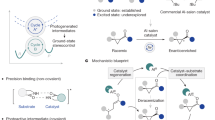
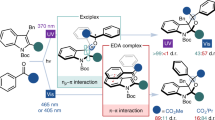
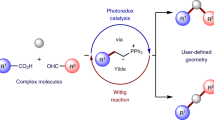
Abstract
Creating, conserving and modifying the stereochemistry of organic compounds has been the subject of significant research efforts in synthetic chemistry. Most synthetic routes are designed according to the stereoselectivity-determining step. Stereochemical editing is an alternative strategy, wherein the chiral-defining or geometry-defining steps are independent of the construction of the major scaffold or complexity. It enables late-stage alterations of stereochemistry and can generate isomers from a single compound. However, in many instances, stereochemical editing processes are contra-thermodynamic, meaning the transformation is unfavourable. To overcome this barrier, photocatalysis uses photogenerated radical species and introduces thermochemical biases. A range of synthetically valuable contra-thermodynamic stereochemical editing processes have been invented, including deracemization of chiral molecules, positional alkene isomerization and dynamic epimerization of sugars and diols. In this Review, we highlight the fundamental mechanisms of visible-light photocatalysis and the general reactivity modes of the photogenerated radical intermediates towards contra-thermodynamic stereochemical editing processes.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Llanos, E. J. et al. Exploration of the chemical space and its three historical regimes. Proc. Natl Acad. Sci. USA 116, 12660–12665 (2019).
Gensch, T. & Glorius, F. The straight dope on the scope of chemical reactions. Science 352, 294–295 (2016).
Mason, S. F. Origins of biomolecular handedness. Nature 311, 19–23 (1984).
Brooks, W. H., Guida, W. C. & Daniel, K. G. The significance of chirality in drug design and development. Curr. Top. Med. Chem. 11, 760–770 (2011).
Calcaterra, A. & D’Acquarica, I. The market of chiral drugs: chiral switches versus de novo enantiomerically pure compounds. J. Pharm. Biomed. Anal. 147, 323–340 (2018).
Reymond, J. L., van Deursen, R., Blum, L. C. & Ruddigkeit, L. Chemical space as a source for new drugs. Med. Chem. Comm. 1, 30–38 (2010).
Jacobsen, E. N., Pfaltz, A. & Yamamoto, H. (eds) Comprehensive Asymmetric Catalysis I−III (Springer-Verlag, 1999).
Andrushko, V. & Andrushko, N. (eds) Stereoselective Synthesis of Drugs and Natural Products (Wiley, 2014).
Carreira, E. M. & Yamamoto, H. (eds) Comprehensive Chirality (Academic, 2012).
Bhat, V., Welin, E. R., Guo, X. & Stoltz, B. M. Advances in stereoconvergent catalysis from 2005 to 2015: transition-metal-mediated stereoablative reactions, dynamic kinetic resolutions, and dynamic kinetic asymmetric transformations. Chem. Rev. 117, 4528–4561 (2017).
Beletskaya, I. P., Najera, C. & Yus, M. Stereodivergent catalysis. Chem. Rev. 118, 5080–5200 (2018).
Zahrt, A. F., Athavale, S. V. & Denmark, S. E. Quantitative structure–selectivity relationships in enantioselective catalysis: past, present, and future. Chem. Rev. 120, 1620–1689 (2020).
Masamune, S., Choy, W., Petersen, J. S. & Sita, L. R. Double asymmetric synthesis and a new strategy for stereochemical control in organic synthesis. Angew. Chem. Int. Ed. 24, 1–30 (1985).
Evans, D. A. Stereoselective organic reactions: catalysts for carbonyl addition processes. Science 240, 420–426 (1988).
Hoveyda, A. H., Evans, D. A. & Fu, G. C. Substrate-directable chemical reactions. Chem. Rev. 93, 1307–1370 (1993).
Taylor, M. S. & Jacobsen, E. N. Asymmetric catalysis in complex target synthesis. Proc. Natl Acad. Sci. USA 101, 5368–5373 (2004).
