Abstract
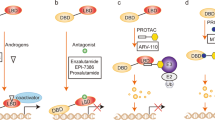
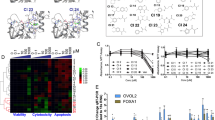
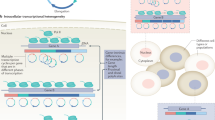
Mutated or dysregulated transcription factors represent a unique class of drug targets that mediate aberrant gene expression, including blockade of differentiation and cell death gene expression programmes, hallmark properties of cancers. Transcription factor activity is altered in numerous cancer types via various direct mechanisms including chromosomal translocations, gene amplification or deletion, point mutations and alteration of expression, as well as indirectly through non-coding DNA mutations that affect transcription factor binding. Multiple approaches to target transcription factor activity have been demonstrated, preclinically and, in some cases, clinically, including inhibition of transcription factor–cofactor protein–protein interactions, inhibition of transcription factor–DNA binding and modulation of levels of transcription factor activity by altering levels of ubiquitylation and subsequent proteasome degradation or by inhibition of regulators of transcription factor expression. In addition, several new approaches to targeting transcription factors have recently emerged including modulation of auto-inhibition, proteolysis targeting chimaeras (PROTACs), use of cysteine reactive inhibitors, targeting intrinsically disordered regions of transcription factors and combinations of transcription factor inhibitors with kinase inhibitors to block the development of resistance. These innovations in drug development hold great promise to yield agents with unique properties that are likely to impact future cancer treatment.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Darnell, J. E. Jr. Transcription factors as targets for cancer therapy. Nat. Rev. Cancer 2, 740–749 (2002).
Lee, T. I. & Young, R. A. Transcriptional regulation and its misregulation in disease. Cell 152, 1237–1251 (2013).
Arkin, M. R., Tang, Y. & Wells, J. A. Small-molecule inhibitors of protein–protein interactions: progressing toward the reality. Chem. Biol. 21, 1102–1114 (2014). This comprehensive review illustrates the extraordinary progress that has been made in the development of protein–protein interaction inhibitors for use in a wide variety of disease settings.
Silvian, L. F. et al. Small molecule inhibition of the TNF family cytokine CD40 ligand through a subunit fracture mechanism. ACS Chem. Biol. 6, 636–647 (2011).
Illendula, A. et al. Small molecule inhibitor of CBFβ–RUNX binding for RUNX transcription factor driven cancers. EBioMedicine 8, 117–131 (2016).
Tovar, C. et al. MDM2 small-molecule antagonist RG7112 activates p53 signaling and regresses human tumors in preclinical cancer models. Cancer Res. 73, 2587–2597 (2013).
Ding, Q. et al. Discovery of RG7388, a potent and selective p53–MDM2 inhibitor in clinical development. J. Med. Chem. 56, 5979–5983 (2013).
Vassilev, L. T. et al. In vivo activation of the p53 pathway by small-molecule antagonists of MDM2. Science 303, 844–848 (2004). This paper describes the development and evaluation of the first MDM2–p53 inhibitor of the Nutlin class.
Zhang, Z. et al. Discovery of potent and selective spiroindolinone MDM2 inhibitor, RO8994, for cancer therapy. Bioorg. Med. Chem. 22, 4001–4009 (2014).
Zhao, Y. et al. A potent small-molecule inhibitor of the MDM2–p53 interaction (MI-888) achieved complete and durable tumor regression in mice. J. Med. Chem. 56, 5553–5561 (2013).
Miyazaki, M. et al. Lead optimization of novel p53–MDM2 interaction inhibitors possessing dihydroimidazothiazole scaffold. Bioorg. Med. Chem. Lett. 23, 728–732 (2013).
Tisato, V., Voltan, R., Gonelli, A., Secchiero, P. & Zauli, G. MDM2/X inhibitors under clinical evaluation: perspectives for the management of hematological malignancies and pediatric cancer. J. Hematol. Oncol. 10, 133 (2017).
Filippakopoulos, P. et al. Selective inhibition of BET bromodomains. Nature 468, 1067–1073 (2010).
Gehling, V. S. et al. Discovery, design, ond Optimization of isoxazole azepine BET inhibitors. ACS Med. Chem. Lett. 4, 835–840 (2013).
Mirguet, O. et al. Discovery of epigenetic regulator I-BET762: lead optimization to afford a clinical candidate inhibitor of the BET bromodomains. J. Med. Chem. 56, 7501–7515 (2013).
Xu, Y. & Vakoc, C. R. Targeting cancer cells with BET bromodomain inhibitors. Cold Spring Harb. Perspect. Med. 7, a026674 (2017).
Helin, K. & Dhanak, D. Chromatin proteins and modifications as drug targets. Nature 502, 480–488 (2013).
Look, A. T. Oncogenic transcription factors in the human acute leukemias. Science 278, 1059–1064 (1997).
Alcalay, M. et al. Acute myeloid leukemia fusion proteins deregulate genes involved in stem cell maintenance and DNA repair. J. Clin. Invest. 112, 1751–1761 (2003).
Martens, J. H. & Stunnenberg, H. G. The molecular signature of oncofusion proteins in acute myeloid leukemia. FEBS Lett. 584, 2662–2669 (2010).
Di Croce, L. Chromatin modifying activity of leukaemia associated fusion proteins. Hum. Mol. Genet. 14, Spec No 1, R77-R84 (2005).
Kottaridis, P. D. et al. Studies of FLT3 mutations in paired presentation and relapse samples from patients with acute myeloid leukemia: implications for the role of FLT3 mutations in leukemogenesis, minimal residual disease detection, and possible therapy with FLT3 inhibitors. Blood 100, 2393–2398 (2002).
Shih, L. Y. et al. Cooperating mutations of receptor tyrosine kinases and Ras genes in childhood core-binding factor acute myeloid leukemia and a comparative analysis on paired diagnosis and relapse samples. Leukemia 22, 303–307 (2008).
