators are attempting to define the optimal combination of ctDNA and imaging assessments for the detection of disease recurrence, which will help in establishing evidence-based management guidelines.
Ongoing therapeutic studies involving patients with stage II colon cancer, such as the NCI–sponsored randomized phase II/III COBRA study (n = 1,408; NCT0406810), the CIRCULATE trial (n = 1,980; NCT04120701) and the DYNAMIC-II study (n = 450; ACTRN12615000381583), are testing the hypothesis that ctDNA will enable the identification of patients with a high risk of disease recurrence (despite having low-risk stage II colon cancer by clinical criteria) who might benefit from adjuvant chemotherapy. The phase II/III DYNAMIC-III study (n = 1,000; ACTRN12617001566325) is currently recruiting patients with stage III colon cancer to evaluate the clinical utility of chemotherapy de-escalation or escalation as informed by ctDNA status. Other de-escalation trials are currently being designed by multiple international groups. Together, these trials will provide data on the clinical utility of ctDNA in monitoring MRD.
In future adjuvant studies, ctDNA could also be used to select patients with a high risk of disease recurrence who could then be enrolled in trials evaluating rational combinations, such as the addition of irinotecan to folinic acid, 5-fluorouracil and oxaliplatin (FOLFOX), or even promising immunotherapeutics based on the premise that the latter therapies might be most effective when the tumour burden is the lowest such as in the MRD state. Such a trial design would require a ctDNA-based assay with a high level of specificity, even at the expense of lower sensitivity, in order to minimize the risks of added toxicities associated with novel therapies (Fig. 2). This technology can also be used in clinical trials of novel agents informed by the specific mutation detected. For example, given the promising phase III data on the efficacy of targeted combination therapies for patients with BRAFV600E-mutant metastatic CRC41,42, the identification of patients with MRD harbouring this mutation introduces an opportunity to extend the testing and treatment of these patients to the adjuvant setting43.
In the MRD setting, patients with persistent ctDNA that is not cleared by initial adjuvant chemotherapy might have an aggressive underlying tumour biology. Whether second-line systemic treatment improves recurrence-free survival and/or OS in these patients could be elucidated through a randomized trial with observation as the control.
In the setting of metastatic CRC, early changes in ctDNA VAF are correlated with radiographic responses to treatment44. Similarly, ctDNA clearance could plausibly be used to identify patients who might be eligible for de-escalation of adjuvant chemotherapy. On the basis of data from the IDEA trial, the majority of patients with stage III colon cancer are deemed to have low-risk disease and might require only 3 months of adjuvant chemotherapy; therefore, an attractive approach to further de-escalation could be to skip adjuvant therapy altogether45. This possibility is supported by historical data suggesting that nearly half of all patients with stage III colon cancer are cured by surgery alone46. Trials designed to evaluate this hypothesis would require an assay with a high level of sensitivity (suggested >95%) for the prediction of disease recurrence, with the allowance of a lower level of specificity (Fig. 3). High sensitivity is a key requirement of the assays used in these trials in order to avoid the undertreatment of patients who have false-negative ctDNA levels owing to assay-related technical factors but have the highest risk of disease recurrence and might benefit from adjuvant therapy. Studies conducted over the past few years using novel assays and serial sampling suggest sensitivity rates approaching 90% for the detection of disease recurrence in certain settings, and these might continue to improve with continued advances in the field37. The sensitivity of blood-based ctDNA assays will also likely hit a ceiling (owing to the inability to detect ctDNA in non-shedders or from certain metastatic sites such as tumours of the peritoneum or nervous system), beyond which further improvements in assay performance would not be possible43. ctDNA is purely prognostic and is not predictive of benefit from adjuvant therapy; therefore, the goal of research in the MRD setting would be to personalize the duration of treatment based on dynamic changes in ctDNA and not on initial TNM staging, which is the current clinical practice (Fig. 3). This goal is reflective of the availability of robust data suggesting that ctDNA outperforms TNM staging and other clinical factors in the stratification of patients with stage II–III CRC by risk of recurrence12,37.
Owing to the high specificity of circulating tumour DNA (ctDNA) for the prediction of disease recurrence, patients with detectable ctDNA might be considered candidates for the escalation of adjuvant therapy over the standard-of-care approach in order to reduce the risk of disease recurrence. Conversely, patients who lack detectable ctDNA, as determined using a sufficiently sensitive assay, and who have a low risk of disease recurrence might benefit from de-escalation to less-intense adjuvant therapies that reduce the risk of toxicities.
CHIP refers to aberrations arising in the DNA of haematopoietic stem cells that can be detected once DNA fragments from these non-malignant cells are released into the circulation47,48. The prevalence of CHIP mutations increases during the ageing process49,50, and CHIP mutations have been reported at low levels in as many as 95% of patients aged 50–60 years who do not have cancer, typically at a VAF <0.1%51. Initial studies focused on sequencing assays demonstrate that CHIP affects mutations in genes that are known to be implicated in haematological malignancies, such as TET2, DNMT3, JAK2 and ASXL1, without much relevance to solid tumours such as CRC. Subsequent unbiased sequencing studies have also revealed mutations in other genes, such as TP53 and KRAS, that might contribute to false-positive results in patients with CRC, especially when testing for MRD52,53,54. These CHIP-related aberrations can be filtered out using novel pipelines developed based on bioinformatics advances that have improved the sensitivity for calling cancer-associated mutations present in ctDNA. Another alternative would be to match ctDNA sequencing with that of leukocytes and/or matched tumour tissues to provide confirmation of such findings. Future studies should focus on the best approach to minimizing false positives related to CHIP with an emphasis on costs and efficiency, keeping in mind the critical time constraints for use of adjuvant therapy in MRD trials.
The rapid turnaround of ctDNA results is crucial given the need for clinical decision-making in the context of the curative potential of these patients. In situations in which tissue sequencing results required for the design of tumour-informed ctDNA assays are delayed, we favour NGS-based multigene assays that can be implemented without prior knowledge of specific genomic aberrations; such assays also have the theoretical advantages of being able to capture subclonal populations that expand over time under the selective pressures of systemic treatment (Box 3).
Management of rectal cancer
Several areas of controversy currently exist relating to the optimal risk stratification and management approach for patients with locally advanced rectal cancer (LARC). The consensus view of the Rectal Working Group is that ctDNA could help address many of these key controversies. Despite this potential, very few studies involving patients with LARC have thus far focused on ctDNA. Data from a study involving 123 patients with LARC show that the total cfDNA at diagnosis is modestly prognostic: patients with cfDNA levels above the 75th percentile had worse DFS and a higher risk of disease recurrence than those below it (HR 2.48, 95% CI 1.3–4.8; P = 0.007)55. Data from another study involving 159 patients with LARC show that ctDNA is detectable in 77%, 8% and 12% of pre-treatment, post-chemoradiotherapy and postoperative plasma samples and that the presence of postoperative ctDNA is a very strong predictive marker for disease recurrence irrespective of the use of adjuvant chemotherapy (chemotherapy: HR 10, 95% CI 3.4–29; P < 0.001; without chemotherapy: HR 22, 95% CI 4.2–110; P < 0.001)56. Furthermore, the findings of a study involving 47 patients with LARC suggest that recurrence-free survival is shorter in patients with detectable ctDNA after completion of chemoradiotherapy (HR 7.1, 95% CI 2.4–21.5; P < 0.001)57.