Chen, D. F. & Gong, L. Z. Organo/transition-metal combined catalysis rejuvenates both in asymmetric synthesis. J. Am. Chem. Soc. 144, 2415–2437 (2022).
Gruber, C. C., Lavandera, I., Faber, K. & Kroutil, W. From a racemate to a single enantiomer: deracemization by stereoinversion. Adv. Synth. Catal. 348, 1789–1805 (2006).
Palmans, A. R. A. Deracemisations under kinetic and thermodynamic control. Mol. Syst. Des. Eng. 2, 34–46 (2017).
Buhse, T. et al. Spontaneous deracemizations. Chem. Rev. 121, 2147–2229 (2021).
Blackmond, D. G. “If pigs could fly” chemistry: a tutorial on the principle of microscopic reversibility. Angew. Chem. Int. Ed. 48, 2648–2654 (2009).
Prier, C. K., Rankic, D. A. & MacMillan, D. W. Visible light photoredox catalysis with transition metal complexes: applications in organic synthesis. Chem. Rev. 113, 5322–5363 (2013).
Schultz, D. M. & Yoon, T. P. Solar synthesis: prospects in visible light photocatalysis. Science 343, 985–994 (2014).
Hossain, A., Bhattacharyya, A. & Reiser, O. Copper’s rapid ascent in visible-light photoredox catalysis. Science 364, 450–461 (2019).
Onsager, L. Reciprocal relations in irreversible processes. I. Phys. Rev. 37, 405–426 (1931).
Metternich, J. & Gilmour, R. Photocatalytic E → Z isomerization of alkenes. Synlett 27, 2541–2552 (2016).
Molloy, J. J., Morack, T. & Gilmour, R. Positional and geometrical isomerisation of alkenes: the pinnacle of atom economy. Angew. Chem. Int. Ed. 58, 13654–13664 (2019).
Zhang, H. & Yu, S. Visible light-promoted isomerization of alkenes. Chin. J. Org. Chem. 39, 95–108 (2019).
Nevesely, T., Wienhold, M., Molloy, J. J. & Gilmour, R. Advances in the E → Z isomerization of alkenes using small molecule photocatalysts. Chem. Rev. 122, 2650–2694 (2022).
Agranat, I., Caner, H. & Caldwell, J. Putting chirality to work: the strategy of chiral switches. Nat. Rev. Drug Disc. 1, 753–768 (2002).
Abram, M., Jakubiec, M. & Kaminski, K. Chirality as an important factor for the development of new antiepileptic drugs. Chem. Med. Chem. 14, 1744–1761 (2019).
Drucker, C. S., Toscano, V. G. & Weiss, R. G. General method for the determination of steric effects during collisional energy transfer. Partial photoresolution of penta-2,3-diene. J. Am. Chem. Soc. 95, 6482–6484 (1973).
Ouannes, C., Beugelmans, R. & Roussi, G. Asymmetric induction during transfer of triplet energy. J. Am. Chem. Soc. 95, 8472–8474 (1973).
Pirkle, W. H. & Reno, D. S. Extension of chromatographically-derived molecular recognition concepts to first order asymmetric transformations. J. Am. Chem. Soc. 109, 7189–7190 (1987).
Hammond, G. S. & Cole, R. S. Asymmetric induction during energy transfer. J. Am. Chem. Soc. 87, 3256–3257 (1965).
Murov, S. L., Cole, R. S. & Hammond, G. S. Mechanisms of photochemical reactions in solution. LIV. A new mechanism of photosensitization. J. Am. Chem. Soc. 90, 2957–2958 (1968).
Shi, Q. & Ye, J. Deracemization enabled by visible-light photocatalysis. Angew. Chem. Int. Ed. 59, 4998–5001 (2020).
Brimioulle, R., Lenhart, D., Maturi, M. M. & Bach, T. Enantioselective catalysis of photochemical reactions. Angew. Chem. Int. Ed. 54, 3872–3890 (2015).
