Abstract
For the design and development of innovative carbon nanotube (CNT)-based tools and applications, an understanding of the molecular interactions between CNTs and biological systems is essential. In this study, a three-dimensional protein-structure-based in silico screen identified the paired immune receptors, sialic acid immunoglobulin-like binding lectin-5 (Siglec-5) and Siglec-14, as CNT-recognizing receptors. Molecular dynamics simulations showed the spatiotemporally stable association of aromatic residues on the extracellular loop of Siglec-5 with CNTs. Siglec-14 mediated spleen tyrosine kinase (Syk)-dependent phagocytosis of multiwalled CNTs and the subsequent secretion of interleukin-1β from human monocytes. Ectopic in vivo expression of human Siglec-14 on mouse alveolar macrophages resulted in enhanced recognition of multiwalled CNTs and exacerbated pulmonary inflammation. Furthermore, fostamatinib, a Syk inhibitor, blocked Siglec-14-mediated proinflammatory responses. These results indicate that Siglec-14 is a human activating receptor recognizing CNTs and that blockade of Siglec-14 and the Syk pathway may overcome CNT-induced inflammation.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The data supporting the findings of this study are available within the Article, Extended Data, Supplementary Information and Source Data files. Structural data obtained from the MD simulations are available via BSM-Arc at https://bsma.pdbj.org/entry/31. Other relevant data are available for research purposes from the corresponding authors upon request. Source data are provided with this paper.
Code availability
The relevant in-house codes applied in this study are available from the corresponding authors upon request.
References
De Volder, M. F., Tawfick, S. H., Baughman, R. H. & Hart, A. J. Carbon nanotubes: present and future commercial applications. Science 339, 535–539 (2013).
Huang, H., Feng, W., Chen, Y. & Shi, J. L. Inorganic nanoparticles in clinical trials and translations. Nano Today 35, 100972 (2020).
Hansen, S. F. & Lennquist, A. SIN list criticism based on misunderstandings. Nat. Nanotechnol. 15, 418 (2020).
Nel, A. E. et al. Understanding biophysicochemical interactions at the nano–bio interface. Nat. Mater. 8, 543–557 (2009).
Rennick, J. J., Johnston, A. P. R. & Parton, R. G. Key principles and methods for studying the endocytosis of biological and nanoparticle therapeutics. Nat. Nanotechnol. 16, 266–276 (2021).
Yang, W., Wang, L., Mettenbrink, E. M., DeAngelis, P. L. & Wilhelm, S. Nanoparticle toxicology. Annu. Rev. Pharmacol. Toxicol. 61, 269–289 (2021).
Poland, C. A. et al. Carbon nanotubes introduced into the abdominal cavity of mice show asbestos-like pathogenicity in a pilot study. Nat. Nanotechnol. 3, 423–428 (2008).
Nagai, H. et al. Diameter and rigidity of multiwalled carbon nanotubes are critical factors in mesothelial injury and carcinogenesis. Proc. Natl Acad. Sci. USA 108, E1330–E1338 (2011).
Donaldson, K., Murphy, F. A., Duffin, R. & Poland, C. A. Asbestos, carbon nanotubes and the pleural mesothelium: a review of the hypothesis regarding the role of long fibre retention in the parietal pleura, inflammation and mesothelioma. Part. Fibre Toxicol. 7, 5 (2010).
Dostert, C. et al. Innate immune activation through Nalp3 inflammasome sensing of asbestos and silica. Science 320, 674–677 (2008).
Franklin, B. S., Mangan, M. S. & Latz, E. Crystal formation in inflammation. Annu. Rev. Immunol. 34, 173–202 (2016).
Palomaki, J. et al. Long, needle-like carbon nanotubes and asbestos activate the NLRP3 inflammasome through a similar mechanism. ACS Nano 5, 6861–6870 (2011).