Together, these data suggest that total cfDNA at the time of diagnosis might have prognostic implications, while binary classifications based on the detection of ctDNA might not (in contrast to the situation at later time points, such as after chemoradiotherapy, curative surgery and/or completion of adjuvant therapy). In fact, a systematic review including data from nine studies and a total of 615 patients suggests a correlation between cfDNA level and clinical outcomes of response to neoadjuvant therapy, including DFS58. However, in another, larger systematic review of data from 25 studies, the investigators concluded that data are as yet inconclusive regarding the utility of pretreatment liquid biopsy or serial (pre-treatment and post-treatment) monitoring, but that the presence of MRD is consistently associated with worse outcomes across studies59. The next steps would be to consider validating the clinical utility of cfDNA and, importantly, to look at additional quantitative assessments (such as VAF) and/or qualitative assessments (such as mutations) as a means of improving the prognostic ability of liquid biopsies at the time of diagnosis. Without such advances, the role of cfDNA at this time point prior to a mandatory intervention might be limited. Conversely, the prognostic implications of detectable ctDNA in patients with earlier-stage II and stage I rectal cancer currently not considered for neoadjuvant therapy are unknown. Whether ctDNA can assist with identifying the minority of these patients with aggressive disease biology and micrometastatic disease within a timeframe that enables them to receive earlier and/or more aggressive interventions is an essential question that should be answered.
The current trimodality approach for patients with LARC involves surgery, chemotherapy and radiotherapy, results in substantial morbidities and might not be required for some patients, yet also fails to prevent disease recurrence in others. The key limitation in the current management of rectal cancer is the lack of reliable and accurate methods of predicting responsiveness to neoadjuvant therapies without surgical resection and subsequent pathological assessments. For example, among patients who demonstrate adequate clinical responses to induction systemic chemotherapy, omitting radiotherapy and its associated toxicities might be possible; this hypothesis is currently being tested in the phase II/III PROSPECT trial (NCT01515787) and in the OPRA trial (NCT02008656) in patients with a complete clinical response to neoadjuvant therapy, in whom standard proctectomy might be avoided (the ‘wait and watch’ approach)60. We hypothesize that ctDNA or changes in ctDNA could provide added value in predicting responsiveness to neoadjuvant therapy in lieu of the current standard pathological assessment criteria — this represents a major research need. For example, a tool designed to increase the level of concordance between clinical and pathological complete response is desperately needed in order to enable the ‘watch and wait’ approach to be adopted as mainstream clinical practice.
The role of adjuvant systemic therapy in patients with rectal cancer receiving neoadjuvant chemoradiotherapy and surgery has not yet been examined rigorously in randomized trials and is largely extrapolated from the experience in those with colon cancer. Whether ctDNA status following surgery can help tailor the need for and the intensity of adjuvant therapy needs to be assessed. Finally, ctDNA-defined MRD might have a crucial role in patients undergoing surveillance, similar to the situation in patients with colon cancer. Thus, ctDNA-based analyses have the potential to radically change our approach to the management of patients with rectal cancer. However, as already highlighted, conclusive data are currently lacking. Thus, we propose guidelines towards the rapid and efficient accumulation of data by obtaining samples across trials at standardized and defined time points in order to overcome these key knowledge gaps (Table 1). Furthermore, given that non-metastatic rectal cancer is typically associated with a relatively lower tumour burden than metastatic CRC and that it might diminish even further with the use of neoadjuvant therapy, selecting a ctDNA platform with very high sensitivity (>95%) while preserving specificity (>99%) is imperative. Similar to the MRD setting, the rapid turnaround of ctDNA results is also vital in the management of patients with rectal cancer given the potential for cure and also the need to evaluate disease status at multiple time points in order to facilitate real-time clinical decisions (Box 4).
Monitoring metastatic disease
Advances in the sensitivity and accuracy of ctDNA-based analyses have enabled the tracking of tumour dynamics in real time61. Such ctDNA-based monitoring for the efficacy of therapies is particularly well suited for the metastatic disease setting: several issues, including assay sensitivity, the status of driver versus passenger alterations, and tissue–ctDNA discordance, all of which restrict the use of ctDNA in other settings, are of lesser concern here61.
Early changes in ctDNA during treatment with standard therapies have been shown to predict later radiological responses in patients with metastatic CRC62,63. For example, in a prospective study (n = 82), reductions in ctDNA concentration of ≥80% after first-line or second-line chemotherapy were associated with a significantly improved objective response rate (47.1% versus 0%; P = 0.03) and longer median progression-free survival (PFS) (8.5 months versus 2.4 months; HR 0.19, 95% CI 0.09–0.40; P < 0.0001) and OS (27.1 months versus 11.2 months; HR 0.25, 95% CI 0.11–0.57; P < 0.001)64. Radiographic imaging and serum carcinoembryonic antigen levels are currently used to monitor disease status in the metastatic setting. However, serum carcinoembryonic antigen levels might only be elevated in 70–80% of patients, and radiographic imaging has several limitations, including costs and inter-operator and/or inter-reader variability. Therefore, data from these early-stage studies suggesting that changes in ctDNA might complement the performance of radiographic imaging in earlier lines of therapy are particularly relevant. Currently, two oral therapies (regorafenib and TAS-102) are approved for clinical use in patients with refractory metastatic CRC with prior disease progression on other cytotoxic and targeted therapies. However, these agents provide limited benefit at the cost of considerable adverse events, with most patients having rapid clinical deterioration owing to disease progression and/or drug-related toxicities. Patients with metastatic CRC who have a longer PFS on regorafenib and TAS-102 have an early decline in mutant DNA fraction, in contrast to those with a shorter PFS who either have an increase or minimal early change in mutant DNA fraction65,66. In patients with metastatic CRC with ctDNA progression, defined as any increase in VAF on regorafenib or TAS-102, the sensitivity, specificity and positive predictive value of ctDNA progression for the detection of subsequent radiographic progressive disease were 61.5%, 100% and 100%, respectively67. Thus, based on the data from these studies, establishing the validity of early changes in ctDNA as surrogate markers for clinical response in patients on later lines of therapy, in whom the prior probability of benefit is low and the risk of harm is high, is imperative. Emerging evidence indicates the feasibility and clinical utility of ctDNA-based monitoring in patients receiving anti-PD-1 antibodies. The monitoring of ctDNA might enable clinicians to differentiate unusual patterns of response, such as pseudo-progression from true disease progression on radiographic imaging, and will likely also be of benefit in patients with CRC68,69,70,71. The ease of serial monitoring using plasma ctDNA genotyping assays, relative to repeat biopsy sampling, means that this technique can also be used as an early predictor of treatment response or resistance in patients enrolled in clinical trials, particularly for those receiving therapies that are expensive and/or likely to be toxic70.
