Abstract
As global temperatures continue to rise, a key uncertainty of climate projections is the microbial decomposition of vast organic carbon stocks in thawing permafrost soils. Decomposition rates can accelerate up to fourfold in the presence of plant roots, and this mechanism—termed the rhizosphere priming effect—may be especially relevant to thawing permafrost soils as rising temperatures also stimulate plant productivity in the Arctic. However, priming is currently not explicitly included in any model projections of future carbon losses from the permafrost area. Here, we combine high-resolution spatial and depth-resolved datasets of key plant and permafrost properties with empirical relationships of priming effects from living plants on microbial respiration. We show that rhizosphere priming amplifies overall soil respiration in permafrost-affected ecosystems by ~12%, which translates to a priming-induced absolute loss of ~40 Pg soil carbon from the northern permafrost area by 2100. Our findings highlight the need to include fine-scale ecological interactions in order to accurately predict large-scale greenhouse gas emissions, and suggest even tighter restrictions on the estimated 200 Pg anthropogenic carbon emission budget to keep global warming below 1.5 °C.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
References to published data used in this study can be found in Supplementary Table 1 (PrimeSCale model), Supplementary Table 2 (meta-analysis of priming studies) and Supplementary Table 3 (meta-analysis of root depth profiles for tundra and boreal), as well as in the main text. Datasets generated for this study are available in the Bolin Centre Database (https://bolin.su.se/data/keuper-wild-2020) and include: (1) intermediate output data of the PrimeSCale model (.xls); and (2) output (Geotiff) and metadata.
Code availability
The custom code for the PrimeSCale 1.0 model, including model scripts, complementary function scripts and input data for the model, is available from the Bolin Centre code repository: https://git.bolin.su.se/bolin/keuper-wild-2020.
References
McGuire, A. D. et al. Dependence of the evolution of carbon dynamics in the northern permafrost region on the trajectory of climate change. Proc. Natl Acad. Sci. USA 115, 3882–2887 (2018).
Schuur, E. A. G. et al. Climate change and the permafrost carbon feedback. Nature 520, 171–179 (2015).
Koven, C. D. et al. A simplified, data-constrained approach to estimate the permafrost carbon-climate feedback. Phil. Trans. R. Soc. A 373, 20140423 (2015).
Xu, L. et al. Temperature and vegetation seasonality diminishment over northern lands. Nat. Clim. Change 3, 581–586 (2013).
Huo, C., Luo, Y. & Cheng, W. Rhizosphere priming effect: a meta-analysis. Soil Biol. Biochem. 111, 78–84 (2017).
Bingeman, C., Varner, J. & Martin, W. The effect of the addition of organic materials on the decomposition of an organic soil. Soil Sci. Soc. Am. J. 17, 34–38 (1953).
Fontaine, S. et al. Stability of organic carbon in deep soil layers controlled by fresh carbon supply. Nature 450, 277–280 (2007).
Kuzyakov, Y., Friedel, J. K. & Stahr, K. Review of mechanisms and quantification of priming effects. Soil Biol. Biochem. 32, 1485–1498 (2000).
Keiluweit, M. et al. Mineral protection of soil carbon counteracted by root exudates. Nat. Clim. Change 5, 588–595 (2015).
Zhang, W., Wang, X. & Wang, S. Addition of external organic carbon and native soil organic carbon decomposition: a meta-analysis. PLoS ONE 8, e54779 (2013).
Dijkstra, F. A. & Cheng, W. Interactions between soil and tree roots accelerate long-term soil carbon decomposition. Ecol. Lett. 10, 1046–1053 (2007).
Hartley, I. P. et al. A potential loss of carbon associated with greater plant growth in the European Arctic. Nat. Clim. Change 2, 875–879 (2012).
Parker, T. C., Subke, J.-A. & Wookey, P. A. Rapid carbon turnover beneath shrub and tree vegetation is associated with low soil carbon stocks at a subarctic treeline. Glob. Change Biol. 21, 2070–2081 (2015).
Sulman, B. N. et al. Feedbacks between plant N demand and rhizosphere priming depend on type of mycorrhizal association. Ecol. Lett. 20, 1043–1053 (2017).
