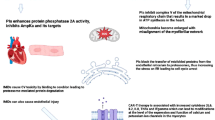
Abstract
Remarkable progress has been made in the development of new therapies for cancer, dramatically changing the landscape of treatment approaches for several malignancies and continuing to increase patient survival. Accordingly, adverse effects of cancer therapies that interfere with the continuation of best-possible care, induce life-threatening risks or lead to long-term morbidity are gaining increasing importance. Cardiovascular toxic effects of cancer therapeutics and radiation therapy are the epitome of such concerns, and proper knowledge, interpretation and management are needed and have to be placed within the context of the overall care of individual patients with cancer. Furthermore, the cardiotoxicity spectrum has broadened to include myocarditis with immune checkpoint inhibitors and cardiac dysfunction in the setting of cytokine release syndrome with chimeric antigen receptor T cell therapy. An increase in the incidence of arrhythmias related to inflammation such as atrial fibrillation can also be expected, in addition to the broadening set of cancer therapeutics that can induce prolongation of the corrected QT interval. Therefore, cardiologists of today have to be familiar not only with the cardiotoxicity associated with traditional cancer therapies, such as anthracycline, trastuzumab or radiation therapy, but even more so with an ever-increasing repertoire of therapeutics. This Review provides this information, summarizing the latest developments at the juncture of cardiology, oncology and haematology.
Key points
Cancer therapy has evolved from the administration of chemical compounds and radiation therapy to the use of targeted agents and immunotherapies.
Along with these developments, the cardiovascular toxicity spectrum of cancer therapies has been changing but cardiac toxicity remains of greatest concern.
Inflammatory and immune mechanisms have to be taken into account when considering cardiotoxicity in patients receiving immune checkpoint inhibitor or chimeric antigen receptor T cell therapies.
With the newer cancer therapies, atrial fibrillation is emerging as the most relevant and practically challenging arrhythmia in patients with cancer.
Corrected QT interval prolongation, ventricular arrhythmias and cardiac arrest can also occur with many of the newer targeted agents.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Global Burden of Disease Cancer Collaboration. The global burden of cancer 2013. JAMA Oncol. 1, 505–527 (2015).
Bluethmann, S. M., Mariotto, A. B. & Rowland, J. H. Anticipating the “silver tsunami”: prevalence trajectories and comorbidity burden among older cancer survivors in the United States. Cancer Epidemiol. Biomarkers Prev. 25, 1029–1036 (2016).
Ewer, M. S. & Ewer, S. M. Cardiotoxicity of anticancer treatments: what the cardiologist needs to know. Nat. Rev. Cardiol. 7, 564–575 (2010).
Ewer, M. S. & Ewer, S. M. Cardiotoxicity of anticancer treatments. Nat. Rev. Cardiol. 12, 547–558 (2015).
Herrmann, J. Vascular toxic effects of cancer therapies. Nat. Rev. Cardiol. https://doi.org/10.1038/s41569-020-0347-2 (2020).
Gianni, L. et al. Anthracycline cardiotoxicity: from bench to bedside. J. Clin. Oncol. 26, 3777–3784 (2008).
Herrmann, J. et al. Evaluation and management of patients with heart disease and cancer: cardio-oncology. Mayo Clin. Proc. 89, 1287–1306 (2014).
Bristow, M. R. et al. Early anthracycline cardiotoxicity. Am. J. Med. 65, 823–832 (1978).
Bristow, M. R., Billingham, M. E., Mason, J. W. & Daniels, J. R. Clinical spectrum of anthracycline antibiotic cardiotoxicity. Cancer Treat. Rep. 62, 873–879 (1978).
Ferrans, V. J. Overview of cardiac pathology in relation to anthracycline cardiotoxicity. Cancer Treat. Rep. 62, 955–961 (1978).
Berry, G. J. & Jorden, M. Pathology of radiation and anthracycline cardiotoxicity. Pediatr. Blood Cancer 44, 630–637 (2005).
Ewer, M. S. et al. A comparison of cardiac biopsy grades and ejection fraction estimations in patients receiving Adriamycin. J. Clin. Oncol. 2, 112–117 (1984).
Nousiainen, T., Jantunen, E., Vanninen, E. & Hartikainen, J. Early decline in left ventricular ejection fraction predicts doxorubicin cardiotoxicity in lymphoma patients. Br. J. Cancer 86, 1697–1700 (2002).
Felker, G. M. et al. Underlying causes and long-term survival in patients with initially unexplained cardiomyopathy. N. Engl. J. Med. 342, 1077–1084 (2000).
Mazur, M. et al. Burden of cardiac arrhythmias in patients with anthracycline-related cardiomyopathy. JACC Clin. Electrophysiol. 3, 139–150 (2017).
Kremer, L. C., van der Pal, H. J., Offringa, M., van Dalen, E. C. & Voute, P. A. Frequency and risk factors of subclinical cardiotoxicity after anthracycline therapy in children: a systematic review. Ann. Oncol. 13, 819–829 (2002).
Zhang, S. et al. Identification of the molecular basis of doxorubicin-induced cardiotoxicity. Nat. Med. 18, 1639–1642 (2012).
Chen, B., Peng, X., Pentassuglia, L., Lim, C. C. & Sawyer, D. B. Molecular and cellular mechanisms of anthracycline cardiotoxicity. Cardiovasc. Toxicol. 7, 114–121 (2007).
Varga, Z. V., Ferdinandy, P., Liaudet, L. & Pacher, P. Drug-induced mitochondrial dysfunction and cardiotoxicity. Am. J. Physiol. Heart Circ. Physiol 309, H1453–H1467 (2015).
Ichikawa, Y. et al. Cardiotoxicity of doxorubicin is mediated through mitochondrial iron accumulation. J. Clin. Invest. 124, 617–630 (2014).
Lebrecht, D., Setzer, B., Ketelsen, U. P., Haberstroh, J. & Walker, U. A. Time-dependent and tissue-specific accumulation of mtDNA and respiratory chain defects in chronic doxorubicin cardiomyopathy. Circulation 108, 2423–2429 (2003).
De Angelis, A. et al. Anthracycline cardiomyopathy is mediated by depletion of the cardiac stem cell pool and is rescued by restoration of progenitor cell function. Circulation 121, 276–292 (2010).
Piegari, E. et al. Doxorubicin induces senescence and impairs function of human cardiac progenitor cells. Basic Res. Cardiol. 108, 334 (2013).
Ali, M. K., Ewer, M. S., Gibbs, H. R., Swafford, J. & Graff, K. L. Late doxorubicin-associated cardiotoxicity in children. The possible role of intercurrent viral infection. Cancer 74, 182–188 (1994).
Carter, P. et al. Humanization of an anti-p185HER2 antibody for human cancer therapy. Proc. Natl Acad. Sci. USA 89, 4285–4289 (1992).
Moasser, M. M. The oncogene HER2: its signaling and transforming functions and its role in human cancer pathogenesis. Oncogene 26, 6469–6487 (2007).
Moasser, M. M. & Krop, I. E. The evolving landscape of HER2 targeting in breast cancer. JAMA Oncol. 1, 1154–1161 (2015).
Slamon, D. J. et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N. Engl. J. Med. 344, 783–792 (2001).