The ideal ctDNA assay to be used for the detection of biomarkers of treatment response will need to have a high level of sensitivity (preferably >90%) in order to detect ctDNA in the majority of patients with metastatic CRC, be reliable and have a rapid turnaround time. Furthermore, the ideal ctDNA assay should involve a multigene panel that enables high-depth sequencing of the most commonly altered genes in order to capture the changes associated with non-targeted as well as targeted therapies (and ideally also capture off-target resistance mutations). With such an assay, the presence of any CRC-related somatic alterations could be used to indicate a positive test, and the highest VAF of the alteration could be used to define ctDNA concentration. The assay would need to be performed prior to the start of therapy and then again soon after starting treatment in order to guide the determination of an early clinical response.
The use of ctDNA for disease monitoring has certain limitations that need to be acknowledged. First, the sensitivity of most ctDNA assays is estimated to be around 85%, and this can vary with tumour location and burden of metastatic disease72. Second, the optimal methodology and modality of ctDNA quantification is as yet unknown; most assays rely on measurements of somatic VAF, which is a mutation-dependent method. Furthermore, a proportion of patients might not have detectable somatic variants in ctDNA owing to a low tumour burden (owing to limited assay sensitivity) or to the true absence of detectable somatic alterations (confirmed by tumour sequencing). The costs of current sequencing methodologies are another key limitation. Mutation-agnostic strategies, such as evaluating ctDNA fragment length or changes in methylation, might also be considered. For example, in a study involving 107 patients with metastatic CRC receiving first-line FOLFOX-based chemotherapy, significant decreases in the levels of hypermethylated ctDNA from the gene encoding neuropeptide Y were found to correlate with improvements in PFS (9.5 months versus 7.4 months; P = 0.002) and OS (25.4 months versus 13.5 months; P = 0.0001)73,74,75,76,77,78,79. Another novel approach is to use non-coding repeat sequences or ‘mobile insertion elements’ present in genomic DNA to overcome this challenge, as these sequences comprise approximately 10% of the genome (ALU, which was originally characterized by the action of the Arthrobacter luteus, Alu restriction endonuclease being the most abundant of these elements)80. ALU elements have been correlated with carcinogenesis and disease progression, leading to efforts in their development as cancer biomarkers81,82,83. Levels of short ALU elements (amplified using a 115 bp primer reflecting total cfDNA) and the integrity index (ratio of long (247 bp) and short (115 bp) fragments) is positively correlated with disease progression and prognosis77,84,85. Future studies might involve the further evaluation of this method given the requirement for only small volumes of plasma77,84. Finally, as discussed elsewhere86,87,88, few ctDNA assays have been prospectively validated; therefore, a concerted effort should be made to incorporate plasma genotyping assays into prospective trials (Box 5).
Tracking clonal dynamics
The current standard of care for metastatic CRC involves testing tumour tissues for three biomarkers: expanded RAS mutations (which are a negative predictor of benefit from anti-EGFR antibodies); BRAFV600E (which is a negative prognostic marker and a positive predictive marker for BRAFV600E-targeted therapies) and MSI status (which has prognostic and predictive value regarding responsiveness to immune-checkpoint inhibitors in addition to being a screening tool for Lynch syndrome)89. Furthermore, other markers, such as ERBB2 amplifications and NTRK fusions, are emerging as positive predictive biomarkers for the use of therapies directed against these aberrations89,90,91. In patients that lack obtainable tumour tissue, ctDNA-based testing might provide a non-invasive solution to the need for molecular diagnosis at baseline. Data from several studies indicate a high level of concordance (>90%) between ctDNA and standard-of-care tumour tissue-based RAS testing63,92.
Resistance to anti-EGFR antibodies
The use of ctDNA to monitor complex and evolving tumour molecular clones has the potential to change the way we treat patients with metastatic CRC using targeted therapies. Among patients with RAS-wild-type metastatic CRCs treated with anti-EGFR antibodies, mutations in genes encoding proteins in the RAS signalling pathway and/or alterations in the extracellular domain (ECD) of EGFR are key mechanisms of resistance92,93,94,95,96,97,98,99. In contrast to tissue-based biopsy sampling of a single lesion, ctDNA-based assays can enable real-time detection of tumour heterogeneity as it evolves as well as the identification of alterations in RAS and the ECD of EGFR that might coexist after anti-EGFR therapy93,94,95,100,101. KRAS mutations can emerge in the blood of patients treated with anti-EGFR antibodies up to 10 months before the emergence of radiological disease progression102. ctDNA-based analyses are not currently ready to replace medical imaging, although trials are warranted to evaluate whether tracking the dynamics of resistance mutations in ctDNA should complement — or even ultimately replace — radiological assessments in guiding anti-EGFR therapy. The withdrawal of anti-EGFR therapy correlates with a decline in KRAS-mutant allelic fraction in ctDNA obtained from patients with metastatic CRC with resistance to anti-EGFR therapies owing to an acquired KRAS mutation, suggesting that ctDNA analysis might enable real-time monitoring of the effects of the selective pressures of targeted therapies on tumour populations101. In the CRICKET trial, investigators assessed the benefits of re-introducing cetuximab after treatment interruption in patients who responded to first-line cetuximab103. Interestingly, data from a retrospective analysis of ctDNA samples showed that patients with persistent RAS-mutant clones (defined by the presence of RAS-mutant clones in ctDNA prior to re-challenge) did not benefit from the re-introduction of cetuximab104. Other ongoing clinical trials, such as CHRONOS (NCT03227926) and FIRE-4 (NCT02934529), are designed to evaluate the use of ctDNA to guide re-challenge with anti-EGFR antibodies. In a phase II trial investigating the efficacy of Sym004 (consisting of two anti-EGFR antibodies, futuximab and modotuximab, which target different epitopes on EGFR) in patients with metastatic CRC with disease progression on cetuximab or panitumumab, ctDNA biomarker profiling revealed a subpopulation of patients (with wild-type RAS, BRAF and EGFR ECDs) who benefited from Sym004 (ref.94).