Wild, B. et al. Input of easily available organic C and N stimulates microbial decomposition of soil organic matter in arctic permafrost soil. Soil Biol. Biochem. 75, 143–151 (2014).
Wild, B. et al. Plant-derived compounds stimulate the decomposition of organic matter in arctic permafrost soils. Sci. Rep. 6, 25607 (2016).
Pegoraro, E. et al. Glucose addition increases the magnitude and decreases the age of soil respired carbon in a long-term permafrost incubation study. Soil Biol. Biochem. 129, 201–211 (2019).
Rousk, K., Michelsen, A. & Rousk, J. Microbial control of soil organic matter mineralization responses to labile carbon in subarctic climate change treatments. Glob. Change Biol. 22, 4150–4161 (2016).
Walz, J., Knoblauch, C., Boehme, L. & Pfeiffer, E.-M. Regulation of soil organic matter decomposition in permafrost-affected Siberian tundra soils—impact of oxygen availability, freezing and thawing, temperature, and labile organic matter. Soil Biol. Biochem. 110, 34–43 (2017).
Hartley, I. P., Hopkins, D. W., Sommerkorn, M. & Wookey, P. A. The response of organic matter mineralisation to nutrient and substrate additions in sub-arctic soils. Soil Biol. Biochem. 42, 92–100 (2010).
Hugelius, G. et al. Estimated stocks of circumpolar permafrost carbon with quantified uncertainty ranges and identified data gaps. Biogeosciences 11, 6573–6593 (2014).
Harden, J. W. et al. Field information links permafrost carbon to physical vulnerabilities of thawing. Geophys. Res. Lett. 39, L15704 (2012).
Beer, C. et al. Terrestrial gross carbon dioxide uptake: global distribution and covariation with climate. Science 329, 834–839 (2010).
Koven, C. D., Lawrence, D. M. & Riley, W. J. Permafrost carbon–climate feedback is sensitive to deep soil carbon decomposability but not deep soil nitrogen dynamics. Proc. Natl Acad. Sci. USA 112, 3752–3757 (2015).
Walker, D. A. et al. The Circumpolar Arctic vegetation map. J. Veg. Sci. 16, 267–282 (2005).
Bengtson, P., Barker, J. & Grayston, S. J. Evidence of a strong coupling between root exudation, C and N availability, and stimulated SOM decomposition caused by rhizosphere priming effects. Ecol. Evol. 2, 1843–1852 (2012).
Walker, T. N. et al. Vascular plants promote ancient peatland carbon loss with climate warming. Glob. Change Biol. 22, 1880–1889 (2016).
Basiliko, N., Stewart, H., Roulet, N. T. & Moore, T. R. Do root exudates enhance peat decomposition? Geomicrobiol. J. 29, 374–378 (2012).
Gavazov, K. et al. Vascular plant-mediated controls on atmospheric carbon assimilation and peat carbon decomposition under climate change. Glob. Change Biol. 24, 3911–3921 (2018).
Knoblauch, C., Beer, C., Liebner, S. & Grigoriev, M. N. Methane production as key to the greenhouse gas budget of thawing permafrost. Nat. Clim. Change 8, 309–312 (2018).
Gentsch, N. et al. Temperature response of permafrost soil carbon is attenuated by mineral protection. Glob. Change Biol. 24, 3401–3415 (2018).
Kuzyakov, Y. Priming effects: interactions between living and dead organic matter. Soil Biol. Biochem. 42, 1363–1371 (2010).
Schenk, H. J. & Jackson, R. B. The global biogeography of roots. Ecol. Monogr. 72, 311–328 (2002).
Keuper, F. et al. Experimentally increased nutrient availability at the permafrost thaw front selectively enhances biomass production of deep-rooting subarctic peatland species. Glob. Change Biol. 23, 4257–4266 (2017).
Blume-Werry, G., Milbau, A., Teuber, L. M., Johansson, M. & Dorrepaal, E. Dwelling in the deep—strongly increased root growth and rooting depth enhance plant interactions with thawing permafrost soil. New Phytol. 223, 1328–1339 (2019).