Nakano, Y. et al. Molecular evolution of acute myeloid leukaemia in relapse: unstable N-ras and FLT3 genes compared with p53 gene. Br. J. Haematol. 104, 659–664 (1999).
Tomlins, S. A. et al. ETS gene fusions in prostate cancer: from discovery to daily clinical practice. Eur. Urol. 56, 275–286 (2009).
Clark, J. P. & Cooper, C. S. ETS gene fusions in prostate cancer. Nat. Rev. Urol. 6, 429–439 (2009).
Tomlins, S. A. et al. Recurrent fusion of TMPRSS2 and ETS transcription factor genes in prostate cancer. Science 310, 644–648 (2005).
Chen, Y. et al. ETS factors reprogram the androgen receptor cistrome and prime prostate tumorigenesis in response to PTEN loss. Nat. Med. 19, 1023–1029 (2013).
Chi, P. et al. ETV1 is a lineage survival factor that cooperates with KIT in gastrointestinal stromal tumours. Nature 467, 849–853 (2010).
Jane-Valbuena, J. et al. An oncogenic role for ETV1 in melanoma. Cancer Res. 70, 2075–2084 (2010).
Scheitz, C. J., Lee, T. S., McDermitt, D. J. & Tumbar, T. Defining a tissue stem cell-driven Runx1/Stat3 signalling axis in epithelial cancer. EMBO J. 31, 4124–4139 (2012).
Morita, K. et al. Genetic regulation of the RUNX transcription factor family has antitumor effects. J. Clin. Invest. 127, 2815–2828 (2017).
Chuang, L. S., Ito, K. & Ito, Y. Roles of RUNX in solid tumors. Adv. Exp. Med. Biol. 962, 299–320 (2017).
Hanahan, D. & Weinberg, R. A. Hallmarks of cancer: the next generation. Cell 144, 646–674 (2011).
Hanahan, D. & Weinberg, R. A. The hallmarks of cancer. Cell 100, 57–70 (2000).
Koehler, A. N. A complex task? Direct modulation of transcription factors with small molecules. Curr. Opin. Chem. Biol. 14, 331–340 (2010).
Majmudar, C. Y. & Mapp, A. K. Chemical approaches to transcriptional regulation. Curr. Opin. Chem. Biol. 9, 467–474 (2005).
Arndt, H. D. Small molecule modulators of transcription. Angew. Chem. 45, 4552–4560 (2006).
Berg, T. Inhibition of transcription factors with small organic molecules. Curr. Opin. Chem. Biol. 12, 464–471 (2008).
Bhagwat, A. S. & Vakoc, C. R. Targeting transcription factors in cancer. Trends Cancer 1, 53–65 (2015).
Burris, T. P. et al. Nuclear receptors and their selective pharmacologic modulators. Pharmacol. Rev. 65, 710–778 (2013).
de The, H. Differentiation therapy revisited. Nat. Rev. Cancer 18, 117–127 (2018).
Mangelsdorf, D. J. et al. The nuclear receptor superfamily: the second decade. Cell 83, 835–839 (1995).
Robinson-Rechavi, M., Escriva Garcia, H. & Laudet, V. The nuclear receptor superfamily. J. Cell Sci. 116, 585–586 (2003).
Pawlak, M., Lefebvre, P. & Staels, B. General molecular biology and architecture of nuclear receptors. Curr. Top. Med. Chem. 12, 486–504 (2012).
Perou, C. M. et al. Molecular portraits of human breast tumours. Nature 406, 747–752 (2000).
Patel, H. K. & Bihani, T. Selective estrogen receptor modulators (SERMs) and selective estrogen receptor degraders (SERDs) in cancer treatment. Pharmacol. Ther. 186, 1–24 (2018).
Shiau, A. K. et al. The structural basis of estrogen receptor/coactivator recognition and the antagonism of this interaction by tamoxifen. Cell 95, 927–937 (1998).
Romano, A. et al. Identification of novel ER-α target genes in breast cancer cells: gene- and cell-selective co-regulator recruitment at target promoters determines the response to 17β-estradiol and tamoxifen. Mol. Cell Endocrinol. 314, 90–100 (2010).
Shang, Y. & Brown, M. Molecular determinants for the tissue specificity of SERMs. Science 295, 2465–2468 (2002).
Dai, C., Heemers, H. & Sharifi, N. Androgen signaling in prostate cancer. Cold Spring Harb. Perspect. Med. 7, a030452 (2017).
Crona, D. J., Milowsky, M. I. & Whang, Y. E. Androgen receptor targeting drugs in castration-resistant prostate cancer and mechanisms of resistance. Clin. Pharmacol. Ther. 98, 582–589 (2015).
Efstathiou, E. et al. Molecular characterization of enzalutamide-treated bone metastatic castration-resistant prostate cancer. Eur. Urol. 67, 53–60 (2015).
Nevedomskaya, E., Baumgart, S. J. & Haendler, B. Recent advances in prostate cancer treatment and drug discovery. Int. J. Mol. Sci. 19 (2018).
Gu, T. L., Goetz, T. L., Graves, B. J. & Speck, N. A. Auto-inhibition and partner proteins, core-binding factor beta (CBFβ) and Ets-1, modulate DNA binding by CBFα2 (AML1). Mol. Cell. Biol. 20, 91–103 (2000).
Goetz, T. L., Gu, T. L., Speck, N. A. & Graves, B. J. Auto-inhibition of Ets-1 is counteracted by DNA binding cooperativity with core-binding factor α2. Mol. Cell. Biol. 20, 81–α90 (2000).
Shrivastava, T. et al. Structural basis of Ets1 activation by Runx1. Leukemia 28, 2040–2048 (2014).
Hollenhorst, P. C., Shah, A. A., Hopkins, C. & Graves, B. J. Genome-wide analyses reveal properties of redundant and specific promoter occupancy within the ETS gene family. Genes Dev. 21, 1882–1894 (2007).