Miyanishi, M. et al. Identification of Tim4 as a phosphatidylserine receptor. Nature 450, 435–439 (2007).
Omori, S. et al. Tim4 recognizes carbon nanotubes and mediates phagocytosis leading to granuloma formation. Cell Rep. 34, 108734 (2021).
Kobayashi, N. et al. TIM-1 and TIM-4 glycoproteins bind phosphatidylserine and mediate uptake of apoptotic cells. Immunity 27, 927–940 (2007).
Berman, H., Henrick, K., Nakamura, H. & Markley, J. L. The worldwide Protein Data Bank (wwPDB): ensuring a single, uniform archive of PDB data. Nucleic Acids Res. 35, D301–D303 (2007).
Duan, S. & Paulson, J. C. Siglecs as immune cell checkpoints in disease. Annu. Rev. Immunol. 38, 365–395 (2020).
Crocker, P. R., Paulson, J. C. & Varki, A. Siglecs and their roles in the immune system. Nat. Rev. Immunol. 7, 255–266 (2007).
Hayami, T., Higo, J., Nakamura, H. & Kasahara, K. Multidimensional virtual-system coupled canonical molecular dynamics to compute free-energy landscapes of peptide multimer assembly. J. Comput. Chem. 40, 2453–2463 (2019).
Jumper, J. et al. Highly accurate protein structure prediction with AlphaFold. Nature 596, 583–589 (2021).
Angata, T., Hingorani, R., Varki, N. M. & Varki, A. Cloning and characterization of a novel mouse Siglec, mSiglec-F: differential evolution of the mouse and human (CD33) Siglec-3-related gene clusters. J. Biol. Chem. 276, 45128–45136 (2001).
Angata, T., Hayakawa, T., Yamanaka, M., Varki, A. & Nakamura, M. Discovery of Siglec-14, a novel sialic acid receptor undergoing concerted evolution with Siglec-5 in primates. FASEB J. 20, 1964–1973 (2006).
Wilson, A. A. et al. Amelioration of emphysema in mice through lentiviral transduction of long-lived pulmonary alveolar macrophages. J. Clin. Invest. 120, 379–389 (2010).
Yamanaka, M., Kato, Y., Angata, T. & Narimatsu, H. Deletion polymorphism of Siglec14 and its functional implications. Glycobiology 19, 841–846 (2009).
Toyokuni, S. Genotoxicity and carcinogenicity risk of carbon nanotubes. Adv. Drug Deliv. Rev. 65, 2098–2110 (2013).
D’Astolfo, D. S. et al. Efficient intracellular delivery of native proteins. Cell 161, 674–690 (2015).
Gonzalez-Durruthy, M., Concu, R., Ruso, J. M. & Cordeiro, M. New mechanistic insights on carbon nanotubes’ nanotoxicity using isolated submitochondrial particles, molecular docking, and nano-QSTR approaches. Biol. (Basel) 10, 171 (2021).
Ge, C. et al. Binding of blood proteins to carbon nanotubes reduces cytotoxicity. Proc. Natl Acad. Sci. USA 108, 16968–16973 (2011).
Zhang, J. et al. Molecular recognition using corona phase complexes made of synthetic polymers adsorbed on carbon nanotubes. Nat. Nanotechnol. 8, 959–968 (2013).
Lanzarotti, E., Defelipe, L. A., Marti, M. A. & Turjanski, A. G. Aromatic clusters in protein–protein and protein–drug complexes. J. Cheminform. 12, 30 (2020).
Westbrook, J., Ito, N., Nakamura, H., Henrick, K. & Berman, H. M. PDBML: the representation of archival macromolecular structure data in XML. Bioinformatics 21, 988–992 (2005).
Kinjo, A. R. et al. Protein Data Bank Japan (PDBj): updated user interfaces, resource description framework, analysis tools for large structures. Nucleic Acids Res. 45, D282–D288 (2017).