Finger, R. A. et al. Effects of permafrost thaw on nitrogen availability and plant–soil interactions in a boreal Alaskan lowland. J. Ecol. 104, 1542–1554 (2016).
Keuper, F. et al. A frozen feast: thawing permafrost increases plant-available nitrogen in subarctic peatlands. Glob. Change Biol. 18, 1998–2007 (2012).
Wild, B. et al. Amino acid production exceeds plant nitrogen demand in Siberian tundra. Environ. Res. Lett. 13, 034002 (2018).
Pearson, R. G. et al. Shifts in Arctic vegetation and associated feedbacks under climate change. Nat. Clim. Change 3, 673–677 (2013).
Lindahl, B. D. & Tunlid, A. Ectomycorrhizal fungi—potential organic matter decomposers, yet not saprotrophs. New Phytol. 205, 1443–1447 (2015).
Zak, D. R. et al. Exploring the role of ectomycorrhizal fungi in soil carbon dynamics. New Phytol. 223, 33–39 (2019).
Soudzilovskaia, N. A. et al. Global mycorrhizal plant distribution linked to terrestrial carbon stocks. Nat. Commun. 10, 5077 (2019).
Tedersoo, L. & Bahram, M. Mycorrhizal types differ in ecophysiology and alter plant nutrition and soil processes. Biol. Rev. 94, 1857–1880 (2019).
Hultman, J. et al. Multi-omics of permafrost, active layer and thermokarst bog soil microbiomes. Nature 521, 208–212 (2015).
Monteux, S. et al. Long-term in situ permafrost thaw effects on bacterial communities and potential aerobic respiration. ISME J. 12, 2129–2141 (2018).
Mackelprang, R. et al. Metagenomic analysis of a permafrost microbial community reveals a rapid response to thaw. Nature 480, 368–371 (2011).
Johnston, E. R. et al. Responses of tundra soil microbial communities to half a decade of experimental warming at two critical depths. Proc. Natl Acad. Sci. USA 116, 15096–15105 (2019).
Monteux, S. A Song of Ice and Mud: Interactions of Microbes with Roots, Fauna and Carbon in Warming Permafrost-Affected Soils. PhD thesis, Umeå Univ. (2018).
Turetsky, M. R. et al. Carbon release through abrupt permafrost thaw. Nat. Geosci. 13, 138–143 (2020).
Wik, M., Varner, R. K., Anthony, K. W., MacIntyre, S. & Bastviken, D. Climate-sensitive northern lakes and ponds are critical components of methane release. Nat. Geosci. 9, 99–105 (2016).
Shakhova, N. et al. Ebullition and storm-induced methane release from the East Siberian Arctic Shelf. Nat. Geosci. 7, 64–70 (2014).
Goodwin, P. et al. Pathways to 1.5 °C and 2 °C warming based on observational and geological constraints. Nat. Geosci. 11, 102–107 (2018).
Brown, J., Ferrians, O. J. Jr, Heginbottom, J. A. & Melnikov, E. S. Circum-Arctic Map of Permafrost and Ground-Ice Conditions Version 2 (National Snow and Ice Data Center, 2002).
Hugelius, G. et al. The Northern Circumpolar Soil Carbon Database: spatially distributed datasets of soil coverage and soil carbon storage in the northern permafrost regions. Earth Syst. Sci. Data 5, 3–13 (2013).
Hugelius, G. et al. A new data set for estimating organic carbon storage to 3 m depth in soils of the northern circumpolar permafrost region. Earth Syst. Sci. Data 5, 393–402 (2013).
Lawrence, D. M., Slater, A. G. & Swenson, S. C. Simulation of present-day and future permafrost and seasonally frozen ground conditions in CCSM4. J. Clim. 25, 2207–2225 (2012).
Fontaine, S., Bardoux, G., Abbadie, L. & Mariotti, A. Carbon input to soil may decrease soil carbon content. Ecol. Lett. 7, 314–320 (2004).
Mooshammer, M. et al. Adjustment of microbial nitrogen use efficiency to carbon:nitrogen imbalances regulates soil nitrogen cycling. Nat. Commun. 5, 3694 (2014).