Farolfi, A. et al. Trastuzumab-induced cardiotoxicity in early breast cancer patients: a retrospective study of possible risk and protective factors. Heart 99, 634–639 (2013).
Nowsheen, S. et al. Trastuzumab in female breast cancer patients with reduced left ventricular ejection fraction. J. Am. Heart Assoc. 7, e008637 (2018).
Ewer, M. S. & Lippman, S. M. Type II chemotherapy-related cardiac dysfunction: time to recognize a new entity. J. Clin. Oncol. 23, 2900–2902 (2005).
Ewer, M. S. et al. Reversibility of trastuzumab-related cardiotoxicity: new insights based on clinical course and response to medical treatment. J. Clin. Oncol. 23, 7820–7826 (2005).
Tan, T. C. et al. Time trends of left ventricular ejection fraction and myocardial deformation indices in a cohort of women with breast cancer treated with anthracyclines, taxanes, and trastuzumab. J. Am. Soc. Echocardiogr. 28, 509–514 (2015).
Yu, A. F. et al. Trastuzumab interruption and treatment-induced cardiotoxicity in early HER2-positive breast cancer. Breast Cancer Res. Treat. 149, 489–495 (2015).
Guarneri, V. et al. Long-term cardiac tolerability of trastuzumab in metastatic breast cancer: the M.D. Anderson Cancer Center experience. J. Clin. Oncol. 24, 4107–4115 (2006).
Cardinale, D. et al. Trastuzumab-induced cardiotoxicity: clinical and prognostic implications of troponin I evaluation. J. Clin. Oncol. 28, 3910–3916 (2010).
ElZarrad, M. K. et al. Trastuzumab alters the expression of genes essential for cardiac function and induces ultrastructural changes of cardiomyocytes in mice. PLoS One 8, e79543 (2013).
de Azambuja, E. et al. Trastuzumab-associated cardiac events at 8 years of median follow-up in the Herceptin Adjuvant trial (BIG 1-01). J. Clin. Oncol. 32, 2159–2165 (2014).
Chen, J. et al. Incidence of heart failure or cardiomyopathy after adjuvant trastuzumab therapy for breast cancer. J. Am. Coll. Cardiol. 60, 2504–2512 (2012).
Bowles, E. J. et al. Risk of heart failure in breast cancer patients after anthracycline and trastuzumab treatment: a retrospective cohort study. J. Natl Cancer Inst. 104, 1293–1305 (2012).
Baselga, J. et al. Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N. Engl. J. Med. 366, 109–119 (2012).
Swain, S. M. et al. Pertuzumab, trastuzumab, and docetaxel in HER2-positive metastatic breast cancer. N. Engl. J. Med. 372, 724–734 (2015).
Piccart-Gebhart, M. et al. Adjuvant lapatinib and trastuzumab for early human epidermal growth factor receptor 2-positive breast cancer: results from the randomized phase III Adjuvant Lapatinib and/or Trastuzumab Treatment Optimization trial. J. Clin. Oncol. 34, 1034–1042 (2016).
Watanabe, H. et al. Congestive heart failure during osimertinib treatment for epidermal growth factor receptor (EGFR)-mutant non-small cell lung cancer (NSCLC). Intern. Med. 56, 2195–2197 (2017).
Bhullar, K. S. et al. Kinase-targeted cancer therapies: progress, challenges and future directions. Mol. Cancer 17, 48 (2018).
Thompson, P. A., Kantarjian, H. M. & Cortes, J. E. Diagnosis and treatment of chronic myeloid leukemia in 2015. Mayo Clin. Proc. 90, 1440–1454 (2015).
Kerkela, R. et al. Cardiotoxicity of the cancer therapeutic agent imatinib mesylate. Nat. Med. 12, 908–916 (2006).
Herman, E. H. et al. A multifaceted evaluation of imatinib-induced cardiotoxicity in the rat. Toxicol. Pathol. 39, 1091–1106 (2011).
Wolf, A. et al. Imatinib does not induce cardiotoxicity at clinically relevant concentrations in preclinical studies. Leuk. Res. 34, 1180–1188 (2010).
Ribeiro, A. L. et al. An evaluation of the cardiotoxicity of imatinib mesylate. Leuk. Res. 32, 1809–1814 (2008).
Estabragh, Z. R. et al. A prospective evaluation of cardiac function in patients with chronic myeloid leukaemia treated with imatinib. Leuk. Res. 35, 49–51 (2011).
Force, T., Krause, D. S. & Van Etten, R. A. Molecular mechanisms of cardiotoxicity of tyrosine kinase inhibition. Nat. Rev. Cancer 7, 332–344 (2007).
Greineder, C. F., Kohnstamm, S. & Ky, B. Heart failure associated with sunitinib: lessons learned from animal models. Curr. Hypertens. Rep. 13, 436–441 (2011).
Hasinoff, B. B., Patel, D. & O’Hara, K. A. Mechanisms of myocyte cytotoxicity induced by the multiple receptor tyrosine kinase inhibitor sunitinib. Mol. Pharmacol. 74, 1722–1728 (2008).
Hasinoff, B. B. & Patel, D. The lack of target specificity of small molecule anticancer kinase inhibitors is correlated with their ability to damage myocytes in vitro. Toxicol. Appl. Pharmacol. 249, 132–139 (2010).
Hasinoff, B. B. The cardiotoxicity and myocyte damage caused by small molecule anticancer tyrosine kinase inhibitors is correlated with lack of target specificity. Toxicol. Appl. Pharmacol. 244, 190–195 (2010).
Will, Y. et al. Effect of the multitargeted tyrosine kinase inhibitors imatinib, dasatinib, sunitinib, and sorafenib on mitochondrial function in isolated rat heart mitochondria and H9c2 cells. Toxicol. Sci. 106, 153–161 (2008).
Jacob, F. et al. Analysis of tyrosine kinase inhibitor-mediated decline in contractile force in rat engineered heart tissue. PLoS One 11, e0145937 (2016).
Lamore, S. D. et al. Deconvoluting kinase inhibitor induced cardiotoxicity. Toxicol. Sci. 158, 213–226 (2017).
Stuhlmiller, T. J. et al. Kinome and transcriptome profiling reveal broad and distinct activities of erlotinib, sunitinib, and sorafenib in the mouse heart and suggest cardiotoxicity from combined signal transducer and activator of transcription and epidermal growth factor receptor inhibition. J. Am. Heart Assoc. 6, e006635 (2017).
Sharma, A. et al. High-throughput screening of tyrosine kinase inhibitor cardiotoxicity with human induced pluripotent stem cells. Sci. Transl Med. 9, eaaf2584 (2017).
Shim, J. V. et al. Mechanistic systems modeling to improve understanding and prediction of cardiotoxicity caused by targeted cancer therapeutics. Front. Physiol. 8, 651 (2017).
Brown, S. A., Sandhu, N. & Herrmann, J. Systems biology approaches to adverse drug effects: the example of cardio-oncology. Nat. Rev. Clin. Oncol. 12, 718–731 (2015).
Brown, S. A., Nhola, L. & Herrmann, J. Cardiovascular toxicities of small molecule tyrosine kinase inhibitors: an opportunity for systems-based approaches. Clin. Pharmacol. Ther. 101, 65–80 (2017).