D Schwinger, D. P. & Bach, T. Chiral 1,3,2-oxazaborolidine catalysts for enantioselective photochemical reactions. Acc. Chem. Res. 53, 1933–1943 (2020).
Grosskopf, J., Kratz, T., Rigotti, T. & Bach, T. Enantioselective photochemical reactions enabled by triplet energy transfer. Chem. Rev. 122, 1626–1653 (2022).
Holzl-Hobmeier, A. et al. Catalytic deracemization of chiral allenes by sensitized excitation with visible light. Nature 564, 240–243 (2018). This work is the first example of visible light-driven highly enantioselective deracemization of chiral allenes via triplet sensitization.
Yang, C. & Inoue, Y. An exciting tool for asymmetric synthesis. Nature 564, 197–199 (2018).
Alonso, R. & Bach, T. A chiral thioxanthone as an organocatalyst for enantioselective [2+2] photocycloaddition reactions induced by visible light. Angew. Chem. Int. Ed. 53, 4368–4371 (2014).
Plaza, M., Jandl, C. & Bach, T. Photochemical deracemization of allenes and subsequent chirality transfer. Angew. Chem. Int. Ed. 59, 12785–12788 (2020). This report describes the extension of the strategy of triplet energy transfer-mediated deracemization to allenes with a pyrrolidin-2-one motif.
Plaza, M., Grosskopf, J., Breitenlechner, S., Bannwarth, C. & Bach, T. Photochemical deracemization of primary allene amides by triplet energy transfer: a combined synthetic and theoretical study. J. Am. Chem. Soc. 143, 11209–11217 (2021).
Wimberger, L., Kratz, T. & Bach, T. Photochemical deracemization of chiral sulfoxides catalyzed by a hydrogen-bonding xanthone sensitizer. Synthesis 51, 4416–4423 (2019).
Cahn, R. S., Ingold, C. & Prelog, V. Specification of molecular chirality. Angew. Chem. Int. Ed. 5, 385–415 (1966).
Grosu, I., Balog, M. & Mager, S. Considerations on the stereochemistry of alkylidenecyclohexanes and their heterocyclic analogs. Rev. Roum. Chim. 50, 333–339 (2005).
Tejedor, R. M., Oriol, L., Serrano, J. L. & Sierra, T. Chiral photochemical induction in liquid crystals. J. Mater. Chem. 18, 2899–2908 (2008).
Kratz, T. et al. Photochemical deracemization of chiral alkenes via triplet energy transfer. J. Am. Chem. Soc. 144, 10133–10138 (2022). This report describes the first example of visible light-driven, highly enantioselective deracemization of axially chiral alkenes, significantly expanding the reaction scope of photochemical deracemization.
Bregent, T., Bouillon, J. P. & Poisson, T. Copper-photocatalyzed contra-thermodynamic isomerization of polarized alkenes. Org. Lett. 22, 7688–7693 (2020).
Cruché, C., Neiderer, W. & Collins, S. K. Heteroleptic copper-based complexes for energy-transfer processes: E → Z isomerization and tandem photocatalytic sequences. ACS Catal. 11, 8829–8836 (2021).
Zhang, H., Huang, C., Yuan, X. A. & Yu, S. Photoexcited chiral copper complex-mediated alkene E → Z isomerization enables kinetic resolution. J. Am. Chem. Soc. 144, 10958–10967 (2022).
Kancherla, R., Muralirajan, K., Sagadevan, A. & Rueping, M. Visible light-induced excited-state transition-metal catalysis. Trends Chem. 1, 510–523 (2019).
Zhu, M., Zhang, X., Zheng, C. & You, S. L. Energy-transfer-enabled dearomative cycloaddition reactions of indoles/pyrroles via excited-state aromatics. Acc. Chem. Res. 55, 2510–2525 (2022).