Slany, R. K. When epigenetics kills: MLL fusion proteins in leukemia. Hematol. Oncol. 23, 1–9 (2005).
Cox, M. C. et al. Chromosomal aberration of the 11q23 locus in acute leukemia and frequency of MLL gene translocation: results in 378 adult patients. Am. J. Clin. Pathol. 122, 298–306 (2004).
Popovic, R. & Zeleznik-Le, N. J. MLL: how complex does it get? J. Cell. Biochem. 95, 234–242 (2005).
Dimartino, J. F. & Cleary, M. L. MLL rearrangements in haematological malignancies: lessons from clinical and biological studies. Br. J. Haematol. 106, 614–626 (1999).
Yokoyama, A. et al. The menin tumor suppressor protein is an essential oncogenic cofactor for MLL-associated leukemogenesis. Cell 123, 207–218 (2005).
Caslini, C. et al. Interaction of MLL amino terminal sequences with menin is required for transformation. Cancer Res. 67, 7275–7283 (2007).
Cierpicki, T. et al. Structure of the MLL CXXC domain–DNA complex and its functional role in MLL–AF9 leukemia. Nat. Struct. Mol. Biol. 17, 62–68 (2010).
Grembecka, J. et al. Menin–MLL inhibitors reverse oncogenic activity of MLL fusion proteins in leukemia. Nat. Chem. Biol. 8, 277–284 (2012). This paper describes the development and demonstration of on-target activity of the first inhibitors of the menin–MLL fusion protein interaction.
He, S. et al. High-affinity small-molecule inhibitors of the menin–mixed lineage leukemia (MLL) interaction closely mimic a natural protein–protein interaction. J. Med. Chem. 57, 1543–1556 (2014).
Borkin, D. et al. Property focused structure-based optimization of small molecule inhibitors of the protein–protein interaction between menin and mixed lineage leukemia (MLL). J. Med. Chem. 59, 892–913 (2016).
Borkin, D. et al. Pharmacologic inhibition of the menin–MLL interaction blocks progression of MLL leukemia in vivo. Cancer Cell 27, 589–602 (2015). This paper demonstrates efficacy of menin–MLL inhibitors in a mouse model of MLL fusion-positive leukaemia as well as with primary MLL fusion-positive leukaemia patient samples.
Borkin, D. et al. Complexity of blocking bivalent protein–protein interactions: development of a highly potent inhibitor of the menin–mixed-lineage leukemia interaction. J. Med. Chem. 61, 4832–4850 (2018).
Liu, P. et al. Fusion between transcription factor CBFβ/PEBP2β and a myosin heavy chain in acute myeloid leukemia. Science 261, 1041–1044 (1993).
Castilla, L. H. et al. Failure of embryonic hematopoiesis and lethal hemorrhages in mouse embryos heterozygous for a knocked-in leukemia gene CBFB–MYH11. Cell 87, 687–696 (1996).
Mandoli, A. et al. CBFB–MYH11/RUNX1 together with a compendium of hematopoietic regulators, chromatin modifiers and basal transcription factors occupies self-renewal genes in inv(16) acute myeloid leukemia. Leukemia 28, 770–778 (2014).
Illendula, A. et al. Chemical biology. A small-molecule inhibitor of the aberrant transcription factor CBFβ–SMMHC delays leukemia in mice. Science 347, 779–784 (2015). This study describes the development of the first inhibitor of the interaction between CBFβ–SMMHC and RUNX, and also demonstrates efficacy of the inhibitor in a mouse model of CBFβ–SMMHC-positive leukaemia and with primary inv(16) leukaemia patient samples.
Pulikkan, J. A. et al. CBFβ–SMMHC inhibition triggers apoptosis by disrupting MYC chromatin dynamics in acute myeloid leukemia. Cell 174, 172–186 (2018). This study describes the mechanism leading to reduced MYC expression seen with the CBFβ–SMMHC inhibitor, which occurs via altered occupancy of BAF and PRC complexes at specific enhancers of MYC.
Choi, A. et al. RUNX1 is required for oncogenic Myb and Myc enhancer activity in T-cell acute lymphoblastic leukemia. Blood 130, 1722–1733 (2017).
Zhou, N. et al. RUNX proteins desensitize multiple myeloma to lenalidomide via protecting IKZFs from degradation. Leukemia 33, 2006–2021 (2019).
Mill, C. P. et al. RUNX1 targeted therapy for AML expressing somatic or germline mutation in RUNX1. Blood 134, 59–73 (2019).
Chimge, N. O. & Frenkel, B. The RUNX family in breast cancer: relationships with estrogen signaling. Oncogene 32, 2121–2130 (2013).
McDonald, L. et al. RUNX2 correlates with subtype-specific breast cancer in a human tissue microarray, and ectopic expression of Runx2 perturbs differentiation in the mouse mammary gland. Dis. Model. Mechanisms 7, 525–534 (2014).
Cancer Genome Atlas Network. Comprehensive molecular portraits of human breast tumours. Nature 490, 61–70 (2012).
Ciriello, G., Cerami, E., Sander, C. & Schultz, N. Mutual exclusivity analysis identifies oncogenic network modules. Genome Res. 22, 398–406 (2012).
Carlton, A. L. et al. Small molecule inhibition of the CBFβ/RUNX interaction decreases ovarian cancer growth and migration through alterations in genes related to epithelial-to-mesenchymal transition. Gynecol. Oncol. 149, 350–360 (2018).
Geng, F., Wenzel, S. & Tansey, W. P. Ubiquitin and proteasomes in transcription. Annu. Rev. Biochem. 81, 177–201 (2012).
Mukouyama, Y. et al. The AML1 transcription factor functions to develop and maintain hematogenic precursor cells in the embryonic aorta–gonad–mesonephros region. Dev. Biol. 220, 27–36 (2000).