Plana, J. C. et al. Expert consensus for multimodality imaging evaluation of adult patients during and after cancer therapy: a report from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 27, 911–939 (2014).
Cardinale, D. et al. Prognostic value of troponin I in cardiac risk stratification of cancer patients undergoing high-dose chemotherapy. Circulation 109, 2749–2754 (2004).
Negishi, T., Thavendiranathan, P., Negishi, K. & Marwick, T. H. Rationale and design of the strain surveillance of chemotherapy for improving cardiovascular outcomes: the SUCCOUR trial. JACC Cardiovasc. Imaging 11, 1098–1105 (2018).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03879629 (2019)
Yancy, C. W. et al. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J. Am. Coll. Cardiol. 70, 776–803 (2017).
Yancy, C. W. et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 128, e240–e327 (2013).
Curigliano, G. et al. Cardiovascular toxicity induced by chemotherapy, targeted agents and radiotherapy: ESMO clinical practice guidelines. Ann. Oncol. 23, vii155–vii166 (2012).
Russell, R. R. et al. The role and clinical effectiveness of multimodality imaging in the management of cardiac complications of cancer and cancer therapy. J. Nucl. Cardiol. 23, 856–884 (2016).
Chang, H. M., Okwuosa, T. M., Scarabelli, T., Moudgil, R. & Yeh, E. T. H. Cardiovascular complications of cancer therapy: best practices in diagnosis, prevention, and management: part 2. J. Am. Coll. Cardiol. 70, 2552–2565 (2017).
Alexander, J. et al. Serial assessment of doxorubicin cardiotoxicity with quantitative radionuclide angiocardiography. N. Engl. J. Med. 300, 278–283 (1979).
Avila, M. S. et al. Carvedilol for prevention of chemotherapy-related cardiotoxicity: the CECCY trial. J. Am. Coll. Cardiol. 71, 2281–2290 (2018).
Urbanek, K. et al. Cardioprotection by targeting the pool of resident and extracardiac progenitors. Curr. Drug Targets 16, 884–894 (2015).
Hamed, S. et al. Erythropoietin improves myocardial performance in doxorubicin-induced cardiomyopathy. Eur. Heart J. 27, 1876–1883 (2006).
Hoch, M. et al. Erythropoietin preserves the endothelial differentiation capacity of cardiac progenitor cells and reduces heart failure during anticancer therapies. Cell Stem Cell 9, 131–143 (2011).
Burridge, P. W. et al. Human induced pluripotent stem cell-derived cardiomyocytes recapitulate the predilection of breast cancer patients to doxorubicin-induced cardiotoxicity. Nat. Med. 22, 547–556 (2016).
Chen, I. Y., Matsa, E. & Wu, J. C. Induced pluripotent stem cells: at the heart of cardiovascular precision medicine. Nat. Rev. Cardiol. 13, 333–349 (2016).
Pituskin, E. et al. Multidisciplinary approach to novel therapies in cardio-oncology research (MANTICORE 101-Breast): a randomized trial for the prevention of trastuzumab-associated cardiotoxicity. J. Clin. Oncol. 35, 870–877 (2017).
Boekhout, A. H. et al. Angiotensin II-receptor inhibition with candesartan to prevent trastuzumab-related cardiotoxic effects in patients with early breast cancer: a randomized clinical trial. JAMA Oncol. 2, 1030–1037 (2016).
Nowsheen, S. et al. Trastuzumab in female breast cancer patients with reduced left ventricular ejection fraction. J. Am. Heart Assoc. 7, e008637 (2018).
Crone, S. A. et al. ErbB2 is essential in the prevention of dilated cardiomyopathy. Nat. Med. 8, 459–465 (2002).
Herrmann, J., Herrmann, S. M. & Haddad, T. C. New-onset heart failure in association with severe hypertension during trastuzumab therapy. Mayo Clin. Proc. 89, 1734–1739 (2014).
Gilchrist, S. C. et al. Cardio-oncology rehabilitation to manage cardiovascular outcomes in cancer patients and survivors: a scientific statement from the American Heart Association. Circulation 139, e997–e1012 (2019).
Squires, R. W., Shultz, A. M. & Herrmann, J. Exercise training and cardiovascular health in cancer patients. Curr. Oncol. Rep. 20, 27 (2018).
Cerny, J., Hassan, A., Smith, C. & Piperdi, B. Coronary vasospasm with myocardial stunning in a patient with colon cancer receiving adjuvant chemotherapy with FOLFOX regimen. Clin. Colorectal Cancer 8, 55–58 (2009).
Basselin, C. et al. 5-Fluorouracil-induced Tako-Tsubo-like syndrome. Pharmacotherapy 31, 226 (2011).
Gianni, M., Dentali, F. & Lonn, E. 5 flourouracil-induced apical ballooning syndrome: a case report. Blood Coagul. Fibrinolysis 20, 306–308 (2009).
Grunwald, M. R., Howie, L. & Diaz, L. A. Jr. Takotsubo cardiomyopathy and fluorouracil: case report and review of the literature. J. Clin. Oncol. 30, e11–e14 (2012).
Kobayashi, N. et al. A case of takotsubo cardiomyopathy during 5-fluorouracil treatment for rectal adenocarcinoma. J. Nippon Med. Sch. 76, 27–33 (2009).
Ozturk, M. A., Ozveren, O., Cinar, V., Erdik, B. & Oyan, B. Takotsubo syndrome: an underdiagnosed complication of 5-fluorouracil mimicking acute myocardial infarction. Blood Coagul. Fibrinolysis 24, 90–94 (2013).
S, Y. H., Tornvall, P., Tornerud, M. & Henareh, L. Capecitabine caused cardiogenic shock through induction of global takotsubo syndrome. Cardiovasc. Revasc. Med. 14, 57–61 (2013).
Stewart, T., Pavlakis, N. & Ward, M. Cardiotoxicity with 5-fluorouracil and capecitabine: more than just vasospastic angina. Intern. Med. J. 40, 303–307 (2010).
Dechant, C. et al. Acute reversible heart failure caused by coronary vasoconstriction due to continuous 5-fluorouracil combination chemotherapy. Case Rep. Oncol. 5, 296–301 (2012).
Tsibiribi, P. et al. Cardiac lesions induced by 5-fluorouracil in the rabbit. Hum. Exp. Toxicol. 25, 305–309 (2006).
Martin, M. et al. Lethal cardiac toxicity after cisplatin and 5-fluorouracil chemotherapy. Report of a case with necropsy study. Am. J. Clin. Oncol. 12, 229–234 (1989).
Eskandari, M. R., Moghaddam, F., Shahraki, J. & Pourahmad, J. A comparison of cardiomyocyte cytotoxic mechanisms for 5-fluorouracil and its pro-drug capecitabine. Xenobiotica 45, 79–87 (2015).
Focaccetti, C. et al. Effects of 5-fluorouracil on morphology, cell cycle, proliferation, apoptosis, autophagy and ROS production in endothelial cells and cardiomyocytes. PLoS One 10, e0115686 (2015).
Lamberti, M. et al. A mechanistic study on the cardiotoxicity of 5-fluorouracil in vitro and clinical and occupational perspectives. Toxicol. Lett. 227, 151–156 (2014).