Troster, A., Bauer, A., Jandl, C. & Bach, T. Enantioselective visible-light-mediated formation of 3-cyclopropylquinolones by triplet-sensitized deracemization. Angew. Chem. Int. Ed. 58, 3538–3541 (2019). This article reports the first example of visible light-driven highly enantioselective deracemization of centrally chiral cyclopropanes via triplet sensitization, and could also be coupled with photoinduced di-π-methane rearrangement reaction.
Zimmerman, H. E. & Armesto, D. Synthetic aspects of the di-π-methane rearrangement. Chem. Rev. 96, 3065–3112 (1996).
Li, X. et al. Photochemically induced ring opening of spirocyclopropyl oxindoles: evidence for a triplet 1,3-diradical intermediate and deracemization by a chiral sensitizer. Angew. Chem. Int. Ed. 59, 21640–21647 (2020).
Hostmann, T., Molloy, J. J., Bussmann, K. & Gilmour, R. Light-enabled enantiodivergence: stereospecific reduction of activated alkenes using a single organocatalyst enantiomer. Org. Lett. 21, 10164–10168 (2019).
Onneken, C., Bussmann, K. & Gilmour, R. Inverting external asymmetric induction via selective energy transfer catalysis: a strategy to β-chiral phosphonate antipodes. Angew. Chem. Int. Ed. 59, 330–334 (2020).
Strieth-Kalthoff, F., James, M. J., Teders, M., Pitzer, L. & Glorius, F. Energy transfer catalysis mediated by visible light: principles, applications, directions. Chem. Soc. Rev. 47, 7190–7202 (2018).
Zhou, Q. Q., Zou, Y. Q., Lu, L. Q. & Xiao, W. J. Visible-light-induced organic photochemical reactions through energy-transfer pathways. Angew. Chem. Int. Ed. 58, 1586–1604 (2019).
Strieth-Kalthoff, F. & Glorius, F. Triplet energy transfer photocatalysis: unlocking the next level. Chem 6, 1888–1903 (2020).
Huang, M., Zhang, L., Pan, T. & Luo, S. Deracemization through photochemical E/Z isomerization of enamines. Science 375, 869–874 (2022). This article describes a highly enantioselective deracemization reaction of α-branched aldehydes for the synthesis of α-tertiary carbonyls by visible light-driven triplet sensitization-mediated E → Z isomerization of the in situ-formed enamines by merging a chiral aminocatalyst and the photocatalyst Ir(ppy)3.
Nicewicz, D. A. & MacMillan, D. W. C. Merging photoredox catalysis with organocatalysis: the direct asymmetric alkylation of aldehydes. Science 322, 77–80 (2008).
Arceo, E., Jurberg, I. D., Alvarez-Fernandez, A. & Melchiorre, P. Photochemical activity of a key donor–acceptor complex can drive stereoselective catalytic α-alkylation of aldehydes. Nat. Chem. 5, 750–756 (2013).
Silvi, M. & Melchiorre, P. Enhancing the potential of enantioselective organocatalysis with light. Nature 554, 41–49 (2018).
Huo, H. et al. Asymmetric photoredox transition-metal catalysis activated by visible light. Nature 515, 100–103 (2014).
Skubi, K. L. et al. Enantioselective excited-state photoreactions controlled by a chiral hydrogen-bonding iridium sensitizer. J. Am. Chem. Soc. 139, 17186–17192 (2017).
Ding, W. et al. Bifunctional photocatalysts for enantioselective aerobic oxidation of β-ketoesters. J. Am. Chem. Soc. 139, 63–66 (2017).
Genzink, M. J., Kidd, J. B., Swords, W. B. & Yoon, T. P. Chiral photocatalyst structures in asymmetric photochemical synthesis. Chem. Rev. 122, 1654–1716 (2022).
Musacchio, A. J. et al. Catalytic intermolecular hydroaminations of unactivated olefins with secondary alkyl amines. Science 355, 727–730 (2017).
Shin, N. Y., Ryss, J. M., Zhang, X., Miller, S. J. & Knowles, R. R. Light-driven deracemization enabled by excited-state electron transfer. Science 366, 364–369 (2019). This article reports the first example of visible light-driven highly enantioselective deracemization of ureas by SET, proton transfer and HAT.