Sinsabaugh, R. L., Manzoni, S., Moorhead, D. L. & Richter, A. Carbon use efficiency of microbial communities: stoichiometry, methodology and modelling. Ecol. Lett. 16, 930–939 (2013).
Sinsabaugh, R. L. et al. Stoichiometry of microbial carbon use efficiency in soils. Ecol. Monogr. 86, 172–189 (2016).
Gentsch, N. et al. Storage and transformation of organic matter fractions in cryoturbated permafrost soils across the Siberian Arctic. Biogeosciences 12, 4525–4542 (2015).
Kuzyakov, Y. Review: factors affecting rhizosphere priming effects. J. Plant Nutr. Soil Sci. 165, 382–396 (2002).
Hinsinger, P., Bengough, A. G., Vetterlein, D. & Young, I. M. Rhizosphere: biophysics, biogeochemistry and ecological relevance. Plant Soil 321, 117–152 (2009).
Jones, D. L. & Murphy, D. V. Microbial response time to sugar and amino acid additions to soil. Soil Biol. Biochem. 39, 2178–2182 (2007).
Boddy, E., Roberts, P., Hill, P. W., Farrar, J. & Jones, D. L. Turnover of low molecular weight dissolved organic C (DOC) and microbial C exhibit different temperature sensitivities in Arctic tundra soils. Soil Biol. Biochem. 40, 1557–1566 (2008).
Global Agro-Ecological Zones (GAEZ v3.0) (FAO/IIASA, 2010).
Zhang, Y., Xu, M., Chen, H. & Adams, J. Global pattern of NPP to GPP ratio derived from MODIS data: effects of ecosystem type, geographical location and climate. Glob. Ecol. Biogeogr. 18, 280–290 (2009).
Jones, D. L., Nguyen, C. & Finlay, R. D. Carbon flow in the rhizosphere: carbon trading at the soil–root interface. Plant Soil 321, 5–33 (2009).
Kuzyakov, Y. & Larionova, A. A. Root and rhizomicrobial respiration: a review of approaches to estimate respiration by autotrophic and heterotrophic organisms in soil. J. Plant Nutr. Soil Sci. 168, 503–520 (2005).
Bond-Lamberty, B., Bailey, V. L., Chen, M., Gough, C. M. & Vargas, R. Globally rising soil heterotrophic respiration over recent decades. Nature 560, 80–83 (2018).
Bond-Lamberty, B., Wang, C. & Gower, S. T. A global relationship between the heterotrophic and autotrophic components of soil respiration? Glob. Change Biol. 10, 1756–1766 (2004).
Hibbard, K. A., Law, B. E., Reichstein, M. & Sulzman, J. An analysis of soil respiration across Northern Hemisphere temperate ecosystems. Biogeochemistry 73, 29–70 (2005).
Buckland, S. T. Monte Carlo confidence intervals. Biometrics 40, 811–817 (1984).
Hijmans, R. J., Cameron, S. E., Parra, J. L., Jones, P. G. & Jarvis, A. Very high resolution interpolated climate surfaces for global land areas. Int. J. Climatol. 25, 1965–1978 (2005).
Kummu, M., de Moel, H., Ward, P. J. & Varis, O. Dryad Data from: How close do we live to water? A global analysis of population distance to freshwater bodies. (Dryad Digital Repository, 2011); https://doi.org/10.5061/dryad.71c6r
Kummu, M., Moel, H., de Ward, P. J. & Varis, O. How close do we live to water? A global analysis of population distance to freshwater bodies. PLoS ONE 6, e20578 (2011).
Iwahashi, J. & Pike, R. J. Automated classifications of topography from DEMs by an unsupervised nested-means algorithm and a three-part geometric signature. Geomorphology 86, 409–440 (2007).