BRAF V600E, HER2 and other alterations
ctDNA-based analyses also enable the characterization of the molecular landscape of ERBB2-altered metastatic CRCs105. For example, ctDNA enabled the identification of alterations potentially associated with resistance in the majority of patients with ERBB2-amplified metastatic CRCs receiving HER2-targeted therapies106,107. Among patients with BRAFV600E-mutant metastatic CRC, the combination of inhibitors of BRAF and EGFR, with or without MEK inhibition, has been shown to improve survival outcomes over standard-of-care chemotherapy108,109. Serial ctDNA-based analyses of samples from patients enrolled in these trials revealed the emergence of multiple alterations in components of the MAPK signalling pathway at the time of development of resistance to these therapies. These observations support the reactivation of MAPK signalling as an important mechanism of acquired resistance that could be tracked in ctDNA. ctDNA-based assays now include the ability to test for MSI status, thus enabling the identification of mismatch repair mutations and a subset of patients with metastatic CRC who are more likely to benefit from immune-checkpoint inhibitors68,69,110,111,112.
These data are promising, although several questions still need to be addressed in order to maximize the utility of ctDNA in tracking clonal evolution and in guiding treatment-related decisions in patients with metastatic CRC. VAFs of mutant alleles detected in ctDNA are dependent on several variables, including clonality and the extent of ctDNA shedding63,92. Data published in 2019 suggest an exponential decay in treatment-emergent ctDNA markers of anti-EGFR therapy, with an estimated ctDNA half-life of 4.3 months. Furthermore, these data also suggest a trend for improvement in the objective response rate (16% for <1 half-life versus 32% for ≥2 half-lives) and PFS (2.6 months versus 3.9 months, respectively, HR 0.8, 95% CI 0.46–1.39; P = 0.43) for re-challenge with anti-EGFR therapies after increasing time intervals from initial exposure104. However, the most appropriate VAF cut-offs that warrant a treatment ‘holiday’ and/or subsequent re-introduction of therapy are currently unknown. This lack of knowledge, along with the potential for multiple mechanisms of treatment-emergent resistance, lends itself to a study enrolling patients with disease progression on prior EGFR-targeted therapies into a basket trial, with patients being placed in one of multiple arms designed to evaluate agents that are active against known mechanisms of resistance and/or downstream targets, with re-challenge based on ctDNA results. Such prospective trials will provide information on multiple unanswered questions regarding the optimal use of ctDNA in the management of patients with CRC who are receiving targeted agents (Fig. 4).
Patients with RAS/RAF-wild-type, HER2-negative colorectal cancer receiving anti-EGFR antibodies demonstrate growth of clones of resistant cells over time, as reflected by increases in the variant allele frequency (VAF) of the resistance mutations present in these clones in circulating tumour DNA (ctDNA) (part a). Of note, the emergence of such aberrations in ctDNA typically predates radiographic or clinical disease progression. Such patients can be managed in several ways, including a ‘holiday’ from the targeted agent during the next line of therapy followed by the subsequent re-introduction of the original targeted agent based on a reduction in the VAF of resistance mutations on ctDNA-based monitoring (part b); by introducing a different biomarker-selected targeted therapy or an agent targeting treatment-emergent mechanisms of resistance, as determined by ctDNA findings (part c); or the introduction of one or more agents with activity against other downstream targets (part d).
Another important issue is that of false-negative results that might limit assay performance and hinder clinical management. Additional features of sampling, such as the presence of an adequate concentration of ctDNA in the plasma specimen and/or the concomitant presence of other detectable mutations, might increase the level of confidence, for example, in that a patient has a KRAS/NRAS-wild-type tumour despite no mutation in these genes being found in ctDNA. Improved clinical reporting with additional data to aid decision-making might enable this complexity to be addressed; for example, providing post-test probability of the accuracy of the findings might aid clinicians in the interpretation of results and their translation into clinical decisions. Finally, current assays are largely geared towards the detection and tracking of mutations. However, an increasing level of need also exists to assess the presence of other molecular alterations such as fusions and/or copy-number alterations. Moreover, ctDNA-based testing should be optimized to guide the use of immune-checkpoint inhibition: whether the number of megabases covered by current assays provides an accurate indication of tumour mutational burden or whether a revised larger set of microsatellites should be included in ctDNA-based assays used to define MSI status remains unknown. These questions need to be addressed in prospective clinical trials (Box 6).
In the absence of results from ongoing clinical trials that include ctDNA-based biomarker screening and monitoring, we propose that the collection and long-term storage of plasma samples be made mandatory for all future studies involving targeted therapies and/or immunotherapy in order to enable retrospective assessments of possible mechanisms of treatment resistance. Such studies should, as a minimum, aim to collect plasma samples prior to treatment, at the time of disease progression and, if possible, at other timepoints.
The ideal application-specific assay to be used to track resistance should include a comprehensive analysis of biomarkers associated with resistance and actionable biomarkers, including point mutations, fusions, gene copy-number alterations, tumour mutational burden and MSI status. The test should be sufficiently sensitive to detect the emergence of subclones with a low VAF and minimize the number of false-negative results.
Conclusions
In summary, the Workshop members identified multiple clinical scenarios in the continuum of CRC management in which ctDNA shows the potential to alter the current status quo (Boxes 2–6). Although the workshop was conducted under the auspices of the NCI Task Forces and hence limited to clinicians and researchers based in the United States, the issues discussed are nevertheless of universal relevance. As assay development and clinical trials are undertaken internationally, it is crucial that these efforts should not take place in silos, but rather be collaborative and involve key stakeholders, ranging from patients and/or their representatives to regulatory agencies. These collaborative initiatives should aim to address the urgent issues raised here by establishing the optimal assay characteristics for each clinical scenario and the standardization of quality control and reference materials for assays, ensuring reliable pre-analytical variables, conducting clinical trials collaboratively, especially those requiring large patient numbers such as de-escalation trials in patients with MRD that might mandate a non-inferiority design, and by establishing platforms that would enable the rapid dissemination of information and data sharing.
References
Holdhoff, M., Schmidt, K. & Donehower, R. et al. Analysis of circulating tumor DNA to confirm somatic KRAS mutations. J. Natl Cancer. Inst. 101, 1284–1285 (2009).
Diehl, F., Li, M. & Dressman, D. et al. Detection and quantification of mutations in the plasma of patients with colorectal tumors. Proc. Natl Acad. Sci. USA 102, 16368–16373 (2005).
Diehl, F., Schmidt, K. & Choti, M. A. et al. Circulating mutant DNA to assess tumor dynamics. Nat. Med. 14, 985–990 (2008).
Diehl, F., Schmidt, K. & Durkee, K. H. et al. Analysis of mutations in DNA isolated from plasma and stool of colorectal cancer patients. Gastroenterology 135, 489–498 (2008).
De Mattos-Arruda, L., Mayor, R. & Ng, C. K. Y. et al. Cerebrospinal fluid-derived circulating tumour DNA better represents the genomic alterations of brain tumours than plasma. Nat. Commun. 6, 8839 (2015).
Kimura, H., Fujiwara, Y. & Sone, T. et al. EGFR mutation status in tumour-derived DNA from pleural effusion fluid is a practical basis for predicting the response to gefitinib. Br. J. Cancer 95, 1390–1395 (2006).