Wendlandt, A. E. Photocatalytic deracemization fixes the mix. Science 366, 304–305 (2019).
Alexeeva, M., Enright, A., Dawson, M. J., Mahmoudian, M. & Turner, N. J. Deracemization of α-methylbenzylamine using an enzyme obtained by in vitro evolution. Angew. Chem. Int. Ed. 41, 3177–3180 (2002).
Lackner, A. D., Samant, A. V. & Toste, F. D. Single-operation deracemization of 3H-indolines and tetrahydroquinolines enabled by phase separation. J. Am. Chem. Soc. 135, 14090–14093 (2013).
Ji, Y., Shi, L., Chen, M. W., Feng, G. S. & Zhou, Y. G. Concise redox deracemization of secondary and tertiary amines with a tetrahydroisoquinoline core via a nonenzymatic process. J. Am. Chem. Soc. 137, 10496–10499 (2015).
Tsunoda, T., Kaku, H., Nagaku, M. & Okuyama, E. Deracemization of 2-alkylcyclohexanones utilizing host–guest molecular association with optically active host compounds in basic suspension media. Tetrahedron Lett. 38, 7759–7760 (1997).
Kaku, H. et al. A method to prepare optically active acyclic α-benzyl ketones by thermodynamically controlled deracemization. Eur. J. Org. Chem. 2013, 8208–8213 (2013).
Kosmrlj, J., Weigel, L. O., Evans, D. A., Downey, C. W. & Wu, J. Unfunctionalized, α-epimerizable nonracemic ketones and aldehydes can be accessed by crystallization-induced dynamic resolution of imines. J. Am. Chem. Soc. 125, 3208–3209 (2003).
Zhang, C. et al. Catalytic α-deracemization of ketones enabled by photoredox deprotonation and enantioselective protonation. J. Am. Chem. Soc. 143, 13393–13400 (2021). This article discloses a novel strategy for photoinduced deracemization of carbonyl compounds using a single chiral-at-rhodium photocatalyst, which involves a sequential SET, HAT and proton transfer process.
Steinlandt, P. S., Zuo, W., Harms, K. & Meggers, E. Bis-cyclometalated indazole chiral-at-rhodium catalyst for asymmetric photoredox cyanoalkylations. Chem. Eur. J. 25, 15333–15340 (2019).
Dorman, G., Nakamura, H., Pulsipher, A. & Prestwich, G. D. The life of pi star: exploring the exciting and forbidden worlds of the benzophenone photophore. Chem. Rev. 116, 15284–15398 (2016).
Capaldo, L., Ravelli, D. & Fagnoni, M. Direct photocatalyzed hydrogen atom transfer (HAT) for aliphatic C–H bonds elaboration. Chem. Rev. 122, 1875–1924 (2022).
Grosskopf, J. et al. Photochemical deracemization at sp3-hybridized carbon centers via a reversible hydrogen atom transfer. J. Am. Chem. Soc. 143, 21241–21245 (2021).
Bauer, A., Westkämper, F., Grimme, S. & Bach, T. Catalytic enantioselective reactions driven by photoinduced electron transfer. Nature 436, 1139–1140 (2005).
Konnert, L., Lamaty, F., Martinez, J. & Colacino, E. Recent advances in the synthesis of hydantoins: the state of the art of a valuable scaffold. Chem. Rev. 117, 13757–13809 (2017).
Wu, S., Snajdrova, R., Moore, J. C., Baldenius, K. & Bornscheuer, U. T. Biocatalysis: enzymatic synthesis for industrial applications. Angew. Chem. Int. Ed. 60, 88–119 (2021).
Skubi, K. L., Blum, T. R. & Yoon, T. P. Dual catalysis strategies in photochemical synthesis. Chem. Rev. 116, 10035–10074 (2016).
Twilton, J. et al. The merger of transition metal and photocatalysis. Nat. Rev. Chem. 1, 0052 (2017).