Venkatachalam, S. et al. Retention of wild-type p53 in tumors from p53 heterozygous mice: reduction of p53 dosage can promote cancer formation. EMBO J. 17, 4657–4667 (1998).
Xie, Y. et al. Reduced Erg dosage impairs survival of hematopoietic stem and progenitor cells. Stem Cells 35, 1773–1785 (2017).
Xu, X. et al. Haploid loss of the tumor suppressor Smad4/Dpc4 initiates gastric polyposis and cancer in mice. Oncogene 19, 1868–1874 (2000).
Senft, D., Qi, J. & Ronai, Z. A. Ubiquitin ligases in oncogenic transformation and cancer therapy. Nat. Rev. Cancer 18, 69–88 (2018).
Buetow, L. & Huang, D. T. Structural insights into the catalysis and regulation of E3 ubiquitin ligases. Nat. Rev. Mol. Cell Biol. 17, 626–642 (2016).
Zheng, N. & Shabek, N. Ubiquitin ligases: structure, function, and regulation. Annu. Rev. Biochem. 86, 129–157 (2017).
Buckley, D. L. et al. Targeting the von Hippel-Lindau E3 ubiquitin ligase using small molecules to disrupt the VHL/HIF-1α interaction. J. Am. Chem. Soc. 134, 4465–4468 (2012).
Buckley, D. L. et al. Small-molecule inhibitors of the interaction between the E3 ligase VHL and HIF1a. Angew. Chem. Int. Ed. 51, 11463–11467 (2012).
Kaelin, W. G. Jr. The von Hippel-Lindau tumour suppressor protein: O2 sensing and cancer. Nat. Rev. Cancer 8, 865–873 (2008).
Lu, G. et al. The myeloma drug lenalidomide promotes the cereblon-dependent destruction of Ikaros proteins. Science 343, 305–309 (2014). This paper describes the elucidation of the mechanism of action of the drug lenalidomide, which enhances binding of cereblon to IKZF to increase ubiquitination and proteasome destruction of IKZF.
Ouchida, A. T. et al. USP10 regulates the stability of the EMT-transcription factor Slug/SNAI2. Biochem. Biophys. Res. Commun. 502, 429–434 (2018).
Wu, Y. et al. Dub3 inhibition suppresses breast cancer invasion and metastasis by promoting Snail1 degradation. Nat. Commun. 8, 14228 (2017).
Lin, Y. et al. Stabilization of the transcription factors slug and twist by the deubiquitinase dub3 is a key requirement for tumor metastasis. Oncotarget 8, 75127–75140 (2017).
Kim, D. et al. Deubiquitinating enzyme USP22 positively regulates c-Myc stability and tumorigenic activity in mammalian and breast cancer cells. J. Cell. Physiol. 232, 3664–3676 (2017).
Tomlins, S. A. et al. Role of the TMPRSS2–ERG gene fusion in prostate cancer. Neoplasia 10, 177–188 (2008).
Carver, B. S. et al. Aberrant ERG expression cooperates with loss of PTEN to promote cancer progression in the prostate. Nat. Genet. 41, 619–624 (2009).
Carver, B. S. et al. ETS rearrangements and prostate cancer initiation. Nature 457, E1; discussion E2–E3 (2009).
Wang, S. et al. Ablation of the oncogenic transcription factor ERG by deubiquitinase inhibition in prostate cancer. Proc. Natl Acad. Sci. USA 111, 4251–4256 (2014).
Wang, S. et al. The ubiquitin ligase TRIM25 targets ERG for degradation in prostate cancer. Oncotarget 7, 64921–64931 (2016).
Kapuria, V. et al. Deubiquitinase inhibition by small-molecule WP1130 triggers aggresome formation and tumor cell apoptosis. Cancer Res. 70, 9265–9276 (2010).
Hainaut, P. & Hollstein, M. p53 and human cancer: the first ten thousand mutations. Adv. Cancer Res. 77, 81–137 (2000).
Vogelstein, B., Lane, D. & Levine, A. J. Surfing the p53 network. Nature 408, 307–310 (2000).
Harris, S. L. & Levine, A. J. The p53 pathway: positive and negative feedback loops. Oncogene 24, 2899–2908 (2005).
Vousden, K. H. & Lane, D. P. p53 in health and disease. Nat. Rev. Mol. Cell Biol. 8, 275–283 (2007).
Freedman, D. A., Wu, L. & Levine, A. J. Functions of the MDM2 oncoprotein. Cell. Mol. Life Sci. 55, 96–107 (1999).
Bond, G. L., Hu, W. & Levine, A. J. MDM2 is a central node in the p53 pathway: 12 years and counting. Curr. Cancer Drug Targets 5, 3–8 (2005).
Wu, X., Bayle, J. H., Olson, D. & Levine, A. J. The p53–mdm-2 autoregulatory feedback loop. Genes Dev. 7, 1126–1132 (1993).
Momand, J., Jung, D., Wilczynski, S. & Niland, J. The MDM2 gene amplification database. Nucleic Acids Res. 26, 3453–3459 (1998).
Fotouhi, N. & Graves, B. Small molecule inhibitors of p53/MDM2 interaction. Curr. Top. Med. Chem. 5, 159–165 (2005).
Lai, A. C. et al. Modular PROTAC design for the degradation of oncogenic BCR–ABL. Angew. Chem. 55, 807–810 (2016).
Bondeson, D. P. et al. Catalytic in vivo protein knockdown by small-molecule PROTACs. Nat. Chem. Biol. 11, 611–617 (2015). This study demonstrates the catalytic behaviour of small-molecule PROTACs.
Winter, G. E. et al. DRUG DEVELOPMENT. Phthalimide conjugation as a strategy for in vivo target protein degradation. Science 348, 1376–1381 (2015). This paper describes the development of a PROTAC targeting BRD4, its mechanism of action via proteasome-mediated reduction in the BRD4 level and its in vivo efficacy in a mouse model.