Wang, Y., Springer, S. & Mulvey, C. L. et al. Detection of somatic mutations and HPV in the saliva and plasma of patients with head and neck squamous cell carcinomas. Sci. Transl Med. 7, 293ra104 (2015).
Reckamp, K. L., Melnikova, V. O. & Karlovich, C. et al. A highly sensitive and Quantitative test platform for detection of NSCLC EGFR mutations in urine and plasma. J. Thorac. Oncol. 11, 1690–1700 (2016).
Bettegowda, C., Sausen, M. & Leary, R. J. et al. Detection of circulating tumor DNA in early- and late-stage human malignancies. Sci. Transl Med. 6, 224ra24 (2014).
Diehn, M., Alizadeh, A. A. & Adams, H.-P. et al. Early prediction of clinical outcomes in resected stage II and III colorectal cancer (CRC) through deep sequencing of circulating tumor DNA (ctDNA). J. Clin. Oncol. 35, 3591–3591 (2017).
Overman, M. J., Vauthey, J.-N. & Aloia, T. A. et al. Circulating tumor DNA (ctDNA) utilizing a high-sensitivity panel to detect minimal residual disease post liver hepatectomy and predict disease recurrence. J. Clin. Oncol. 35, 3522–3522 (2017).
Tie, J., Wang, Y. & Tomasetti, C. et al. Circulating tumor DNA analysis detects minimal residual disease and predicts recurrence in patients with stage II colon cancer. Sci. Transl Med. 8, 346ra92 (2016).
Tie, J. C., Cohen, J. D. & Wang, Y. et al. Serial circulating tumor DNA analysis as a prognostic marker and a real-time indicator of adjuvant chemotherapy efficacy in stage III colon cancer. J. Clin. Oncol. 36 (Suppl. 15), 3516 (2018).
Wang, J. Y., Hsieh, J. S. & Chang, M. Y. et al. Molecular detection of APC, K- ras, and p53 mutations in the serum of colorectal cancer patients as circulating biomarkers. World J. Surg. 28, 721–726 (2004).
Sun, X., Huang, T. & Cheng, F. et al. Monitoring colorectal cancer following surgery using plasma circulating tumor DNA. Oncol. Lett. 15, 4365–4375 (2018).
Bazan, V., Bruno, L. & Augello, C. et al. Molecular detection of TP53, Ki-Ras and p16INK4A promoter methylation in plasma of patients with colorectal cancer and its association with prognosis. Results of a 3-year GOIM (Gruppo Oncologico dell’Italia Meridionale) prospective study. Ann. Oncol. 17, vii84–vii90 (2006).
Petit, J., Carroll, G. & Gould, T. et al. Cell-free DNA as a diagnostic blood-based biomarker for colorectal cancer: a systematic review. J. Surg. Res. 236, 184–197 (2019).
Cree, I. A., Uttley, L. & Buckley Woods, H. et al. The evidence base for circulating tumour DNA blood-based biomarkers for the early detection of cancer: a systematic mapping review. BMC Cancer 17, 697 (2017).
Siravegna, G., Marsoni, S. & Siena, S. et al. Integrating liquid biopsies into the management of cancer. Nat. Rev. Clin. Oncol. 14, 531–548 (2017).
Meddeb, R., Pisareva, E. & Thierry, A. R. Guidelines for the preanalytical conditions for analyzing circulating cell-free DNA. Clin. Chem. 65, 623–633 (2019).
Merker, J. D., Oxnard, G. R. & Compton, C. et al. Circulating tumor DNA analysis in patients with cancer: American Society of clinical oncology and college of American pathologists joint review. Arch. Pathol. Lab. Med. 142, 1242–1253 (2018).
Merker, J. D., Oxnard, G. R. & Compton, C. et al. Circulating tumor DNA Analysis in Patients with cancer: American Society of Clinical Oncology and College of American Pathologists joint review. J. Clin. Oncol. 36, 1631–1641 (2018).
van Dessel, L. F., Beije, N. & Helmijr, J. C. et al. Application of circulating tumor DNA in prospective clinical oncology trials - standardization of preanalytical conditions. Mol. Oncol. 11, 295–304 (2017).
Medina Diaz, I., Nocon, A. & Mehnert, D. H. et al. Performance of streck cfDNA blood collection tubes for liquid biopsy testing. PLoS One 11, e0166354 (2016).
Kang, Q., Henry, N. L. & Paoletti, C. et al. Comparative analysis of circulating tumor DNA stability In K3EDTA, streck, and cellsave blood collection tubes. Clin. Biochem. 49, 1354–1360 (2016).
Page, K., Powles, T. & Slade, M. J. et al. The importance of careful blood processing in isolation of cell-free DNA. Ann. N. Y. Acad. Sci. 1075, 313–317 (2006).
Corcoran, R. B. & Chabner, B. A. Application of cell-free DNA analysis to cancer treatment. N. Engl. J. Med. 379, 1754–1765 (2018).
Oxnard, G. R., Paweletz, C. P. & Sholl, L. M. Genomic analysis of plasma cell-free DNA in patients with cancer. JAMA Oncol. 3, 740–741 (2017).
Saiyed, Z. M., Bochiwal, C. & Gorasia, H. et al. Application of magnetic particles (Fe3O4) for isolation of genomic DNA from mammalian cells. Anal. Biochem. 356, 306–308 (2006).
Benson, A. B., Venook, A. P. & Al-Hawary, M. M. et al. NCCN guidelines insights: colon cancer, version 2.2018. J. Natl Compr. Canc. Netw. 16, 359–369 (2018).
Ellervik, C. & Vaught, J. Preanalytical variables affecting the integrity of human biospecimens in biobanking. Clin. Chem. 61, 914–934 (2015).
Koessler, T., Addeo, A. & Nouspikel, T. Implementing circulating tumor DNA analysis in a clinical laboratory: a user manual. Adv. Clin. Chem. 89, 131–188 (2019).
Hojbjerg, J. A., Madsen, A. T. & Schmidt, H. H. et al. Intra-individual variation of circulating tumour DNA in lung cancer patients. Mol. Oncol. 13, 2098–2106 (2019).
Compton, C. C., Fielding, L. P. & Burgart, L. J. et al. Prognostic factors in colorectal cancer. College of American pathologists consensus statement 1999. Arch. Pathol. Lab. Med. 124, 979–994 (2000).
Li, L., Zhou, W. & Li, C. et al. Analysis of circulating tumor DNA to monitor disease status in colorectal cancer after surgery. J. Clin. Oncol. 36, e15583 (2018).
Overman, M. J., Vauthey, J.-N. & Aloia, T. A. et al. Circulating tumor DNA (ctDNA) utilizing a high-sensitivity panel to detect minimal residual disease post liver hepatectomy and predict disease recurrence. J. Clin. Oncol. 35 (Suppl. 15), 3522 (2017).