Lischke, J., Lang, C., Sawodny, O. & Feuer, R. Impairment of energy metabolism in cardiomyocytes caused by 5-FU catabolites can be compensated by administration of amino acids. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2015, 5363–5366 (2015).
Polk, A., Vistisen, K., Vaage-Nilsen, M. & Nielsen, D. L. A systematic review of the pathophysiology of 5-fluorouracil-induced cardiotoxicity. BMC Pharmacol. Toxicol. 15, 47 (2014).
Sara, J. D. et al. 5-fluorouracil and cardiotoxicity: a review. Ther. Adv. Med. Oncol. 10, 1758835918780140 (2018).
Arellano, M., Malet-Martino, M., Martino, R. & Gires, P. The anti-cancer drug 5-fluorouracil is metabolized by the isolated perfused rat liver and in rats into highly toxic fluoroacetate. Br. J. Cancer 77, 79–86 (1998).
Matsubara, I., Kamiya, J. & Imai, S. Cardiotoxic effects of 5-fluorouracil in the guinea pig. Jpn. J. Pharmacol. 30, 871–879 (1980).
Diasio, R. B. The role of dihydropyrimidine dehydrogenase (DPD) modulation in 5-FU pharmacology. Oncology 12, 23–27 (1998).
Papanastasopoulos, P. & Stebbing, J. Molecular basis of 5-fluorouracil-related toxicity: lessons from clinical practice. Anticancer Res. 34, 1531–1535 (2014).
Franco, T. H., Khan, A., Joshi, V. & Thomas, B. Takotsubo cardiomyopathy in two men receiving bevacizumab for metastatic cancer. Ther. Clin. Risk Manag. 4, 1367–1370 (2008).
Numico, G. et al. Takotsubo syndrome in a patient treated with sunitinib for renal cancer. J. Clin. Oncol. 30, e218–e220 (2012).
Touyz, R. M. & Herrmann, J. Cardiotoxicity with vascular endothelial growth factor inhibitor therapy. NPJ Precis. Oncol. 2, 13 (2018).
Baffert, F. et al. Cellular changes in normal blood capillaries undergoing regression after inhibition of VEGF signaling. Am. J. Physiol. Heart Circ. Physiol. 290, H547–H559 (2006).
Kamba, T. et al. VEGF-dependent plasticity of fenestrated capillaries in the normal adult microvasculature. Am. J. Physiol. Heart Circ. Physiol 290, H560–H576 (2006).
Lazarus, A. & Keshet, E. Vascular endothelial growth factor and vascular homeostasis. Proc. Am. Thorac. Soc. 8, 508–511 (2011).
Maharaj, A. S. & D’Amore, P. A. Roles for VEGF in the adult. Microvasc. Res. 74, 100–113 (2007).
June, C. H. & Sadelain, M. Chimeric antigen receptor therapy. N. Engl. J. Med. 379, 64–73 (2018).
Brudno, J. N. & Kochenderfer, J. N. Chimeric antigen receptor T-cell therapies for lymphoma. Nat. Rev. Clin. Oncol. 15, 31–46 (2018).
Brichard, V. G. & Godechal, Q. MAGE-A3-specific anticancer immunotherapy in the clinical practice. Oncoimmunology 2, e25995 (2013).
Linette, G. P. et al. Cardiovascular toxicity and titin cross-reactivity of affinity-enhanced T cells in myeloma and melanoma. Blood 122, 863–871 (2013).
Cameron, B. J. et al. Identification of a titin-derived HLA-A1-presented peptide as a cross-reactive target for engineered MAGE A3-directed T cells. Sci. Transl Med. 5, 197ra103 (2013).
Morgan, R. A. et al. Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol. Ther. 18, 843–851 (2010).
Ahmed, N. et al. Human epidermal growth factor receptor 2 (HER2) -specific chimeric antigen receptor-modified T cells for the immunotherapy of HER2-positive sarcoma. J. Clin. Oncol. 33, 1688–1696 (2015).
Brudno, J. N. & Kochenderfer, J. N. Toxicities of chimeric antigen receptor T cells: recognition and management. Blood 127, 3321–3330 (2016).
Bonifant, C. L., Jackson, H. J., Brentjens, R. J. & Curran, K. J. Toxicity and management in CAR T-cell therapy. Mol. Ther. Oncolytics 3, 16011 (2016).
Neelapu, S. S. et al. Chimeric antigen receptor T-cell therapy - assessment and management of toxicities. Nat. Rev. Clin. Oncol. 15, 47–62 (2018).
Sato, R. & Nasu, M. A review of sepsis-induced cardiomyopathy. J. Intensive Care 3, 48 (2015).
Krishnagopalan, S., Kumar, A., Parrillo, J. E. & Kumar, A. Myocardial dysfunction in the patient with sepsis. Curr. Opin. Crit. Care 8, 376–388 (2002).
Vallabhajosyula, S. et al. New-onset heart failure and mortality in hospital survivors of sepsis-related left ventricular dysfunction. Shock 49, 144–149 (2018).
Court, O., Kumar, A., Parrillo, J. E. & Kumar, A. Clinical review: myocardial depression in sepsis and septic shock. Crit. Care 6, 500–508 (2002).
Morelli, A. et al. Effect of heart rate control with esmolol on hemodynamic and clinical outcomes in patients with septic shock: a randomized clinical trial. JAMA 310, 1683–1691 (2013).
Kumar, A. et al. Cardiovascular response to dobutamine stress predicts outcome in severe sepsis and septic shock. Crit. Care 12, R35 (2008).
Przepiorka, D. et al. FDA approval: blinatumomab. Clin. Cancer Res. 21, 4035–4039 (2015).
Slaney, C. Y., Wang, P., Darcy, P. K. & Kershaw, M. H. CARs versus BiTEs: a comparison between T cell-redirection strategies for cancer treatment. Cancer Discov. 8, 924–934 (2018).
Pardoll, D. M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 12, 252–264 (2012).
Boutros, C. et al. Safety profiles of anti-CTLA-4 and anti-PD-1 antibodies alone and in combination. Nat. Rev. Clin. Oncol. 13, 473–486 (2016).
Sury, K., Perazella, M. A. & Shirali, A. C. Cardiorenal complications of immune checkpoint inhibitors. Nat. Rev. Nephrol. 14, 571–588 (2018).
Yang, S. & Asnani, A. Cardiotoxicities associated with immune checkpoint inhibitors. Curr. Probl. Cancer 42, 422–432 (2018).
Ederhy, S. et al. Takotsubo-like syndrome in cancer patients treated with immune checkpoint inhibitors. JACC Cardiovasc. Imaging 11, 1187–1190 (2018).
Giza, D. E. et al. Stress-induced cardiomyopathy in cancer patients. Am. J. Cardiol. 120, 2284–2288 (2017).
Ogawa, Y. Paradigm shift in radiation biology/radiation oncology-exploitation of the “H2O2 effect” for radiotherapy using low-LET (linear energy transfer) radiation such as X-rays and high-energy electrons. Cancers 8, 28 (2016).
Saiki, H. et al. Risk of heart failure with preserved ejection fraction in older women after contemporary radiotherapy for breast cancer. Circulation 135, 1388–1396 (2017).