Burslem, G. M. et al. The advantages of targeted protein degradation over inhibition: an RTK case study. Cell Chem. Biol. 25, 67–77 e63 (2018).
Lai, A. C. & Crews, C. M. Induced protein degradation: an emerging drug discovery paradigm. Nat. Rev. Drug Discov. 16, 101–114 (2017). This work is a comprehensive review of PROTACs by one of the original developers of this approach.
Akhtar, M. S. et al. TFIIH kinase places bivalent marks on the carboxy-terminal domain of RNA polymerase II. Mol. Cell 34, 387–393 (2009).
Drapkin, R., Le Roy, G., Cho, H., Akoulitchev, S. & Reinberg, D. Human cyclin-dependent kinase-activating kinase exists in three distinct complexes. Proc. Natl Acad. Sci. USA 93, 6488–6493 (1996).
Glover-Cutter, K. et al. TFIIH-associated Cdk7 kinase functions in phosphorylation of C-terminal domain Ser7 residues, promoter-proximal pausing, and termination by RNA polymerase II. Mol. Cell. Biol. 29, 5455–5464 (2009).
Kwiatkowski, N. et al. Targeting transcription regulation in cancer with a covalent CDK7 inhibitor. Nature 511, 616–620 (2014). This paper describes the development of a CDK7 inhibitor, its mechanism of action via changes in the level of the transcription factor RUNX1 and its in vivo efficacy in a mouse model of T-ALL.
Chipumuro, E. et al. CDK7 inhibition suppresses super-enhancer-linked oncogenic transcription in MYCN-driven cancer. Cell 159, 1126–1139 (2014).
Christensen, C. L. et al. Targeting transcriptional addictions in small cell lung cancer with a covalent CDK7 inhibitor. Cancer Cell 26, 909–922 (2014).
Ahuja, N., Sharma, A. R. & Baylin, S. B. Epigenetic therapeutics: a new weapon in the war against cancer. Annu. Rev Med 67, 73–89 (2016).
Bennett, R. L. & Licht, J. D. Targeting epigenetics in cancer. Annu. Rev. Pharmacol. Toxicol. 58, 187–207 (2018).
Mohammad, H. P., Barbash, O. & Creasy, C. L. Targeting epigenetic modifications in cancer therapy: erasing the roadmap to cancer. Nat. Med. 25, 403–418 (2019).
Stathis, A. & Bertoni, F. BET proteins as targets for anticancer treatment. Cancer Discov. 8, 24–36 (2018).
Stonestrom, A. J. et al. Functions of BET proteins in erythroid gene expression. Blood 125, 2825–2834 (2015).
Roe, J. S., Mercan, F., Rivera, K., Pappin, D. J. & Vakoc, C. R. BET bromodomain inhibition suppresses the function of hematopoietic transcription factors in acute myeloid leukemia. Mol. Cell 58, 1028–1039 (2015). This paper describes the effects of a BRD4 bromodomain inhibitor on binding of BRD4 to specific haematopoietic transcription factors as well as the inhibition of the activity of these transcription factors.
Lamonica, J. M. et al. Bromodomain protein Brd3 associates with acetylated GATA1 to promote its chromatin occupancy at erythroid target genes. Proc. Natl Acad. Sci. USA 108, E159–E168 (2011).
Shi, J. et al. Disrupting the interaction of BRD4 with diacetylated Twist suppresses tumorigenesis in basal-like breast cancer. Cancer Cell 25, 210–225 (2014).
French, C. A. Small-molecule targeting of BET proteins in cancer. Adv. Cancer Res. 131, 21–58 (2016).
Delmore, J. E. et al. BET bromodomain inhibition as a therapeutic strategy to target c-Myc. Cell 146, 904–917 (2011).
Loven, J. et al. Selective inhibition of tumor oncogenes by disruption of super-enhancers. Cell 153, 320–334 (2013).
Horiuchi, D., Anderton, B. & Goga, A. Taking on challenging targets: making MYC druggable. Am. Soc. Clin. Oncol. Educ. Book, e497-e502, https://doi.org/10.14694/EdBook_AM.2014.34.e497 (2014).
Carabet, L. A., Rennie, P. S. & Cherkasov, A. Therapeutic inhibition of MYC in cancer. Structural bases and computer-aided drug discovery approaches. Int. J. Mol. Sci. 20, E120 (2018).
Castell, A. et al. A selective high affinity MYC-binding compound inhibits MYC:MAX interaction and MYC-dependent tumor cell proliferation. Sci. Rep. 8, (10064 (2018).
Struntz, N. B. et al. Stabilization of the Max homodimer with a small molecule attenuates Myc-driven transcription. Cell Chem. Biol. 26, 711–723 e714 (2019).
Doroshow, D. B., Eder, J. P. & LoRusso, P. M. BET inhibitors: a novel epigenetic approach. Ann. Oncol. 28, 1776–1787 (2017).
Leung, C. H., Chan, D. S., Ma, V. P. & Ma, D. L. DNA-binding small molecules as inhibitors of transcription factors. Med. Res. Rev. 33, 823–846 (2013).
Gniazdowski, M., Denny, W. A., Nelson, S. M. & Czyz, M. Transcription factors as targets for DNA-interacting drugs. Curr. Med. Chem. 10, 909–924 (2003).
Gniazdowski, M., Denny, W. A., Nelson, S. M. & Czyz, M. Effects of anticancer drugs on transcription factor–DNA interactions. Expert Opin. Ther. Targets 9, 471–489 (2005).
Dervan, P. B. Molecular recognition of DNA by small molecules. Bioorg. Med. Chem. 9, 2215–2235 (2001).
Trauger, J. W., Baird, E. E. & Dervan, P. B. Recognition of DNA by designed ligands at subnanomolar concentrations. Nature 382, 559–561 (1996).