Lu, F. D., He, G. F., Lu, L. Q. & Xiao, W. J. Metallaphotoredox catalysis for multicomponent coupling reactions. Green. Chem. 23, 5379–5393 (2021).
Zhang, Z. & Hu, X. Visible-light-driven catalytic deracemization of secondary alcohols. Angew. Chem. Int. Ed. 60, 22833–22838 (2021).
Bierbaumer, S. et al. Synthesis of enantiopure sulfoxides by concurrent photocatalytic oxidation and biocatalytic reduction. Angew. Chem. Int. Ed. 61, e202117103 (2022).
Fiorito, D., Scaringi, S. & Mazet, C. Transition metal-catalyzed alkene isomerization as an enabling technology in tandem, sequential and domino processes. Chem. Soc. Rev. 50, 1391–1406 (2021).
Massad, I. & Marek, I. Alkene isomerization through allylmetals as a strategic tool in stereoselective synthesis. ACS Catal. 10, 5793–5804 (2020).
Singh, K., Staig, S. J. & Weaver, J. D. Facile synthesis of Z-alkenes via uphill catalysis. J. Am. Chem. Soc. 136, 5275–5278 (2014).
Metternich, J. B. & Gilmour, R. A bio-inspired, catalytic E → Z isomerization of activated olefins. J. Am. Chem. Soc. 137, 11254–11257 (2015).
DeHovitz, J. S. & Hyster, T. K. Photoinduced dynamic radical processes for isomerizations, deracemizations, and dynamic kinetic resolutions. ACS Catal. 12, 8911–8924 (2022).
Piva, O. & Pete, J. P. Highly enantioselective protonation of photodienols an unusual substituent effect on the induced chirality. Tetrahedron Lett. 31, 5157–5160 (1990).
Piva, O., Mortezaei, R., Henin, F., Muzart, J. & Pete, J. P. Highly enantioselective photodeconjugation of α,β-unsaturated esters. origin of the chiral discrimination. J. Am. Chem. Soc. 112, 9263–9272 (1990).
Morack, T., Onneken, C., Nakakohara, H., Mück-Lichtenfeld, C. & Gilmour, R. Enantiodivergent prenylation via deconjugative isomerization. ACS Catal. 11, 11929–11937 (2021). This article describes a practical visible light-driven deconjugative isomerization reaction of α,β-unsaturated ketones, and an enantioselective variant is also achieved using a chiral aminocatalyst.
Schwarz, J. L., Schafers, F., Tlahuext-Aca, A., Luckemeier, L. & Glorius, F. Diastereoselective allylation of aldehydes by dual photoredox and chromium catalysis. J. Am. Chem. Soc. 140, 12705–12709 (2018).
Schäfers, F. et al. Direct access to monoprotected homoallylic 1,2-diols via dual chromium/photoredox catalysis. ACS Catal. 10, 11841–11847 (2020).
Mitsunuma, H., Tanabe, S., Fuse, H., Ohkubo, K. & Kanai, M. Catalytic asymmetric allylation of aldehydes with alkenes through allylic C(sp3)–H functionalization mediated by organophotoredox and chiral chromium hybrid catalysis. Chem. Sci. 10, 3459–3465 (2019).
Tanabe, S., Mitsunuma, H. & Kanai, M. Catalytic allylation of aldehydes using unactivated alkenes. J. Am. Chem. Soc. 142, 12374–12381 (2020).
Castro, C. E. & Kray, W. C. The cleavage of bonds by low valent transition metal ions. The homogeneous reduction of alkyl halides by chromous sulfate. J. Am. Chem. Soc. 85, 2768–2773 (1963).
Omoto, M., Kato, N., Sogon, T. & Mori, A. Revisit to the reduction of allylic chlorides to less substituted olefins by a low-valent chromium species in the presence of a proton source. Tetrahedron Lett. 42, 939–941 (2001).
Zhao, K. & Knowles, R. R. Contra-thermodynamic positional isomerization of olefins. J. Am. Chem. Soc. 144, 137–144 (2022). This article reports a highly efficient method for contra-thermodynamic positional isomerization of alkenes by visible light-driven allylmetallation and protodemetallation.