Heselich, A. et al. High LET radiation shows no major cellular and functional effects on primary cardiomyocytes in vitro. Life Sci. Space Res. 16, 93–100 (2018).
Hughson, R. L., Helm, A. & Durante, M. Heart in space: effect of the extraterrestrial environment on the cardiovascular system. Nat. Rev. Cardiol. 15, 167–180 (2018).
Fajardo, L. F. & Stewart, J. R. Experimental radiation-induced heart disease. I. Light microscopic studies. Am. J. Pathol. 59, 299–316 (1970).
Khan, M. Y. Radiation-induced cardiomyopathy. I. An electron microscopic study of cardiac muscle cells. Am. J. Pathol. 73, 131–146 (1973).
Fajardo, L. F. & Stewart, J. R. Capillary injury preceding radiation-induced myocardial fibrosis. Radiology 101, 429–433 (1971).
Stewart, F. A., Seemann, I., Hoving, S. & Russell, N. S. Understanding radiation-induced cardiovascular damage and strategies for intervention. Clin. Oncol. 25, 617–624 (2013).
Cuomo, J. R. et al. How to prevent and manage radiation-induced coronary artery disease. Heart 104, 1647–1653 (2018).
Taunk, N. K., Haffty, B. G., Kostis, J. B. & Goyal, S. Radiation-induced heart disease: pathologic abnormalities and putative mechanisms. Front. Oncol. 5, 39 (2015).
Cosset, J. M. et al. Pericarditis and myocardial infarctions after Hodgkin’s disease therapy. Int. J. Radiat. Oncol. Biol. Phys. 21, 447–449 (1991).
Jensen, S. A. & Sorensen, J. B. Risk factors and prevention of cardiotoxicity induced by 5-fluorouracil or capecitabine. Cancer Chemother. Pharmacol. 58, 487–493 (2006).
Meyer, C. C., Calis, K. A., Burke, L. B., Walawander, C. A. & Grasela, T. H. Symptomatic cardiotoxicity associated with 5-fluorouracil. Pharmacotherapy 17, 729–736 (1997).
Clasen, S. C. et al. Fluoropyrimidine-induced cardiac toxicity: challenging the current paradigm. J. Gastrointest. Oncol. 8, 970–979 (2017).
Ma, W. W. et al. Emergency use of uridine triacetate for the prevention and treatment of life-threatening 5-fluorouracil and capecitabine toxicity. Cancer 123, 345–356 (2017).
Truitt, R. et al. Increased afterload augments sunitinib-induced cardiotoxicity in an engineered cardiac microtissue model. JACC Basic Transl Sci. 3, 265–276 (2018).
Touyz, R. M., Herrmann, S. M. S. & Herrmann, J. Vascular toxicities with VEGF inhibitor therapies-focus on hypertension and arterial thrombotic events. J. Am. Soc. Hypertens. 12, 409–425 (2018).
Chu, T. F. et al. Cardiotoxicity associated with tyrosine kinase inhibitor sunitinib. Lancet 370, 2011–2019 (2007).
Di Lorenzo, G. et al. Cardiovascular toxicity following sunitinib therapy in metastatic renal cell carcinoma: a multicenter analysis. Ann. Oncol. 20, 1535–1542 (2009).
Abdel-Qadir, H., Ethier, J. L., Lee, D. S., Thavendiranathan, P. & Amir, E. Cardiovascular toxicity of angiogenesis inhibitors in treatment of malignancy: a systematic review and meta-analysis. Cancer Treat. Rev. 53, 120–127 (2017).
Annane, D. et al. A global perspective on vasoactive agents in shock. Intensive Care Med. 44, 833–846 (2018).
Donis, N., Oury, C., Moonen, M. & Lancellotti, P. Treating cardiovascular complications of radiotherapy: a role for new pharmacotherapies. Expert Opin. Pharmacother. 19, 431–442 (2018).
Lancellotti, P. et al. Expert consensus for multi-modality imaging evaluation of cardiovascular complications of radiotherapy in adults: a report from the European Association of Cardiovascular Imaging and the American Society of Echocardiography. J. Am. Soc. Echocardiogr. 26, 1013–1032 (2013).
Iliescu, C. A. et al. SCAI expert consensus statement: evaluation, management, and special considerations of cardio-oncology patients in the cardiac catheterization laboratory (endorsed by the Cardiological Society of India, and Sociedad Latino Americana de Cardiologia Intervencionista). Catheter. Cardiovasc. Interv. 87, E202–E223 (2016).
Appelbaum, F. et al. Acute lethal carditis caused by high-dose combination chemotherapy. A unique clinical and pathological entity. Lancet 1, 58–62 (1976).
O’Connell, T. X. & Berenbaum, M. C. Cardiac and pulmonary effects of high doses of cyclophosphamide and isophosphamide. Cancer Res. 34, 1586–1591 (1974).
Dhesi, S. et al. Cyclophosphamide-induced cardiomyopathy: a case report, review, and recommendations for management. J. Investig. Med. High Impact Case Rep. 1, 2324709613480346 (2013).
Bianchini, G. & Gianni, L. The immune system and response to HER2-targeted treatment in breast cancer. Lancet Oncol. 15, e58–e68 (2014).
Yousif, N. G. & Al-Amran, F. G. Novel Toll-like receptor-4 deficiency attenuates trastuzumab (Herceptin) induced cardiac injury in mice. BMC Cardiovasc. Disord. 11, 62 (2011).
Asawaeer, M., Barton, D., Radio, S. & Chatzizisis, Y. S. Tyrosine kinase inhibitor-induced acute myocarditis, myositis, and cardiogenic shock. Methodist Debakey Cardiovasc. J. 14, e5–e6 (2018).
Palmieri, D. J. & Carlino, M. S. Immune checkpoint inhibitor toxicity. Curr. Oncol. Rep. 20, 72 (2018).
Puzanov, I. et al. Managing toxicities associated with immune checkpoint inhibitors: consensus recommendations from the Society for Immunotherapy of Cancer (SITC) Toxicity Management Working Group. J. Immunother. Cancer 5, 95 (2017).
Spain, L., Diem, S. & Larkin, J. Management of toxicities of immune checkpoint inhibitors. Cancer Treat. Rev. 44, 51–60 (2016).
Eigentler, T. K. et al. Diagnosis, monitoring and management of immune-related adverse drug reactions of anti-PD-1 antibody therapy. Cancer Treat. Rev. 45, 7–18 (2016).
Wang, D. Y. et al. Fatal toxic effects associated with immune checkpoint inhibitors: a systematic review and meta-analysis. JAMA Oncol. 4, 1721–1728 (2018).
Lyon, A. R., Yousaf, N., Battisti, N. M. L., Moslehi, J. & Larkin, J. Immune checkpoint inhibitors and cardiovascular toxicity. Lancet Oncol. 19, e447–e458 (2018).
Mir, H. et al. Cardiac complications associated with checkpoint inhibition: a systematic review of the literature in an important emerging area. Can. J. Cardiol. 34, 1059–1068 (2018).
Escudier, M. et al. Clinical features, management, and outcomes of immune checkpoint inhibitor-related cardiotoxicity. Circulation 136, 2085–2087 (2017).