Best, T. P., Edelson, B. S., Nickols, N. G. & Dervan, P. B. Nuclear localization of pyrrole–imidazole polyamide–fluorescein conjugates in cell culture. Proc. Natl Acad. Sci. USA 100, 12063–12068 (2003).
Antony-Debre, I. et al. Pharmacological inhibition of the transcription factor PU.1 in leukemia. J. Clin. Invest. 127, 4297–4313 (2017).
Chen, Y. N. et al. Allosteric inhibition of SHP2 phosphatase inhibits cancers driven by receptor tyrosine kinases. Nature 535, 148–152 (2016). This study describes the development of an inhibitor of SHP2 based on stabilization of the auto-inhibited state of SHP2.
Graves, B. J. et al. Autoinhibition as a transcriptional regulatory mechanism. Cold Spring Harb. Symp. Quant. Biol. 63, 621–629 (1998).
Pufall, M. A. & Graves, B. J. Autoinhibitory domains: modular effectors of cellular regulation. Annu. Rev. Cell Dev. Biol. 18, 421–462 (2002).
Hollenhorst, P. C., McIntosh, L. P. & Graves, B. J. Genomic and biochemical insights into the specificity of ETS transcription factors. Annu. Rev. Biochem. 80, 437–471 (2011).
Lanning, B. R. et al. A road map to evaluate the proteome-wide selectivity of covalent kinase inhibitors. Nat. Chem. Biol. 10, 760–767 (2014).
Keating, G. M. Afatinib: a review of its use in the treatment of advanced non-small cell lung cancer. Drugs 74, 207–221 (2014).
Dungo, R. T. & Keating, G. M. Afatinib: first global approval. Drugs 73, 1503–1515 (2013).
Cramer, P., Hallek, M. & Eichhorst, B. State-of-the-art treatment and novel agents in chronic lymphocytic leukemia. Oncol. Res. Treat. 39, 25–32 (2016).
Shlomai, J. Redox control of protein–DNA interactions: from molecular mechanisms to significance in signal transduction, gene expression, and DNA replication. Antioxid. Redox Signal. 13, 1429–1476 (2010).
Akamatsu, Y. et al. Redox regulation of the DNA binding activity in transcription factor PEBP2. The roles of two conserved cysteine residues. J. Biol. Chem. 272, 14497–14500 (1997).
DeHart, C. J., Chahal, J. S., Flint, S. J. & Perlman, D. H. Extensive post-translational modification of active and inactivated forms of endogenous p53. Mol. Cell. Proteom. 13, 1–17 (2014).
Blumenthal, E. et al. Covalent modifications of RUNX proteins: structure affects function. Adv. Exp. Med. Biol. 962, 33–44 (2017).
Uversky, V. N. Intrinsic disorder, protein–protein interactions, and disease. Adv. Protein Chem. Struct. Biol. 110, 85–121 (2018).
Dunker, A. K., Brown, C. J., Lawson, J. D., Iakoucheva, L. M. & Obradovic, Z. Intrinsic disorder and protein function. Biochemistry 41, 6573–6582 (2002).
Dyson, H. J. & Wright, P. E. Intrinsically unstructured proteins and their functions. Nat. Rev. Mol. Cell Biol. 6, 197–208 (2005).
Iakoucheva, L. M., Brown, C. J., Lawson, J. D., Obradovic, Z. & Dunker, A. K. Intrinsic disorder in cell-signaling and cancer-associated proteins. J. Mol. Biol. 323, 573–584 (2002).
Dyson, H. J. & Wright, P. E. Role of intrinsic protein disorder in the function and interactions of the transcriptional coactivators CREB-binding protein (CBP) and p300. J. Biol. Chem. 291, 6714–p6722 (2016).
Leach, B. I. et al. Leukemia fusion target AF9 is an intrinsically disordered transcriptional regulator that recruits multiple partners via coupled folding and binding. Structure 21, 176–183 (2013). This paper provides an example of an intrinsically disordered region that mediates specific cofactor binding to the leukaemia fusion protein MLL–AF9.
Kuntimaddi, A. et al. Degree of recruitment of DOT1L to MLL–AF9 defines level of H3K79 di- and tri-methylation on target genes and transformation potential. Cell Rep. 11, 808–820 (2015). This paper provides an example of the binding of a cofactor (DOT1L) to an intrinsically disordered region (the AF9 portion of the MLL–AF9 fusion protein) that is essential for leukaemia development.
Lokken, A. A. et al. Importance of a specific amino acid pairing for murine MLL leukemias driven by MLLT1/3 or AFF1/4. Leukemia Res. 38, 1309–1315 (2014).
Zhang, Y., Cao, H. & Liu, Z. Binding cavities and druggability of intrinsically disordered proteins. Protein Sci. 24, 688–705 (2015).
Berg, T. et al. Small-molecule antagonists of Myc/Max dimerization inhibit Myc-induced transformation of chicken embryo fibroblasts. Proc. Natl Acad. Sci. USA 99, 3830–3835 (2002).
Shi, J., Stover, J. S., Whitby, L. R., Vogt, P. K. & Boger, D. L. Small molecule inhibitors of Myc/Max dimerization and Myc-induced cell transformation. Bioorg. Med. Chem. Lett. 19, 6038–6041 (2009).
Erkizan, H. V. et al. A small molecule blocking oncogenic protein EWS–FLI1 interaction with RNA helicase A inhibits growth of Ewing's sarcoma. Nat. Med. 15, 750–756 (2009).
Zhang, Z. et al. Chemical perturbation of an intrinsically disordered region of TFIID distinguishes two modes of transcription initiation. eLife 4, e07777 (2015).
Srinivasan, R. S. et al. The synthetic peptide PFWT disrupts AF4–AF9 protein complexes and induces apoptosis in t(4;11) leukemia cells. Leukemia 18, 1364–1372 (2004).