Fu, Q. et al. Stimuli-responsive plasmonic assemblies and their biomedical applications. Nano Today 36, 101014 (2021).
Jorgensen, W. L., Maxwell, D. S. & TiradoRives, J. Development and testing of the OPLS all-atom force field on conformational energetics and properties of organic liquids. J. Am. Chem. Soc. 118, 11225–11236 (1996).
Jorgensen, W. L., Chandrasekhar, J., Madura, J. D., Impey, R. W. & Klein, M. L. Comparison of simple potential functions for simulating liquid water. J. Chem. Phys. 79, 926–935 (1983).
Darden, T., York, D. & Pedersen, L. Particle mesh Ewald—an N.log(N) method for Ewald sums in large systems. J. Chem. Phys. 98, 10089–10092 (1993).
Essmann, U. et al. A smooth particle mesh Ewald method. J. Chem. Phys. 103, 8577–8593 (1995).
Abraham, M. J. et al. Gromacs: high performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX 1-2, 19–25 (2015).
Berendsen, H. J. C., Postma, J. P. M., Vangunsteren, W. F., Dinola, A. & Haak, J. R. Molecular-dynamics with coupling to an external bath. J. Chem. Phys. 81, 3684–3690 (1984).
Nose, S. A molecular-dynamics method for simulations in the canonical ensemble. Mol. Phys. 52, 255–268 (1984).
Hess, B., Bekker, H., Berendsen, H. J. C. & Fraaije, J. G. E. M. LINCS: a linear constraint solver for molecular simulations. J. Comput. Chem. 18, 1463–1472 (1997).
Daszykowski, M & Walczak, B. In Comprehensive Chemometrics (eds Brown, S. D., Tauler, R. & Walczak, B.), 635–654 (Elsevier, 2009).
Huerta-Cepas, J., Serra, F. & Bork, P. ETE 3: reconstruction, analysis, and visualization of phylogenomic data. Mol. Biol. Evol. 33, 1635–1638 (2016).
Price, M. N., Dehal, P. S. & Arkin, A. P. FastTree 2—approximately maximum-likelihood trees for large alignments. PLoS One 5, e9490 (2010).
Varadi, M. et al. AlphaFold Protein Structure Database: massively expanding the structural coverage of protein-sequence space with high-accuracy models. Nucleic Acids Res. 50, D439–D444 (2022).
Tsugita, M., Morimoto, N., Tashiro, M., Kinoshita, K. & Nakayama, M. SR-B1 Is a silica receptor that mediates canonical inflammasome activation. Cell Rep. 18, 1298–1311 (2017).
Kitamura, T. et al. Retrovirus-mediated gene transfer and expression cloning: powerful tools in functional genomics. Exp. Hematol. 31, 1007–1014 (2003).
Naito, Y., Hino, K., Bono, H. & Ui-Tei, K. CRISPRdirect: software for designing CRISPR/Cas guide RNA with reduced off-target sites. Bioinformatics 31, 1120–1123 (2015).
Sanjana, N. E., Shalem, O. & Zhang, F. Improved vectors and genome-wide libraries for CRISPR screening. Nat. Methods 11, 783–784 (2014).
Lam, C. W., James, J. T., McCluskey, R., Arepalli, S. & Hunter, R. L. A review of carbon nanotube toxicity and assessment of potential occupational and environmental health risks. Crit. Rev. Toxicol. 36, 189–217 (2006).
Flynn, R. et al. Targeting Syk-activated B cells in murine and human chronic graft-versus-host disease. Blood 125, 4085–4094 (2015).
Agarwal, K. et al. Inhibition of mucin-type O-glycosylation through metabolic processing and incorporation of N-thioglycolyl-d-galactosamine peracetate (Ac(5)GalNTGc). J. Am. Chem. Soc. 135, 14189–14197 (2013).