Johnson, D. B. et al. Fulminant myocarditis with combination immune checkpoint blockade. N. Engl. J. Med. 375, 1749–1755 (2016).
Nishimura, H. et al. Autoimmune dilated cardiomyopathy in PD-1 receptor-deficient mice. Science 291, 319–322 (2001).
Okazaki, T. et al. Autoantibodies against cardiac troponin I are responsible for dilated cardiomyopathy in PD-1-deficient mice. Nat. Med. 9, 1477–1483 (2003).
Grabie, N. et al. Endothelial programmed death-1 ligand 1 (PD-L1) regulates CD8+ T-cell mediated injury in the heart. Circulation 116, 2062–2071 (2007).
Lichtman, A. H. The heart of the matter: protection of the myocardium from T cells. J. Autoimmun. 45, 90–96 (2013).
Rodig, N. et al. Endothelial expression of PD-L1 and PD-L2 down-regulates CD8+ T cell activation and cytolysis. Eur. J. Immunol. 33, 3117–3126 (2003).
Tarrio, M. L., Grabie, N., Bu, D. X., Sharpe, A. H. & Lichtman, A. H. PD-1 protects against inflammation and myocyte damage in T cell-mediated myocarditis. J. Immunol. 188, 4876–4884 (2012).
Baban, B., Liu, J. Y., Qin, X., Weintraub, N. L. & Mozaffari, M. S. Upregulation of programmed death-1 and Its ligand in cardiac injury models: interaction with GADD153. PLoS One 10, e0124059 (2015).
Varricchi, G., Galdiero, M. R. & Tocchetti, C. G. Cardiac toxicity of immune checkpoint inhibitors: cardio-oncology meets immunology. Circulation 136, 1989–1992 (2017).
Freilich, M. et al. Recovery from anthracycline cardiomyopathy after long-term support with a continuous flow left ventricular assist device. J. Heart Lung Transpl. 28, 101–103 (2009).
Arangalage, D. et al. Survival after fulminant myocarditis induced by immune-checkpoint inhibitors. Ann. Intern. Med. 167, 683–684 (2017).
Mahajan, V. S. & Jarolim, P. How to interpret elevated cardiac troponin levels. Circulation 124, 2350–2354 (2011).
Mahmood, S. S. et al. Myocarditis in patients treated with immune checkpoint inhibitors. J. Am. Coll. Cardiol. 71, 1755–1764 (2018).
O’Regan, D. P. & Cook, S. A. Myocarditis or myocardial infarction? MRI can help. Heart 97, 1283 (2011).
Miller, E. J. & Culver, D. A. Establishing an evidence-based method to diagnose cardiac sarcoidosis: the complementary use of cardiac magnetic resonance imaging and FDG-PET. Circ. Cardiovasc. Imaging 11, e007408 (2018).
Wang, D. Y., Okoye, G. D., Neilan, T. G., Johnson, D. B. & Moslehi, J. J. Cardiovascular toxicities associated with cancer immunotherapies. Curr. Cardiol. Rep. 19, 21 (2017).
Salem, J. E. et al. Abatacept for severe immune checkpoint inhibitor-associated myocarditis. N. Engl. J. Med. 380, 2377–2379 (2019).
Arbuck, S. G. et al. A reassessment of cardiac toxicity associated with Taxol. J. Natl Cancer Inst. Monogr. 15, 117–130 (1993).
Pai, V. B. & Nahata, M. C. Cardiotoxicity of chemotherapeutic agents: incidence, treatment and prevention. Drug Saf. 22, 263–302 (2000).
Tamargo, J., Caballero, R. & Delpon, E. Cancer chemotherapy and cardiac arrhythmias: a review. Drug Saf. 38, 129–152 (2015).
Ghiaseddin, A. et al. Phase II study of bevacizumab and vorinostat for patients with recurrent World Health Organization grade 4 malignant glioma. Oncologist 23, 157–e121 (2018).
Lele, A. V., Clutter, S., Price, E. & De Ruyter, M. L. Severe hypothyroidism presenting as myxedema coma in the postoperative period in a patient taking sunitinib: case report and review of literature. J. Clin. Anesth. 25, 47–51 (2013).
Herrmann, J. Tyrosine kinase inhibitors and vascular toxicity: impetus for a classification system? Curr. Oncol. Rep. 18, 33 (2016).
Mathur, K., Saini, A., Ellenbogen, K. A. & Shepard, R. K. Profound sinoatrial arrest associated with Ibrutinib. Case Rep. Oncol. Med. 2017, 7304021 (2017).
Cooper, L. T. Jr. Myocarditis. N. Engl. J. Med. 360, 1526–1538 (2009).
Kaplan, B. M., Miller, A. J., Bharati, S., Lev, M. & Martin Grais, I. Complete AV block following mediastinal radiation therapy: electrocardiographic and pathologic correlation and review of the world literature. J. Interv. Card. Electrophysiol. 1, 175–188 (1997).
Orzan, F. et al. Associated cardiac lesions in patients with radiation-induced complete heart block. Int. J. Cardiol. 39, 151–156 (1993).
Tzivoni, D., Ratzkowski, E., Biran, S., Brook, J. G. & Stern, S. Complete heart block following therapeutic irradiation of the left side of the chest. Chest 71, 231–234 (1977).
Cohen, S. I., Bharati, S., Glass, J. & Lev, M. Radiotherapy as a cause of complete atrioventricular block in Hodgkin’s disease. An electrophysiological-pathological correlation. Arch. Intern. Med. 141, 676–679 (1981).
Santoro, F. et al. Late calcification of the mitral-aortic junction causing transient complete atrio-ventricular block after mediastinal radiation of Hodgkin lymphoma: multimodal visualization. Int. J. Cardiol. 155, e49–e50 (2012).
Nair, C. K. et al. Conduction defects and mitral annulus calcification. Br. Heart J. 44, 162–167 (1980).
Barbey, J. T., Pezzullo, J. C. & Soignet, S. L. Effect of arsenic trioxide on QT interval in patients with advanced malignancies. J. Clin. Oncol. 21, 3609–3615 (2003).
Roboz, G. J. et al. Prevalence, management, and clinical consequences of QT interval prolongation during treatment with arsenic trioxide. J. Clin. Oncol. 32, 3723–3728 (2014).
Duan, J. et al. Anticancer drugs-related QTc prolongation, torsade de pointes and sudden death: current evidence and future research perspectives. Oncotarget 9, 25738–25749 (2018).
Porta-Sanchez, A. et al. Incidence, diagnosis, and management of QT prolongation induced by cancer therapies: a systematic review. J. Am. Heart Assoc. 6, e007724 (2017).
Cheng, C., Woronow, D., Nayernama, A., Wroblewski, T. & Jones, S. C. Ibrutinib-associated ventricular arrhythmia in the FDA adverse event reporting system. Leuk. Lymphoma 59, 3016–3017 (2018).
Tomcsanyi, J., Matrai, Z. & Tomcsanyi, K. Ventricular tachycardia caused by ibrutinib. J. Emerg. Med. 53, e27 (2017).
Lampson, B. L. et al. Ventricular arrhythmias and sudden death in patients taking ibrutinib. Blood 129, 2581–2584 (2017).