Jin, F., Yu, C., Lai, L. & Liu, Z. Ligand clouds around protein clouds: a scenario of ligand binding with intrinsically disordered proteins. PLOS Comp. Biol. 9, e1003249 (2013).
Cohen-Solal, K. A., Kaufman, H. L. & Lasfar, A. Transcription factors as critical players in melanoma invasiveness, drug resistance, and opportunities for therapeutic drug development. Pigment. Cell Melanoma Res. 31, 241–252 (2018).
Garcia-Alonso, L. et al. Transcription factor activities enhance markers of drug sensitivity in cancer. Cancer Res. 78, 769–780 (2018).
Zecena, H. et al. Systems biology analysis of mitogen activated protein kinase inhibitor resistance in malignant melanoma. BMC Syst. Biol. 12, 33 (2018).
Yao, S., Fan, L. Y. & Lam, E. W. The FOXO3–FOXM1 axis: a key cancer drug target and a modulator of cancer drug resistance. Semin. Cancer Biol. 50, 77–89 (2018).
Barabe, F., Kennedy, J. A., Hope, K. J. & Dick, J. E. Modeling the initiation and progression of human acute leukemia in mice. Science 316, 600–604 (2007).
Krivtsov, A. V. et al. Transformation from committed progenitor to leukaemia stem cell initiated by MLL–AF9. Nature 442, 818–822 (2006).
Kuo, Y. H. et al. Cbfβ–SMMHC induces distinct abnormal myeloid progenitors able to develop acute myeloid leukemia. Cancer Cell 9, 57–68 (2006).
Bell, R. J. et al. Cancer. The transcription factor GABP selectively binds and activates the mutant TERT promoter in cancer. Science 348, 1036–1039 (2015).
Makowski, M. M. et al. An interaction proteomics survey of transcription factor binding at recurrent TERT promoter mutations. Proteomics 16, 417–426 (2016).
Ptasinska, A. et al. Depletion of RUNX1/ETO in t(8;21) AML cells leads to genome-wide changes in chromatin structure and transcription factor binding. Leukemia 26, 1829–1841 (2012).
Zhang, H. et al. KLF8 involves in TGF-β-induced EMT and promotes invasion and migration in gastric cancer cells. J. Cancer Res. Clin. Oncol. 139, 1033–1042 (2013).
Micalizzi, D. S. et al. The Six1 homeoprotein induces human mammary carcinoma cells to undergo epithelial–mesenchymal transition and metastasis in mice through increasing TGF-β signaling. J. Clin. Invest. 119, 2678–2690 (2009).
Pratap, J. et al. The Runx2 osteogenic transcription factor regulates matrix metalloproteinase 9 in bone metastatic cancer cells and controls cell invasion. Mol. Cell. Biol. 25, 8581–8591 (2005).
Chimge, N. O. et al. Regulation of breast cancer metastasis by Runx2 and estrogen signaling: the role of SNAI2. Breast Cancer Res. 13, R127 (2011).
Mendoza-Villanueva, D., Deng, W., Lopez-Camacho, C. & Shore, P. The Runx transcriptional co-activator, CBFβ, is essential for invasion of breast cancer cells. Mol. Cancer 9, 171 (2010).
Baniwal, S. K. et al. Runx2 transcriptome of prostate cancer cells: insights into invasiveness and bone metastasis. Mol. Cancer 9, 258 (2010).
Little, G. H. et al. Differential effects of RUNX2 on the androgen receptor in prostate cancer: synergistic stimulation of a gene set exemplified by SNAI2 and subsequent invasiveness. Cancer Res. 74, 2857–2868 (2014).
Little, G. H. et al. Genome-wide Runx2 occupancy in prostate cancer cells suggests a role in regulating secretion. Nucleic Acids Res. 40, 3538–3547 (2012).
de The, H., Pandolfi, P. P. & Chen, Z. Acute promyelocytic leukemia: a paradigm for oncoprotein-targeted cure. Cancer Cell 32, 552–560 (2017).
Matkar, S. et al. An epigenetic pathway regulates sensitivity of breast cancer cells to HER2 inhibition via FOXO/c-Myc axis. Cancer Cell 28, 472–485 (2015).
Hirade, T. et al. Internal tandem duplication of FLT3 deregulates proliferation and differentiation and confers resistance to the FLT3 inhibitor AC220 by up-regulating RUNX1 expression in hematopoietic cells. Int. J. Hematol. 103, 95–106 (2016).
Cauchy, P. et al. Chronic FLT3–ITD signaling in acute myeloid leukemia is connected to a specific chromatin signature. Cell Rep. 12, 821–836 (2015).
Boregowda, R. K. et al. The transcription factor RUNX2 regulates receptor tyrosine kinase expression in melanoma. Oncotarget 7, 29689–29707 (2016).
Sanda, T. et al. Core transcriptional regulatory circuit controlled by the TAL1 complex in human T cell acute lymphoblastic leukemia. Cancer Cell 22, 209–221 (2012).
Morita, K. et al. Autonomous feedback loop of RUNX1–p53–CBFB in acute myeloid leukemia cells. Sci. Rep. 7, 16604 (2017).
Tetsu, O. & McCormick, F. ETS-targeted therapy: can it substitute for MEK inhibitors? Clin. Transl. Med. 6, 16 (2017).
Rakhra, K. et al. CD4(+) T cells contribute to the remodeling of the microenvironment required for sustained tumor regression upon oncogene inactivation. Cancer Cell 18, 485–498 (2010).
Xu, Y. et al. Translation control of the immune checkpoint in cancer and its therapeutic targeting. Nat. Med. 25, 301–311 (2019).
Cerezo, M. et al. Translational control of tumor immune escape via the eIF4F–STAT1–PD-L1 axis in melanoma. Nat. Med. 24, 1877–1886 (2018).
Elias, S. et al. Immune evasion by oncogenic proteins of acute myeloid leukemia. Blood 123, 1535–1543 (2014).