Tie, J., Cohen, J. D. & Wang, Y. et al. Circulating tumor DNA analyses as markers of recurrence risk and benefit of adjuvant therapy for stage III colon cancer. JAMA Oncol. 5, 1710–1717 (2019).
Wang, Y., Li, L. & Cohen, J. D. et al. Prognostic potential of circulating tumor DNA measurement in postoperative surveillance of nonmetastatic colorectal cancer. JAMA Oncol. 5, 1118–1123 (2019).
Reinert, T., Henriksen, T. V. & Christensen, E. et al. Analysis of plasma cell-free DNA by ultradeep sequencing in patients with stages I to III colorectal cancer. JAMA Oncol. 5, 1124–1131 (2019).
Wan, J. C. M., Massie, C. & Garcia-Corbacho, J. et al. Liquid biopsies come of age: towards implementation of circulating tumour DNA. Nat. Rev. Cancer 17, 223–238 (2017).
Kopetz, S., McDonough, S. L. & Morris, V. K. et al. Randomized trial of irinotecan and cetuximab with or without vemurafenib in BRAF-mutant metastatic colorectal cancer (SWOG 1406). J. Clin. Oncol. 35, 520 (2017).
Kopetz, S., Grothey, A. & Yaeger, R. et al. Encorafenib, binimetinib, and cetuximab in BRAF V600E-mutated colorectal cancer. N. Engl. J. Med. 381, 1632–1643 (2019).
Dasari, A., Grothey, A. & Kopetz, S. Circulating tumor DNA-defined minimal residual disease in solid tumors: opportunities to accelerate the development of adjuvant therapies. J. Clin. Oncol. 36, JCO2018789032 (2018).
Hong, D. S., Morris, V. K. & El Osta, B. et al. Phase IB study of vemurafenib in combination with irinotecan and cetuximab in patients with metastatic colorectal cancer with BRAFV600E mutation. Cancer Discov. 6, 1352–1365 (2016).
Grothey, A., Sobrero, A. F. & Shields, A. F. et al. Duration of adjuvant chemotherapy for stage III colon cancer. N. Engl. J. Med. 378, 1177–1188 (2018).
Sargent, D., Sobrero, A. & Grothey, A. et al. Evidence for cure by adjuvant therapy in colon cancer: observations based on individual patient data from 20,898 patients on 18 randomized trials. J. Clin. Oncol. 27, 872–877 (2009).
Sawai, C. M., Babovic, S. & Upadhaya, S. et al. Hematopoietic stem cells are the major source of multilineage hematopoiesis in adult animals. Immunity 45, 597–609 (2016).
Pang, W. W., Price, E. A. & Sahoo, D. et al. Human bone marrow hematopoietic stem cells are increased in frequency and myeloid-biased with age. Proc. Natl Acad. Sci. USA 108, 20012–20007 (2011).
Genovese, G., Kahler, A. K. & Handsaker, R. E. et al. Clonal hematopoiesis and blood-cancer risk inferred from blood DNA sequence. N. Engl. J. Med. 371, 2477–2487 (2014).
Jaiswal, S., Fontanillas, P. & Flannick, J. et al. Age-related clonal hematopoiesis associated with adverse outcomes. N. Engl. J. Med. 371, 2488–2498 (2014).
Young, A. L., Challen, G. A. & Birmann, B. M. et al. Clonal haematopoiesis harbouring AML-associated mutations is ubiquitous in healthy adults. Nat. Commun. 7, 12484 (2016).
Chen, S., Wang, Q. & Yu, H. et al. Mutant p53 drives clonal hematopoiesis through modulating epigenetic pathway. Nat. Commun. 10, 5649 (2019).
Gibson, C. J. & Steensma, D. P. New insights from studies of clonal hematopoiesis. Clin. Cancer Res. 24, 4633–4642 (2018).
Severson, E. A., Riedlinger, G. M. & Connelly, C. F. et al. Detection of clonal hematopoiesis of indeterminate potential in clinical sequencing of solid tumor specimens. Blood 131, 2501–2505 (2018).
Schou, J. V., Larsen, F. O. & Sorensen, B. S. et al. Circulating cell-free DNA as predictor of treatment failure after neoadjuvant chemo-radiotherapy before surgery in patients with locally advanced rectal cancer. Ann. Oncol. 29, 610–615 (2018).
Tie, J., Cohen, J. D. & Wang, Y. et al. Serial circulating tumour DNA analysis during multimodality treatment of locally advanced rectal cancer: a prospective biomarker study. Gut 68, 663–671 (2019).
Khakoo, S., Carter, P. D. & Brown, G. et al. MRI tumor regression grade and circulating tumor DNA as complementary tools to assess response and guide therapy adaptation in rectal cancer. Clin. Cancer Res. 26, 183–192 (2020).
Boysen, A. K., Schou, J. V. & Spindler, K. G. Cell-free DNA and preoperative chemoradiotherapy for rectal cancer: a systematic review. Clin. Transl Oncol. 21, 874–880 (2019).
Massihnia, D., Pizzutilo, E. G. & Amatu, A. et al. Liquid biopsy for rectal cancer: a systematic review. Cancer Treat. Rev. 79, 101893 (2019).
van der Valk, M. J. M., Hilling, D. E. & Bastiaannet, E. et al. Long-term outcomes of clinical complete responders after neoadjuvant treatment for rectal cancer in the International Watch & Wait Database (IWWD): an international multicentre registry study. Lancet 391, 2537–2545 (2018).
Diaz, L. A. Jr & Bardelli, A. Liquid biopsies: genotyping circulating tumor DNA. J. Clin. Oncol. 32, 579–586 (2014).
Tie, J., Kinde, I. & Wang, Y. et al. Circulating tumor DNA as an early marker of therapeutic response in patients with metastatic colorectal cancer. Ann. Oncol. 26, 1715–1722 (2015).
Vidal, J., Muinelo, L. & Dalmases, A. et al. Plasma ctDNA RAS mutation analysis for the diagnosis and treatment monitoring of metastatic colorectal cancer patients. Ann. Oncol. 28, 1325–1332 (2017).
Garlan, F., Laurent-Puig, P. & Sefrioui, D. et al. Early evaluation of circulating tumor DNA as marker of therapeutic efficacy in metastatic colorectal cancer patients (PLACOL Study). Clin. Cancer Res. 23, 5416–5425 (2017).
Wong, A. L., Lim, J. S. & Sinha, A. et al. Tumour pharmacodynamics and circulating cell free DNA in patients with refractory colorectal carcinoma treated with regorafenib. J. Transl Med. 13, 57 (2015).
Vandeputte, C., Kehagias, P. & El Housni, H. et al. Circulating tumor DNA in early response assessment and monitoring of advanced colorectal cancer treated with a multi-kinase inhibitor. Oncotarget 9, 17756–17769 (2018).