Ito, F. et al. Asbestos conceives Fe(II)-dependent mutagenic stromal milieu through ceaseless macrophage ferroptosis and β-catenin induction in mesothelium. Redox Biol. 36, 101616 (2020).
Maejima, I. et al. Autophagy sequesters damaged lysosomes to control lysosomal biogenesis and kidney injury. EMBO J. 32, 2336–2347 (2013).
Acknowledgements
We thank M. Ema (Shiga University of Medical Science) for advice and technical support on mouse experiments. We also thank the members of our laboratories for helpful suggestions. This work was supported by the Japan Science and Technology Agency (JST), PRESTO under grant number JPMJPR17H9 to M.N., JST CREST under grant number JPMJCR19H4 to S.T., the Japan Society for the Promotion of Sciences (JSPS) under grant numbers JP19H03880 and JP22H03340 to M.N., JP20K12069 and JP21K06052 to K. Kasahara, and the Uehara Memorial Foundation to M.N. This research was partially supported by the Platform Project for Supporting Drug Discovery and Life Science Research (Basis for Supporting Innovative Drug Discovery and Life Science Research (BINDS)) from AMED under grant number JP19am0101067 (support number 0314). The computational resources were provided by the HPCI System Research Project (project IDs hp200063, hp200090, hp210005 and hp210008), the NIG supercomputer at ROIS National Institute of Genetics and the Human Genome Center (University of Tokyo).
Author information
Authors and Affiliations
Contributions
K. Kasahara and M.N. designed the experiments. S.-I.Y., Q.X., F.I., K.T., Y.K., M.K., K. Kasahara and M.N. performed the experiments and analysed data. S.O., H.T., K. Kinoshita, T.T. and S.T. provided critical materials and advice. K. Kasahara and M.N. wrote the manuscript. All the authors discussed the results and assisted in the preparation of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests. K. Kasahara is now an employee of Japan Tobacco Inc. This company is not involved in this study.
Peer review
Peer review information
Nature Nanotechnology thanks Yuliang Zhao and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Siglec-5, but not Siglec-3, recognizes MWCNTs.
a, Siglec-5, Siglec-3, and Tim4 expressions on NIH-3T3 cells were analyzed by flow cytometry. b, Parental NIH-3T3 cells and NIH-3T3 cells stably expressing Siglec-5, Siglec-3, or Tim4 were cultured with (shaded histograms) or without (open histograms) MWCNTs (10 or 30 μg/ml) for 30 min. Binding was analyzed by flow cytometry. Numbers indicate the median side scatter intensity (MSI). The delta median side scatter intensity (∆MSI) was calculated by subtracting MSI of MWCNT-treated cells from MSI of untreated cells in Fig. 1c. The gating strategy is shown in Supplementary Fig. 12.
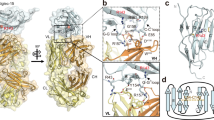
Extended Data Fig. 2 Schematic diagrams of architecture of Siglec-5 with the CNT interfaces.
a, Extracellular domains of Siglec-5 are abstracted as two connected cylinders. The four interfaces (1) through (4) are placed at the extracellular bottom of the cylinder. b, The topology diagram emphasizes the positions of interfaces along the sequence.
Extended Data Fig. 3 A cluster analysis of Siglec-5-CNT binding modes observed in the MD simulations.
a, Relative contact frequency of each Siglec-5 residue in each cluster shows that modes 1 and 2 recognize CNT via V-set Ig-like domain. Mode 4 and 5 contact CNT via C2-set Ig-like domain neighboring a cytoplasmic domain. Mode 3 is the unbound form. b, Schematic diagram presents the characteristics of each mode.
Extended Data Fig. 4 Snapshots of Siglec-5 and −3 in the VcMD simulations.
a and b, Snapshots of Siglec-5 (a) and −3 (b) binding to CNT with the mode 2. All the four interfaces (labelled as (1) to (4)) directed to the CNT surface. However, Siglec-3 does not have side chains with π-electron system which attractively interact with the CNT surface. c and d, Snapshots of Siglec-5 (c) and −3 (d) binding to CNT with the mode 1*, which makes contact with CNT via the interface (2).