Acknowledgements
We thank P. Thornton, F. Dijkstra, Y. Carrillo and R. E. Hewitt for providing additional information on published data. Figure 1a–c is courtesy of R. Miedema (IN Produktie, Amsterdam). This study was supported by funding from: the Swedish Research Council (VR) (grant number 621-2011-5444), Formas (grant number 214-2011-788) and the Knut and Alice Wallenberg Foundation (grant number KAW 2012.0152) (all awarded to E.D.); Academy of Finland-funded projects SCART (grant number 267463) and WASCO (grant number 305471), Emil Aaltonen Foundation-funded project ‘eat-less-water’, the European Research Council under the European Union’s Horizon 2020 Research and Innovation Programme (grant agreement number 819202), and Maa- ja vesitekniikan tuki ry (all awarded to M.K.); the JPI Climate Project COUP-Austria (BMWFW-6.020/0008) (awarded to A.R.); two projects funded by the Swedish Research Council, the EU JPI Climate COUP project (E0689701) and the Project INCA (E0641701)–Marie Sklodowska-Curie Actions cofund (600398) (awarded to G.H.); the Deutsche Forschungsgemeinschaft (BE 6485/1-1) (to C.B.); and the US DOE BER RGMA programme through the RUBISCO SFA and ECRP projects (to C.K.).
Author information
Authors and Affiliations
Contributions
F.K. and E.D. conceived of the idea. F.K., B.W. and E.D. led the conceptual model development in collaboration with M.K., C.B., G.B.-W., S.F., K.G., G.G., G.H., E.J.K., P.K., S.M., A.R. and J.T.W. The model was implemented by M.K. and M.J. C.B., N.G., G.H., C.K. and P.K. provided additional data. M.K., G.H., C.K., J.T.W. and E.D. performed additional statistical analyses. F.K. and B.W. wrote the manuscript with contributions from all authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Primary Handling Editors: Tamara Goldin; Xujia Jiang.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
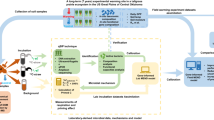
Extended Data Fig. 1 The PrimeSCale model estimates rhizosphere priming effect (RPE)-induced SOC losses and consists of three modules: Soil, Plant, and Soil Respiration.
SOIL: Soil grid cubes potentially susceptible to the RPE were identified by first quantifying SOC stocks at 0–3 m depth21,54,55, then excluding continuously frozen (depth > ALT)24 or non vegetated grid cubes (GPP = 0)23,24, as the RPE depends on the presence of living plant roots. An additional sensitivity analysis was performed using only the fraction of the SOC stock with C/N below 20. PLANT: RPE ratios (the ratio of SOC respiration from plant-affected soil divided by SOC respiration from not plant-affected soil) were estimated for each grid cube. The relationship between the RPE ratio and root respiration as a proxy for plant belowground C allocation, was based on a meta-analysis of studies that quantify RPE in experiments with intact plants. Root respiration was estimated using current and future GPP data for each grid cell in our study area and spread over 5 cm depth increments using root depth distribution functions. These functions were based on a meta-analysis of rooting depth patterns in permafrost-affected boreal forests as well as different tundra vegetation types25,39. SOIL RESPIRATION: Basal SOC respiration (that is, SOC respiration without RPE) was calculated using a relationship between the fraction of SOC that is respired (Rh) and GPP, derived from the Community Land Model (CLM)24, in combination with high resolution SOC21,54,55 and GPP spatial databases23,24. The GPP serves here as a proxy for climatic conditions that favour both GPP and basal SOC respiration. SOC respiration from plant-affected soil (that is, SOC respiration with RPE) was calculated by combining the RPE ratios with the basal SOC respiration values (Rh) for each individual grid cube. For more details on the Soil, Plant and Soil Respiration Modules see Methods.
Extended Data Fig. 2 Relative root depth distribution in five tundra and boreal forest vegetation types for the northern circumpolar permafrost area for 2010 and 2100 (RCP 8.5).
Relative root distribution was calculated over 60 soil layers (5 cm increments) for erect-shrub, graminoid, prostrate-shrub and wetland tundra as well as boreal forest on permafrost, reflecting depth distributions depending on vegetation type specific root depth distribution, and observed and projected area specific active layer thickness. Shaded areas depict uncertainty ranges (10th–90th percentile), and the spatial distribution of vegetation types for years 2010 and 2100 is presented in Extended Data Fig. 9.