Lima Pereira, A., Lam, M., Marie, P. K., Raghav, K. & Morris, V. K. et al. Circulating tumor DNA (ctDNA) as an early marker to monitor clinical benefit of regorafenib and TAS-102 in patients with metastatic colorectal cancer (mCRC). J. Clin. Oncol. 36, 3533 (2018).
Snyder, A., Morrissey, M. P. & Hellmann, M. D. Use of circulating tumor DNA for cancer immunotherapy. Clin. Cancer Res. 25, 6909–6915 (2019).
Cabel, L., Proudhon, C. & Romano, E. et al. Clinical potential of circulating tumour DNA in patients receiving anticancer immunotherapy. Nat. Rev. Clin. Oncol. 15, 639–650 (2018).
Lee, J. H., Long, G. V. & Menzies, A. M. et al. Association between circulating tumor DNA and pseudoprogression in patients with metastatic melanoma treated with anti-programmed cell death 1 antibodies. JAMA Oncol. 4, 717–721 (2018).
Cabel, L., Riva, F. & Servois, V. et al. Circulating tumor DNA changes for early monitoring of anti-PD1 immunotherapy: a proof-of-concept study. Ann. Oncol. 28, 1996–2001 (2017).
Bachet, J. B., Bouche, O. & Taieb, J. et al. RAS mutation analysis in circulating tumor DNA from patients with metastatic colorectal cancer: the AGEO RASANC prospective multicenter study. Ann. Oncol. 29, 1211–1219 (2018).
Luo, H., Zhao, Q. & Wei, W. et al. Circulating tumor DNA methylation profiles enable early diagnosis, prognosis prediction, and screening for colorectal cancer. Sci. Transl Med. 12, eaax7533 (2020).
Symonds, E. L., Pedersen, S. K. & Murray, D. et al. Circulating epigenetic biomarkers for detection of recurrent colorectal cancer. Cancer 126, 1460–1469 (2020).
Gai, W., Ji, L. & Lam, W. K. J. et al. Liver- and colon-specific DNA methylation markers in plasma for investigation of colorectal cancers with or without liver metastases. Clin. Chem. 64, 1239–1249 (2018).
Mouliere, F., Chandrananda, D. & Piskorz, A. M. et al. Enhanced detection of circulating tumor DNA by fragment size analysis. Sci. Transl Med. 10, eaat4921 (2018).
Wang, X., Shi, X. Q. & Zeng, P. W. et al. Circulating cell free DNA as the diagnostic marker for colorectal cancer: a systematic review and meta-analysis. Oncotarget 9, 24514–24524 (2018).
Thomsen, C. B., Hansen, T. F. & Andersen, R. F. et al. Early identification of treatment effect by methylated circulating tumour DNA in metastatic colorectal cancer. Ann. Oncol. 30 (Suppl. 5), V201 (2019).
Strickler, J. H., Zemla, T. & Ou, F.-S. et al. Trastuzumab and tucatinib for the treatment of HER2 amplified metastatic colorectal cancer (mCRC): initial results from the MOUNTAINEER trial. Ann. Oncol. 30 (Suppl. 5), V200 (2019).
Batzer, M. A. & Deininger, P. L. Alu repeats and human genomic diversity. Nat. Rev. Genet. 3, 370–379 (2002).
Paz, N., Levanon, E. Y. & Amariglio, N. et al. Altered adenosine-to-inosine RNA editing in human cancer. Genome Res. 17, 1586–1595 (2007).
Di Ruocco, F., Basso, V. & Rivoire, M. et al. Alu RNA accumulation induces epithelial-to-mesenchymal transition by modulating miR-566 and is associated with cancer progression. Oncogene 37, 627–637 (2018).
Lazzari, E., Mondala, P. K. & Santos, N. D. et al. Alu-dependent RNA editing of GLI1 promotes malignant regeneration in multiple myeloma. Nat. Commun. 8, 1922 (2017).
Hao, T. B., Shi, W. & Shen, X. J. et al. Circulating cell-free DNA in serum as a biomarker for diagnosis and prognostic prediction of colorectal cancer. Br. J. Cancer 111, 1482–1489 (2014).
El-Gayar, D., El-Abd, N. & Hassan, N. et al. Increased free circulating DNA integrity Index as a serum biomarker in patients with colorectal carcinoma. Asian Pac. J. Cancer Prev. 17, 939–944 (2016).
Lanman, R. B., Mortimer, S. A. & Zill, O. A. et al. Analytical and clinical validation of a digital sequencing panel for quantitative, highly accurate evaluation of cell-free circulating tumor DNA. PLoS One 10, e0140712 (2015).
Sacher, A. G., Paweletz, C. & Dahlberg, S. E. et al. Prospective validation of rapid plasma genotyping for the detection of EGFR and KRAS mutations in advanced lung cancer. JAMA Oncol. 2, 1014–1022 (2016).
Kim, S. T., Lee, W. S. & Lanman, R. B. et al. Prospective blinded study of somatic mutation detection in cell-free DNA utilizing a targeted 54-gene next generation sequencing panel in metastatic solid tumor patients. Oncotarget 6, 40360–40369 (2015).
Sepulveda, A. R., Hamilton, S. R. & Allegra, C. J. et al. Molecular biomarkers for the evaluation of colorectal cancer: guideline from the American society for clinical pathology, college of American pathologists, association for molecular pathology and American society of clinical oncology. J. Mol. Diagn. 19, 187–225 (2017).
Meric-Bernstam, F., Hurwitz, H. & Raghav, K. P. S. et al. Pertuzumab plus trastuzumab for HER2-amplified metastatic colorectal cancer (MyPathway): an updated report from a multicentre, open-label, phase 2a, multiple basket study. Lancet Oncol. 20, 518–530 (2019).
Nakamura, Y., Okamoto, W. & Kato, T. et al. 526PD - TRIUMPH: primary efficacy of a phase II trial of trastuzumab (T) and pertuzumab (P) in patients (pts) with metastatic colorectal cancer (mCRC) with HER2 (ERBB2) amplification (amp) in tumour tissue or circulating tumour DNA (ctDNA): a GOZILA sub-study. Ann. Oncol. 30, v199–v200 (2019).
Montagut, C., Tsui, D. W. & Diaz, L. A. Jr Detection of somatic RAS mutations in circulating tumor DNA from metastatic colorectal cancer patients: are we ready for clinical use? Ann. Oncol. 29, 1083–1084 (2018).
Russo, M., Siravegna, G. & Blaszkowsky, L. S. et al. Tumor heterogeneity and lesion-specific response to targeted therapy in colorectal cancer. Cancer Discov. 6, 147–153 (2016).