Beyer, A., Ganti, B., Majkrzak, A. & Theyyunni, N. A perfect storm: tyrosine kinase inhibitor-associated polymorphic ventricular tachycardia. J. Emerg. Med. 52, e123–e127 (2017).
Wallace, N., Wong, E., Cooper, D. & Chao, H. A case of new-onset cardiomyopathy and ventricular tachycardia in a patient receiving ibrutinib for relapsed mantle cell lymphoma. Clin. Case Rep. 4, 1120–1121 (2016).
Tomcsanyi, J., Nenyei, Z., Matrai, Z. & Bozsik, B. Ibrutinib, an approved tyrosine kinase inhibitor as a potential cause of recurrent polymorphic ventricular tachycardia. JACC Clin. Electrophysiol. 2, 847–849 (2016).
Tuomi, J. M., Xenocostas, A. & Jones, D. L. Increased susceptibility for atrial and ventricular cardiac arrhythmias in mice treated with a single high dose of ibrutinib. Can. J. Cardiol. 34, 337–341 (2018).
Thill, M. & Schmidt, M. Management of adverse events during cyclin-dependent kinase 4/6 (CDK4/6) inhibitor-based treatment in breast cancer. Ther. Adv. Med. Oncol. 10, 1758835918793326 (2018).
US Food and Drug Administration. Package insert Kisqali (ribociclib) tablets prescribing information (FDA, 2018).
Shah, A. et al. FDA approval: ribociclib for the treatment of postmenopausal women with hormone receptor-positive, HER2-negative advanced or metastatic breast cancer. Clin. Cancer Res. 24, 2999–3004 (2018).
Bellet, M. et al. Palbociclib and ribociclib in breast cancer: consensus workshop on the management of concomitant medication. Ther. Adv. Med. Oncol. 11, 1758835919833867 (2019).
Cuculich, P. S. et al. Noninvasive cardiac radiation for ablation of ventricular tachycardia. N. Engl. J. Med. 377, 2325–2336 (2017).
Larsen, R. L. et al. Electrocardiographic changes and arrhythmias after cancer therapy in children and young adults. Am. J. Cardiol. 70, 73–77 (1992).
Al-Khatib, S. M. et al. 2017 AHA/ACC/HRS guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J. Am. Coll. Cardiol. 72, e91–e220 (2018).
Conen, D. et al. Risk of malignant cancer among women with new-onset atrial fibrillation. JAMA Cardiol. 1, 389–396 (2016).
Wallis, C. J. D. et al. Association between use of antithrombotic medication and hematuria-related complications. JAMA 318, 1260–1271 (2017).
Sekiguchi, H. et al. Cancer antigen-125 plasma level as a biomarker of new-onset atrial fibrillation in postmenopausal women. Heart 103, 1368–1373 (2017).
Farmakis, D., Parissis, J. & Filippatos, G. Insights into onco-cardiology: atrial fibrillation in cancer. J. Am. Coll. Cardiol. 63, 945–953 (2014).
Feliz, V. et al. Melphalan-induced supraventricular tachycardia: incidence and risk factors. Clin. Cardiol. 34, 356–359 (2011).
Zhao, D. et al. Atrial fibrillation following treatment with paclitaxel: a case report. Biomed. Rep. 9, 540–544 (2018).
Shanafelt, T. D. et al. Atrial fibrillation in patients with chronic lymphocytic leukemia (CLL). Leuk. Lymphoma 58, 1630–1639 (2017).
Leong, D. P. et al. The risk of atrial fibrillation with ibrutinib use: a systematic review and meta-analysis. Blood 128, 138–140 (2016).
Yun, S., Vincelette, N. D., Acharya, U. & Abraham, I. Risk of atrial fibrillation and bleeding diathesis associated with ibrutinib treatment: a systematic review and pooled analysis of four randomized controlled trials. Clin. Lymphoma Myeloma Leuk. 17, 31–37 e13 (2017).
Visentin, A. et al. A scoring system to predict the risk of atrial fibrillation in chronic lymphocytic leukemia. Hematol. Oncol. 37, 508–512 (2019).
Archibald, W. et al. Atrial fibrillation (AF) in patients with CLL treated with ibrutinib: assessing prediction models and clinical outcomes. J. Clin. Oncol. 37, 7522–7522 (2019).
Wiczer, T. E. et al. Cumulative incidence, risk factors, and management of atrial fibrillation in patients receiving ibrutinib. Blood Adv. 1, 1739–1748 (2017).
Thompson, P. A. et al. Atrial fibrillation in CLL patients treated with ibrutinib. An international retrospective study. Br. J. Haematol. 175, 462–466 (2016).
Pretorius, L. et al. Reduced phosphoinositide 3-kinase (p110alpha) activation increases the susceptibility to atrial fibrillation. Am. J. Pathol. 175, 998–1009 (2009).
Ganatra, S. et al. Ibrutinib-associated atrial fibrillation. JACC Clin. Electrophysiol. 4, 1491–1500 (2018).
Patel, V. et al. Comparison of acalabrutinib, a selective bruton tyrosine kinase inhibitor, with Ibrutinib in chronic lymphocytic leukemia cells. Clin. Cancer Res. 23, 3734–3743 (2017).
Owen, C., Berinstein, N. L., Christofides, A. & Sehn, L. H. Review of Bruton tyrosine kinase inhibitors for the treatment of relapsed or refractory mantle cell lymphoma. Curr. Oncol. 26, e233–e240 (2019).
Boos, C. J., Anderson, R. A. & Lip, G. Y. Is atrial fibrillation an inflammatory disorder? Eur. Heart J. 27, 136–149 (2006).
Pastori, D. et al. Inflammation and the risk of atrial high-rate episodes (AHREs) in patients with cardiac implantable electronic devices. Clin. Res. Cardiol. 107, 772–777 (2018).
Hu, Y. F., Chen, Y. J., Lin, Y. J. & Chen, S. A. Inflammation and the pathogenesis of atrial fibrillation. Nat. Rev. Cardiol. 12, 230–243 (2015).
Aviles, R. J. et al. Inflammation as a risk factor for atrial fibrillation. Circulation 108, 3006–3010 (2003).
Davila, M. L. et al. Efficacy and toxicity management of 19-28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Sci. Transl Med. 6, 224ra225 (2014).
Palla, A. R., Kennedy, D., Mosharraf, H. & Doll, D. Autoimmune hemolytic anemia as a complication of nivolumab therapy. Case Rep. Oncol. 9, 691–697 (2016).
Dein, E. et al. Two cases of sinusitis induced by immune checkpoint inhibition. J. Immunother. 40, 312–314 (2017).
Vaidya, V. et al. Atrial fibrillation after thoracic radiotherapy for cancer: examining differences in clinical characteristics at time of diagnosis compared with the general population. J. Am. Coll. Cardiol. 65, A318 (2015).
Zei, P. C. & Soltys, S. Ablative radiotherapy as a noninvasive alternative to catheter ablation for cardiac arrhythmias. Curr. Cardiol. Rep. 19, 79 (2017).
Vrontikis, A. et al. Proposed algorithm for managing Ibrutinib-related atrial fibrillation. Oncology 30, 970–974, 980-971, C973 (2016).