Extended Data Fig. 5 Multiple sequence alignments and AlphaFold-predicted structures of Siglecs.
a, Amino acid sequences of Siglec V-set Ig-like domain were aligned with CLUSTALW. Red solid squares indicate aromatic clusters. Red dot squares indicate an incomplete aromatic cluster. Numbers indicate amino acid positions of the N-terminal V-set Ig-like domain without signal sequence of Siglec-7. b, A phylogenetic tree was generated with CLUSTALW. Numbers indicate branch lengths. c, Protein structures of Siglecs were predicted by AlphaFold. The extracellular loops are surrounded by black dot squares. Aromatic residues in the extracellular loops are indicated by black arrowheads.
Extended Data Fig. 6 Generation of anti-Siglec-5/14 neutralizing mAbs.
a, Parental Jurkat.EcoR cells, and Jurkat.EcoR cells stably expressing Siglec-5 or Siglec-14 were pretreated with control mouse IgG1 (cIg), or anti-Siglec-5/14 neutralizing mAbs, SY1 and SY2 (10 μg/ml each), and then these cells were cultured with MWCNTs (30 μg/ml) for 30 min. MWCNT recognition was analyzed as in Fig. 1c. b, The indicated Jurkat.EcoR cells and NIH-3T3 cells were stained with biotinylated cIg, SY1, or SY2, followed by PE-streptavidin. Cells were analyzed by flow cytometry.
Extended Data Fig. 7 Effect of sialidase on Siglec-14 responses to MWCNTs.
a, Siglec-14/THP-1 cells were treated with the indicated activity of sialidase for 1 hr and were then stained with indicated mAbs. Desialylation was assessed by flow cytometry using anti-sialylated CD43 mAb (clone 1G10). b, Cells and MWCNTs (30 μg/ml) were each pretreated with sialidase as in a, and then were combined and cultured for 30 min. MWCNT binding was analyzed by flow cytometry. c, MWCNTs (30 μg/ml) and PMA-primed cells were each pretreated with sialidase as in a, and then were combined and cultured for 5 hr in serum-free medium containing 1% BSA. IL-1β secretion was analyzed by ELISA. Data are shown as mean ± SD (n = 3). ***p < 0.001, two-way ANOVA with Tukey-Kramer test.
Extended Data Fig. 8 Siglec-14 recognize SWCNTs resulting in induction of IL-8, but not IL-1β, secretion.
a, b, Size and shape of MWCNTs and SWCNTs were analyzed by transmission electron microscopy (a), and by light microscopy in 0.5% BSA/PBS (b). c, MWCNTs and SWCNTs were stained with cIg (black lines) or Siglec-14-Ig (blue lines), followed by AF647-anti-mouse IgG in 0.5% BSA/PBS. Siglec-14-Ig binding was analyzed by flow cytometry. d, Indicated THP-1 cells were primed with PMA (0.5 μM) for 12 hr and were treated with the indicated dose of MWCNTs or SWCNTs for 5 hr. IL-1β secretion was analyzed by ELISA. Data are shown as mean ± SD (n = 3). e, Indicated THP-1 cells were stimulated with the indicated dose of MWCNTs or SWCNTs for 5 hr. IL-8 secretion was analyzed by ELISA. See also Method section. Data are shown as mean ± SD (n = 3). **p = 0.0364, ***p < 0.01, two-way ANOVA with Tukey-Kramer test to compare each mean with every other mean.