Occhialini, G., Palani, V. & Wendlandt, A. E. Catalytic, contra-thermodynamic positional alkene isomerization. J. Am. Chem. Soc. 144, 145–152 (2022). This article describes an efficient method for contra-thermodynamic internal to terminal alkene isomerization by a visible light-driven sequential HAT/allylmetallation/biomolecular homolytic substitution process.
de Leder Kremer, R. M. & Gallo-Rodriguez, C. Naturally occurring monosaccharides: properties and synthesis. Adv. Carbohydr. Chem. Biochem. 59, 9–67 (2004).
Kudo, F., Hoshi, S., Kawashima, T., Kamachi, T. & Eguchi, T. Characterization of a radical S-adenosyl-L-methionine epimerase, NeoN, in the last step of neomycin B biosynthesis. J. Am. Chem. Soc. 136, 13909–13915 (2014).
Wang, Y., Carder, H. M. & Wendlandt, A. E. Synthesis of rare sugar isomers through site-selective epimerization. Nature 578, 403–408 (2020). This article establishes a conceptual feasibility of contra-thermodynamic dynamic epimerization of biomass-derived sugars by visible light-driven hydrogen atom abstraction and hydrogen atom donation.
Zhang, Y. A., Gu, X. & Wendlandt, A. E. A change from kinetic to thermodynamic control enables trans-selective stereochemical editing of vicinal diols. J. Am. Chem. Soc. 144, 599–605 (2022).
Oswood, C. J. & MacMillan, D. W. C. Selective isomerization via transient thermodynamic control: dynamic epimerization of trans to cis diols. J. Am. Chem. Soc. 144, 93–98 (2022).
Alektiar, S. N., Williams, O. P. & Wickens, Z. K. An alkene, a photon, and a catalyst walk into a bar; Zaitsev wasn’t invited. Trends Chem. 4, 467–470 (2022).
Buglioni, L., Raymenants, F., Slattery, A., Zondag, S. D. A. & Noel, T. Technological innovations in photochemistry for organic synthesis: flow chemistry, high-throughput experimentation, scale-up, and photoelectrochemistry. Chem. Rev. 122, 2752–2906 (2022).
Rehm, T. H. Reactor technology concepts for flow photochemistry. ChemPhotoChem 4, 235–254 (2020).
Wang, P.-Z., Xiao, W.-J. & Chen, J.-R. Light empowers contra-thermodynamic stereochemical editing. Preprint at ChemRxiv https://chemrxiv.org/engage/chemrxiv/article-details/628464bd6b12b66a7875dae8 (2022).
Acknowledgements
The authors are grateful for financial support from the National Natural Science Foundation of China (22171099, 21971081, 22171099, 91856119, 21772053, 21820102003 and 91956201), the Program of Introducing Talents of Discipline to Universities of China (111 Program, B17019) and the Excellent Doctoral Dissertation Cultivation Grant to P.-Z.W. from CCNU (2022YBZZ003). They also thank all of the anonymous referees for their invaluable suggestions and comments on preparing this manuscript.
Author information
Authors and Affiliations
Contributions
P.-Z.W. and J.-R.C. contributed to the literature search, the preparation of figures and writing of the article. All authors contributed to editing the manuscript prior to submission. W.-J.X. and J.-R.C. conceived and directed the project.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Chemistry thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, PZ., Xiao, WJ. & Chen, JR. Light-empowered contra-thermodynamic stereochemical editing. Nat Rev Chem 7, 35–50 (2023). https://doi.org/10.1038/s41570-022-00441-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41570-022-00441-2
This article is cited by
-
Endergonic synthesis driven by chemical fuelling
Nature Synthesis (2024)
-
Photocatalytic Z/E isomerization unlocking the stereodivergent construction of axially chiral alkene frameworks
Nature Communications (2024)
-
Ratcheting synthesis
Nature Reviews Chemistry (2023)