Extended Data Fig. 3 Relative root depth distribution in five tundra and boreal forest vegetation types normalized to active layer thickness (ALT) for present and 2100 (RCP 8.5).
Relative root distribution was calculated over 60 soil layers (5 cm increments) with standardized ALT in erect-shrub, graminoid, prostrate-shrub and wetland tundra as well as and boreal forest on permafrost, shaded areas depict 10th -90th percentile.
Extended Data Fig. 4 Relative root depth distribution in tundra and boreal forest with varying active layer thickness (ALT).
Relative root distribution was calculated over 60 soil layers (5 cm increments) with varying ALT in erect-shrub, graminoid, prostrate-shrub and wetland tundra as well as boreal forest on permafrost.
Extended Data Fig. 5 Depth distribution of absolute root respiration, averaged across the northern circumpolar permafrost area for 2010 and 2100 (RCP 8.5).
Shaded areas depict 10th–90th percentile uncertainty range.
Extended Data Fig. 6 Spatial distribution of coefficients of variation for the RPE-ratio and the absolute RPE in the northern circumpolar permafrost region.
a-i, Spatial distribution of the coefficients of variation for the RPE-ratio (a–c, for 2010 and 2100, RCP 4.5 and 8.5); Spatial distribution of the coefficients of variation of the absolute rhizosphere priming effect under two scenarios, either assuming that all plant-affected SOC is susceptible to the RPE (d–f, no C/N threshold scenario) or that microbial carbon limitation is required (g–i, C/N threshold scenario) for 2010 and 2100, RCP 4.5 and 8.5.
Extended Data Fig. 7 Spatial patterns of model input data.
Gross primary production (GPP) for the years 2010 and 2100 (RCP4.5 and 8.5 scenario) (a–c); GPP without CO2-fertilization, used in soil respiration estimates (d–f); Active layer thickness (ALT) for the years 2010 and 2100 (RCP4.5 and RCP8.5 scenarios) to a depth of 3 m. (g–i); SOC stocks in Gelisols to a depth of 3 m, for organic, cryoturbated, and mineral horizons (j–l); and (m–o) soil type percentage of each Gelisol suborder (Histels, Orthels and Turbels). Note that maps show percentage of full soil coverage and will not sum to 100% in locations where permafrost-free soils occur.
Extended Data Fig. 8 Source studies used to constrain the RPE ratio function.
RPE ratio plotted against root respiration. RPE ratio was calculated as ratio of SOC respiration from planted over unplanted soil, and root respiration was estimated from plant-associated respiration. Data were derived from a meta-analysis of studies quantifying RPE in experiments with intact plants (12 studies comprising 65 individual treatment combinations, see Supplementary Table 2 for details and source references). The solid line shows the saturating function corresponding to the posterior medians; the shaded area indicates the 95% posterior credible region for the parameters of this function. The dotted line indicates an RPE ratio of 1, that is no RPE.
Extended Data Fig. 10
Active layer dependent relative depth distribution curves for soil respiration (Rh). Data were extracted from the CLM model, specific for each active layer depth.
Supplementary information
Supplementary Information
Supplementary Tables 1–9 and methods.
Rights and permissions
About this article
Cite this article
Keuper, F., Wild, B., Kummu, M. et al. Carbon loss from northern circumpolar permafrost soils amplified by rhizosphere priming. Nat. Geosci. 13, 560–565 (2020). https://doi.org/10.1038/s41561-020-0607-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41561-020-0607-0
This article is cited by
-
Projected soil carbon loss with warming in constrained Earth system models
Nature Communications (2024)
-
Experimental warming accelerates positive soil priming in a temperate grassland ecosystem
Nature Communications (2024)
-
Response of Boreal Plant Communities and Forest Floor Carbon Fluxes to Experimental Nutrient Additions
Ecosystems (2024)
-
Hydrological Regime and Plant Functional Traits Jointly Mediate the Influence of Salix spp. on Soil Organic Carbon Stocks in a High Arctic Tundra
Ecosystems (2023)
-
Close coupling of plant functional types with soil microbial community composition drives soil carbon and nutrient cycling in tundra heath
Plant and Soil (2023)