Montagut, C., Argiles, G. & Ciardiello, F. et al. Efficacy of Sym004 in patients with metastatic colorectal cancer with acquired resistance to anti-EGFR therapy and molecularly selected by circulating tumor DNA Analyses: a phase 2 randomized clinical trial. JAMA Oncol. 4, e175245 (2018).
Strickler, J. H., Loree, J. M. & Ahronian, L. G. et al. Genomic landscape of cell-free DNA in patients with colorectal cancer. Cancer Discov. 8, 164–173 (2018).
Morelli, M. P., Overman, M. J. & Dasari, A. et al. Characterizing the patterns of clonal selection in circulating tumor DNA from patients with colorectal cancer refractory to anti-EGFR treatment. Ann. Oncol. 26, 731–736 (2015).
Arena, S., Bellosillo, B. & Siravegna, G. et al. Emergence of multiple EGFR extracellular mutations during cetuximab treatment in colorectal cancer. Clin. Cancer Res. 21, 2157–2166 (2015).
Misale, S., Yaeger, R. & Hobor, S. et al. Emergence of KRAS mutations and acquired resistance to anti-EGFR therapy in colorectal cancer. Nature 486, 532–536 (2012).
Diaz, L. A. Jr., Williams, R. T. & Wu, J. et al. The molecular evolution of acquired resistance to targeted EGFR blockade in colorectal cancers. Nature 486, 537–540 (2012).
Van Emburgh, B. O., Arena, S. & Siravegna, G. et al. Acquired RAS or EGFR mutations and duration of response to EGFR blockade in colorectal cancer. Nat. Commun. 7, 13665 (2016).
Siravegna, G., Mussolin, B. & Buscarino, M. et al. Clonal evolution and resistance to EGFR blockade in the blood of colorectal cancer patients. Nat. Med. 21, 795–801 (2015).
Montagut, C., Dalmases, A. & Bellosillo, B. et al. Identification of a mutation in the extracellular domain of the Epidermal Growth Factor Receptor conferring cetuximab resistance in colorectal cancer. Nat. Med. 18, 221–223 (2012).
Santini, D., Vincenzi, B. & Addeo, R. et al. Cetuximab rechallenge in metastatic colorectal cancer patients: how to come away from acquired resistance? Ann. Oncol. 23, 2313–2318 (2012).
Parseghian, C. M., Loree, J. M. & Morris, V. K. et al. Anti-EGFR-resistant clones decay exponentially after progression: implications for anti-EGFR re-challenge. Ann. Oncol. 30, 243–249 (2019).
Loree, J. M., Bailey, A. M. & Johnson, A. M. et al. Molecular landscape of ERBB2/ERBB3 mutated colorectal cancer. J. Natl Cancer. Inst. 110, 1409–1417 (2018).
Sartore-Bianchi, A., Trusolino, L. & Martino, C. et al. Dual-targeted therapy with trastuzumab and lapatinib in treatment-refractory, KRAS codon 12/13 wild-type, HER2-positive metastatic colorectal cancer (HERACLES): a proof-of-concept, multicentre, open-label, phase 2 trial. Lancet Oncol. 17, 738–746 (2016).
Siravegna, G., Lazzari, L. & Crisafulli, G. et al. Radiologic and genomic evolution of individual metastases during HER2 blockade in colorectal cancer. Cancer Cell 34, 148–162.e7 (2018).
Corcoran, R. B., Andre, T. & Atreya, C. E. et al. Combined BRAF, EGFR, and MEK inhibition in patients with BRAFV600E-mutant colorectal cancer. Cancer Discov. 8, 428–443 (2018).
Van Cutsem, E., Huijberts, S. & Grothey, A. et al. Binimetinib, encorafenib, and cetuximab triplet therapy for patients with BRAF V600E-mutant metastatic colorectal cancer: safety lead-in results from the phase III BEACON colorectal cancer study. J. Clin. Oncol. 37, 1460–1469 (2019).
Pabla, S., Andreas, J. & Lenzo, F. L. et al. Development and analytical validation of a next-generation sequencing based microsatellite instability (MSI) assay. Oncotarget 10, 5181–5193 (2019).
Georgiadis, A., Durham, J. N. & Keefer, L. A. et al. Noninvasive detection of microsatellite instability and high tumor mutation burden in cancer patients treated with PD-1 blockade. Clin. Cancer Res. 25, 7024–7034 (2019).
Willis, J., Lefterova, M. I. & Artyomenko, A. et al. Validation of microsatellite instability detection using a comprehensive plasma-based genotyping panel. Clin. Cancer Res. 25, 7035–7045 (2019).
Author information
Authors and Affiliations
Contributions
A.D., V.M., M.D., R.A.G., S.R.H., H.H., T.S.H., H.F.K., K.R., Q.S., J.H.S., A.V., R.Y., G.Y., Y.N.Y. and S.K. researched data for this manuscript. All authors made a substantial contribution to discussions of content. A.V., V.M., C.A., A.B.B., R.B.C., D.D., M.J.G., R.A.G., S.R.H., J.F.H., T.S.H., A.I., H.F.K., W.O.L., F.-S.O., L.P., K.R., R.Y., Y.N.Y., and S.K. wrote the manuscript and A.D., V.M., C.A., C.A., A.B.B., P.B., K.C., R.B.C., D.D., M.D., A.D., C.E., T.J.G., M.J.G., R.A.G., S.R.H., J.F.H., H.H., T.S.H., F.I., A.I., S.A.J., J.L., C.H.L., H-J.L., W.O.L., C.M., B.C.O., F.-S.O, L.P., D.S., A.J.S., Q.S., J.H.S., R.Y., G.Y., Y.N.N., J.Z., and S.K. reviewed and/or edited the manuscript prior to submission.
Corresponding author
Ethics declarations
Competing interests
C.A. has received research funding from Guardant Health. R.C. is an advisory board member of Guardant Health and holds equity interests in nRichDx. M.D. has acted as a consultant of Quanticell. C.L. has acted as a consultant of Foundation Medicine. C.M. has acted as a consultant of Guardant Health. The other authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Dasari, A., Morris, V.K., Allegra, C.J. et al. ctDNA applications and integration in colorectal cancer: an NCI Colon and Rectal–Anal Task Forces whitepaper. Nat Rev Clin Oncol 17, 757–770 (2020). https://doi.org/10.1038/s41571-020-0392-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41571-020-0392-0
This article is cited by
-
Clinical applications and perspectives of circulating tumor DNA in gastric cancer
Cancer Cell International (2024)
-
Liquid biopsy techniques and lung cancer: diagnosis, monitoring and evaluation
Journal of Experimental & Clinical Cancer Research (2024)
-
Total neoadjuvant therapy in oesophageal and gastro-oesophageal junctional adenocarcinoma
British Journal of Cancer (2024)
-
Diagnostic liquid biopsy biomarkers in renal cell cancer
Nature Reviews Urology (2024)
-
Redefining precision radiotherapy through liquid biopsy
British Journal of Cancer (2023)