Extended Data Fig. 9 Galectin-3-fluorescent reporter system for detection of lysosomal damage.
a, Galectin-3 is normally distributed throughout cytoplasm and nucleus. Upon lysosomal membrane rupture, Galectin-3 is rapidly recruited lysosomes to access the luminal β-galactoside sugar-containing carbohydrates. Galectin-3 fused with monomeric azami-green fluorescent protein (mAG-Gal3) allows visualization of galectin-3 re-localization and is used as a tool to monitor vesicle rupture. Figure was drawn with BioRender.com. b, THP-1 cells stably expressing Siglec-14 and mAG-Gal3 were stimulated with MWCNTs, SWCNTs (100 μg/ml each), or a lysosomotropic compound L-Leucyl-L-leucine methy ester (LLOMe; 3 mM) for 3 hr. Cells were stained with DAPI and were analyzed by fluorescence microcopy.
Extended Data Fig. 10 Model of Siglec-14-mediated pro-inflammatory responses to MWCNTs and SWCNTs.
a, Upon MWCNT recognition, Siglec-14 transmits activation signals leading to phagocytosis and IL-8 induction. Phagocytosed MWCNTs cause phagosomal rupture, resulting in NLRP3 inflammasome activation and caspase-1 activation leading to IL-1β secretion. b, Upon SWCNT recognition, Siglec-14 transmits activation signals leading to phagocytosis and IL-8 induction. Since phagocytosed SWCNTs do not cause phagosomal rupture, IL-1β is not secreted. Figure was drawn with BioRender.com.
Supplementary information
Supplementary Information
Supplementary Note and Figs. 1-13.
Supplementary Video 1
A side-view movie of an MD simulation trajectory of the WT model. One of ten 300 ns trajectories is shown as a side-view movie.
Supplementary Video 2
A long shot movie of an MD simulation trajectory of the WT model. The same trajectory as Supplementary Movie 1 is presented from a different perspective.
Supplementary Video 3
A side-view movie of an MD simulation trajectory of the WY50/51AA model. One of ten 300 ns trajectories is shown as a side-view movie.
Supplementary Video 4
A long shot movie of an MD simulation trajectory of the WY50/51AA model. The same trajectory as Supplementary Movie 3 is presented from a different perspective.
Supplementary Video 5
A side-view movie of an MD simulation trajectory of the WYYY50/51/68/69AAAA model. One of ten 300 ns trajectories is shown as a side-view movie.
Supplementary Video 6
A long shot movie of an MD simulation trajectory of the WYYY50/51/68/69AAAA model. The same trajectory as Supplementary Movie 5 is presented from a different perspective.
Source data
Source Data Fig. 1
Statistical Source Data, In silico Source Data
Source Data Fig. 2
Statistical Source Data, In silico Source Data
Source Data Fig. 3
Statistical Source Data, Uncropped western blots
Source Data Fig. 4
Statistical Source Data
Source Data Fig. 5
Statistical Source Data, Uncropped western blots
Source Data Extended Data Fig. 3
In silico Source Data
Source Data Extended Data Fig. 7
Statistical Source Data
Source Data Extended Data Fig. 8
Statistical Source Data
Source Data Supplementary Fig. 1
Statistical Source Data
Source Data Supplementary Fig. 2
Statistical Source Data
Source Data Supplementary Fig. 3
In silico Source Data
Source Data Supplementary Fig. 5
In silico Source Data
Source Data Supplementary Fig. 7
In silico Source Data
Source Data Supplementary Fig. 8
In silico Source Data
Source Data Supplementary Fig. 10
Statistical Source Data, Uncropped western blots
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yamaguchi, SI., Xie, Q., Ito, F. et al. Carbon nanotube recognition by human Siglec-14 provokes inflammation. Nat. Nanotechnol. 18, 628–636 (2023). https://doi.org/10.1038/s41565-023-01363-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41565-023-01363-w
This article is cited by
-
Carbon nanotubes activate inflammatory signalling through binding to Siglec-14
Nature Nanotechnology (2023)
-
Human and environmental safety of carbon nanotubes across their life cycle
Nature Reviews Materials (2023)