US National Library of Medicine. ClinicalTrials.gov, http://www.clinicaltrials.gov/ct2/show/NCT02633059 (2015).
US National Library of Medicine. ClinicalTrials.gov, http://www.clinicaltrials.gov/ct2/show/NCT03287245 (2017).
US National Library of Medicine. ClinicalTrials.gov, http://www.clinicaltrials.gov/ct2/show/NCT02670044 (2016).
US National Library of Medicine. ClinicalTrials.gov, http://www.clinicaltrials.gov/ct2/show/NCT03135262 (2017).
US National Library of Medicine. ClinicalTrials.gov, http://www.clinicaltrials.gov/ct2/show/NCT03566485 (2018).
US National Library of Medicine. ClinicalTrials.gov, http://www.clinicaltrials.gov/ct2/show/NCT03850535 (2019).
US National Library of Medicine. ClinicalTrials.gov, http://www.clinicaltrials.gov/ct2/show/NCT02890069 (2016).
US National Library of Medicine. ClinicalTrials.gov, http://www.clinicaltrials.gov/ct2/show/NCT02780128 (2016).
US National Library of Medicine. ClinicalTrials.gov, http://www.clinicaltrials.gov/ct2/show/NCT02601378 (2016).
US National Library of Medicine. ClinicalTrials.gov, http://www.clinicaltrials.gov/ct2/show/NCT03888612 (2019).
US National Library of Medicine. ClinicalTrials.gov, http://www.clinicaltrials.gov/ct2/show/NCT03134638 (2019).
US National Library of Medicine. ClinicalTrials.gov, http://www.clinicaltrials.gov/ct2/show/NCT02959437 (2016).
US National Library of Medicine. ClinicalTrials.gov, http://www.clinicaltrials.gov/ct2/show/NCT02711137 (2016).
US National Library of Medicine. ClinicalTrials.gov, http://www.clinicaltrials.gov/ct2/show/NCT02419417 (2015).
Acknowledgements
The author thanks the many outstanding trainees in the laboratory over the years who facilitated the work and whose many stimulating discussions guided the composition of this Review, and D. Brautigan at University of Virginia, USA, for helping produce a readable meaningful scientific review. Apologies to those whose important contributions have not been highlighted owing to space limitations.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing Interests
J.H.B. has a licensing agreement with Systems Oncology for the CBFβ–SMMHC inhibitor AI-10-49 (LeuSO).
Additional information
Peer review information
Nature Reviews Cancer thanks A. Koehler, D. Tenen and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Hotspot residues
-
Specific amino acids on a protein–protein interaction surface that contribute the most energy to the binding of the two proteins.
- Interaction energy
-
The energy, typically measured in kilocalories per mole, associated with the binding of two species to one another (protein–protein, protein–nucleic acid, protein–small molecule).
- Allosteric modulation
-
Acting by binding at a site distinct from the primary site of activity of a protein, for example, at a site distinct from the active site of an enzyme.
- Epigenetic reader
-
A protein that binds to peptide elements, typically from histones or transcription factors, that have specific post-translational modifications, for example, methylation, acetylation or phosphorylation.
- Epigenetic writer
-
A protein that adds specific post-translational modifications, including methylation and acetylation, to peptide elements in histones and transcription factors.
- Bioavailability
-
The proportion of a drug that enters the circulation after administration and can have an active effect.
- Castration-resistant prostate cancer
-
(CRPC). Prostate cancer that progresses despite androgen depletion therapy.
- Absorption, distribution, metabolism, excretion, toxicity
-
(ADMET). Important properties of drugs that determine their efficacy.
- Definitive haematopoiesis
-
Blood cell development involving haematopoietic stem cells that differentiate to produce all of the lineages of the haematopoietic system. In contrast to primitive (embryonic) haematopoiesis, this process occurs later in development.
- Nuclear magnetic resonance (NMR) spectroscopy
-
A technique that relies on energy differences of nuclear spins in a magnetic field that is used for determining protein 3D structure, protein dynamics and drug binding to proteins
- Deubiquitinases
-
(DUBs). Enzymes that remove ubiquitin from proteins.
- Michael acceptor
-
A chemical moiety that can react with amino acid side chains in a protein to form a covalent bond with the protein
- K d
-
The equilibrium dissociation constant, a measure of the affinity of binding of two molecules to one another.
- DNA minor groove
-
Along with the major groove, the minor groove makes up the 3D structure of DNA and provides contacts to bind proteins or small molecules.
- Chemoproteomics
-
The use of proteomics approaches, typically based on functionalized chemical probes in conjunction with mass spectrometry, to identify the targets of action of molecules in cells.
- pK a
-
The negative log of the equilibrium association constant, used for characterizing the acidity of exchangeable protons on the side chains of amino acids in proteins.
- X-ray crystallography
-
A technique that utilizes the diffraction of X-rays to determine the 3D structure of proteins and nucleic acids.
Rights and permissions
About this article
Cite this article
Bushweller, J.H. Targeting transcription factors in cancer — from undruggable to reality. Nat Rev Cancer 19, 611–624 (2019). https://doi.org/10.1038/s41568-019-0196-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41568-019-0196-7
This article is cited by
-
EGR1 suppresses HCC growth and aerobic glycolysis by transcriptionally downregulating PFKL
Journal of Experimental & Clinical Cancer Research (2024)
-
Transcription factor ZIC2 regulates the tumorigenic phenotypes associated with both bulk and cancer stem cells in epithelial ovarian cancer
Oncogene (2024)
-
An integrated ceRNA network identifies miR-375 as an upregulated miRNA playing a tumor suppressive role in aggressive prostate cancer
Oncogene (2024)
-
Synthetic biology approaches for improving the specificity and efficacy of cancer immunotherapy
Cellular & Molecular Immunology (2024)
-
Decoding gene regulatory circuitry underlying TNBC chemoresistance reveals biomarkers for therapy response and therapeutic targets
npj Precision Oncology (2024)