Aguilar, C. Ibrutinib-related bleeding: pathogenesis, clinical implications and management. Blood Coagul. Fibrinolysis 29, 481–487 (2018).
Busygina, K. et al. Oral Bruton tyrosine kinase inhibitors selectively block atherosclerotic plaque-triggered thrombus formation in humans. Blood 131, 2605–2616 (2018).
Shatzel, J. J. et al. Ibrutinib-associated bleeding: pathogenesis, management and risk reduction strategies. J. Thromb. Haemost. 15, 835–847 (2017).
Laube, E. S. et al. Rivaroxaban for stroke prevention in patients with nonvalvular atrial fibrillation and active cancer. Am. J. Cardiol. 120, 213–217 (2017).
Melloni, C. et al. Efficacy and safety of apixaban versus warfarin in patients with atrial fibrillation and a history of cancer: insights from the Aristotle trial. Am. J. Med. 130, 1440–1448.e1 (2017).
Russo, V. et al. Use of non-vitamin k antagonist oral anticoagulants in atrial fibrillation patients with malignancy: clinical practice experience in a single institution and literature review. Semin. Thromb. Hemost. 44, 370–376 (2018).
Shah, S. et al. Comparative effectiveness of direct oral anticoagulants and warfarin in patients with cancer and atrial fibrillation. Blood Adv. 2, 200–209 (2018).
Vedovati, M. C. et al. Patients with cancer and atrial fibrillation treated with doacs: a prospective cohort study. Int. J. Cardiol. 269, 152–157 (2018).
Xiang, E. et al. Anticoagulation prescribing patterns in patients with cancer. J. Thromb. Thrombolysis 45, 89–98 (2018).
Mulligan, S. P., Ward, C. M., Whalley, D. & Hilmer, S. N. Atrial fibrillation, anticoagulant stroke prophylaxis and bleeding risk with ibrutinib therapy for chronic lymphocytic leukaemia and lymphoproliferative disorders. Br. J. Haematol. 175, 359–364 (2016).
Ahn, I. E. et al. Depth and durability of response to ibrutinib in CLL: 5-year follow-up of a phase 2 study. Blood 131, 2357–2366 (2018).
Lee, Y. J. et al. Bleeding risk and major adverse events in patients with cancer on oral anticoagulation therapy. Int. J. Cardiol. 203, 372–378 (2016).
Friberg, L., Skeppholm, M. & Terent, A. Benefit of anticoagulation unlikely in patients with atrial fibrillation and a CHA2DS2-VASc score of 1. J. Am. Coll. Cardiol. 65, 225–232 (2015).
Hu, Y. F. et al. Incident thromboembolism and heart failure associated with new-onset atrial fibrillation in cancer patients. Int. J. Cardiol. 165, 355–357 (2013).
D’Souza, M. et al. CHA2DS2-VASc score and risk of thromboembolism and bleeding in patients with atrial fibrillation and recent cancer. Eur. J. Prev. Cardiol. 25, 651–658 (2018).
Hu, W. S. & Lin, C. L. Comparison of CHA2DS2-VASc, CHADS2 and HATCH scores for the prediction of new-onset atrial fibrillation in cancer patients: a nationwide cohort study of 760,339 study participants with competing risk analysis. Atherosclerosis 266, 205–211 (2017).
Hu, W. S. & Lin, C. L. Impact of atrial fibrillation on the development of ischemic stroke among cancer patients classified by CHA2DS2-VASc score-a nationwide cohort study. Oncotarget 9, 7623–7630 (2018).
O’Neal, W. T. et al. Provider specialty, anticoagulation, and stroke risk in patients with atrial fibrillation and cancer. J. Am. Coll. Cardiol. 72, 1913–1922 (2018).
Acknowledgements
The author receives support from the US National Institutes of Health (HL116952 and CA233610) and the Miami Heart Research Institute/Florida Heart Research Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The author declares no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Glossary
- Oncogenes
-
Oncogenes encode proteins that can transform cells into tumour cells. All but a few are derived from normal cellular genes (proto-oncogenes), and activation of a proto-oncogene into an oncogene generally involves a gain-of-function mutation.
- Philadelphia chromosome
-
Named after the city in which it was discovered in 1960 as the first tumour-specific chromosomal change in the form of a shortened chromosome 22 as a result of a reciprocal translocation that leads to the oncogenic BCR–ABL1 gene fusion, which has a causal role in the malignant transformation of white blood cell precursors; the Philadelphia chromosome is found in 90% of patients with chronic myeloid leukaemia.
- Endoplasmic reticulum stress response
-
Disruption of endoplasmic reticulum function leads to impairment of protein folding, accumulation of unfolded and misfolded proteins and risk of cell toxicity. The cell reacts to this endoplasmic reticulum stress by initiating the unfolded protein response to increase the capacity of the cell to handle and/or eliminate the accumulating unfolded or misfolded proteins or to initiate apoptosis.
- Sentinel kinase theory
-
The theory that inhibition of one specific enzyme among all the enzymes that catalyse the transfer of a phosphate group from ATP onto a tyrosine, serine or threonine residue of a protein (kinome) is responsible for a specific action.
- Cytokine release syndrome
-
(CRS). A systemic inflammatory response that can be triggered by a variety of factors such as infections, antibody-based immunotherapies and chimeric antigen receptor T cell therapy. CRS is caused by the rapid release of a large amount of cytokines into the circulation, leading to fever, nausea, headache, rash, tachycardia, hypotension and respiratory distress.
- Bispecific T cell engager therapy
-
(BiTE therapy). BiTE antibody constructs are designed to create an immunologic synapse between an effector T cell and a tumour cell by simultaneously binding to the T cell activation molecule CD3 and a tumour-associated antigen, which is CD19 on B cells in the case of blinatumomab (approved for the treatment of B cell acute lymphoblastic leukaemia).
- Cell senescence
-
A process defined as irreversible cell cycle arrest, driven by a variety of mechanisms, including telomere shortening, other forms of genotoxic stress, mitogens or inflammatory cytokines, that culminate in the activation of the tumour suppressor p53 and/or the cyclin-dependent kinase inhibitor p16.
- Cardiovascular flow reserve
-
The capacity of the coronary vascular bed to increase blood flow maximally to the myocardium, often expressed as a ratio with regard to baseline blood flow.
Rights and permissions
About this article
Cite this article
Herrmann, J. Adverse cardiac effects of cancer therapies: cardiotoxicity and arrhythmia. Nat Rev Cardiol 17, 474–502 (2020). https://doi.org/10.1038/s41569-020-0348-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41569-020-0348-1
This article is cited by
-
Pharmacological properties of Polygonatum and its active ingredients for the prevention and treatment of cardiovascular diseases
Chinese Medicine (2024)
-
Cardiovascular health assessment in routine cancer follow-up in community settings: survivor risk awareness and perspectives
BMC Cancer (2024)
-
Cardiovascular disease and cancer: shared risk factors and mechanisms
Nature Reviews Cardiology (2024)
-
Associations between HIFs and tumor immune checkpoints: mechanism and therapy
Discover Oncology (2024)
-
Characterization of anthracycline-induced cardiotoxicity by diffusion tensor magnetic resonance imaging
Basic Research in Cardiology (2024)