Abstract
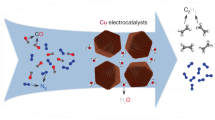
Although progress has been made in producing multi-carbon products from the electrochemical reduction of CO2, the modest selectivity for ethylene (C2H4) leads to low energy efficiency and high downstream separation costs. Here we functionalize Cu catalysts with a variety of substituted aryl diazonium salts to improve selectivity towards multi-carbon products. Using computation and operando spectroscopy, we find that Cu surface oxidation state (δ+ where 0 < δ < 1) can be tuned by functionalization and that it influences the selectivity to C2H4. We report a Faradaic efficiency and a specific current density for C2H4 as large as 83 ± 2% and 212 mA cm−2, respectively, on partially oxidized Cu0.26+. Using a CO gas feed, we demonstrate an energy efficiency of ~40% with a C2H4 Faradaic efficiency of 86 ± 2%, corresponding to a low electrical power consumption of 25.6 kWh Nm−3 for the CO to C2H4 conversion reaction. Our findings provide a route towards practical electrosynthesis of C2H4 using valence engineering of copper.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
The authors declare that the data supporting the findings of this study are available within the paper and its Supplementary Information. Source data are available at https://doi.org/10.6084/m9.figshare.24903033. Source data are provided with this paper.
References
Jouny, M., Luc, W. & Jiao, F. General techno-economic analysis of CO2 electrolysis systems. Ind. Eng. Chem. Res. 57, 2165–2177 (2018).
Verma, S., Lu, S. & Kenis, P. J. Co-electrolysis of CO2 and glycerol as a pathway to carbon chemicals with improved technoeconomics due to low electricity consumption. Nat. Energy 4, 466–474 (2019).
Li, F. et al. Molecular tuning of CO2-to-ethylene conversion. Nature 577, 509–513 (2020).
García de Arquer, F. P. et al. CO2 electrolysis to multicarbon products at activities greater than 1 A cm−2. Science 367, 661–666 (2020).
Zhong, M. et al. Accelerated discovery of CO2 electrocatalysts using active machine learning. Nature 581, 178–183 (2020).
Liu, W. et al. Electrochemical CO2 reduction to ethylene by ultrathin CuO nanoplate arrays. Nat. Commun. 13, 1877 (2022).
Ma, S., Luo, R., Moniri, S., Lan, Y. & Kenis, P. J. Efficient electrochemical flow system with improved anode for the conversion of CO2 to CO. J. Electrochem. Soc. 161, F1124 (2014).
Verma, S., Lu, X., Ma, S., Masel, R. I. & Kenis, P. J. The effect of electrolyte composition on the electroreduction of CO2 to CO on Ag based gas diffusion electrodes. Phys. Chem. Chem. Phys. 18, 7075–7084 (2016).
Dinh, C.-T. et al. CO2 electroreduction to ethylene via hydroxide-mediated copper catalysis at an abrupt interface. Science 360, 783–787 (2018).
Zhang, T. et al. Highly selective and productive reduction of carbon dioxide to multicarbon products via in situ CO management using segmented tandem electrodes. Nat. Catal. 5, 202–211 (2022).
De Luna, P. et al. Catalyst electro-redeposition controls morphology and oxidation state for selective carbon dioxide reduction. Nat. Catal. 1, 103–110 (2018).
Xiao, H., Goddard, W. A., Cheng, T. & Liu, Y. Cu metal embedded in oxidized matrix catalyst to promote CO2 activation and CO dimerization for electrochemical reduction of CO2. Proc. Natl Acad. Sci. USA 114, 6685–6688 (2017).
Arán-Ais, R. M., Scholten, F., Kunze, S., Rizo, R. & Roldan Cuenya, B. The role of in situ generated morphological motifs and Cu (i) species in C2+ product selectivity during CO2 pulsed electroreduction. Nat. Energy 5, 317–325 (2020).
Lee, S., Kim, D. & Lee, J. Electrocatalytic production of C3‐C4 compounds by conversion of CO2 on a chloride‐induced bi‐phasic Cu2O‐Cu catalyst. Angew. Chem. Int. Ed. 127, 14914–14918 (2015).
Cao, L., Wu, Y., Hang, T. & Li, M. Covalent grafting of dielectric films on Cu (111) surface via electrochemical reduction of aryl diazonium salts. Langmuir 38, 14969–14980 (2022).
Pinson, J. in Aryl Diazonium Salts: New Coupling Agents in Polymer and Surface Science (ed. Chehimi, M. M.) Ch. 1, 1–35 (Wiley, 2012).
Berisha, A., Chehimi, M. M., Pinson, J. & Podvorica, F. Electrode surface modification using diazonium salts. Electroanal. Chem. 26, 115–224 (2015).
Mooste, M. et al. Surface and electrochemical characterization of aryl films grafted on polycrystalline copper from the diazonium compounds using the rotating disk electrode method. Electroanal. Chem. 817, 89–100 (2018).
Chira, A., Bucur, B. & Radu, G.-L. Electrodeposited organic layers formed from aryl diazonium salts for inhibition of copper corrosion. Materials 10, 235 (2017).
Hollins, P. The influence of surface defects on the infrared spectra of adsorbed species. Surf. Sci. Rep. 16, 51–94 (1992).
Hammett, L. P. The effect of structure upon the reactions of organic compounds. Benzene derivatives. J. Am. Chem. Soc. 59, 96–103 (1937).
Hansch, C., Leo, A. & Taft, R. A survey of Hammett substituent constants and resonance and field parameters. Chem. Rev. 91, 165–195 (1991).
Li, Y. C. et al. Binding site diversity promotes CO2 electroreduction to ethanol. J. Am. Chem. Soc. 141, 8584–8591 (2019).
Hurley, B. L. & McCreery, R. L. Covalent bonding of organic molecules to Cu and Al alloy 2024 T3 surfaces via diazonium ion reduction. J. Electrochem. Soc. 151, B252 (2004).
Doppelt, P., Hallais, G., Pinson, J., Podvorica, F. & Verneyre, S. Surface modification of conducting substrates. Existence of azo bonds in the structure of organic layers obtained from diazonium salts. Chem. Mater. 19, 4570–4575 (2007).
Menanteau, T., Dias, M. N., Levillain, E., Downard, A. J. & Breton, T. Electrografting via diazonium chemistry: the key role of the aryl substituent in the layer growth mechanism. J. Phys. Chem. C. 120, 4423–4429 (2016).
Ozden, A. et al. High-rate and efficient ethylene electrosynthesis using a catalyst/promoter/transport layer. ACS Energy Lett. 5, 2811–2818 (2020).
Chen, X. et al. Electrochemical CO2-to-ethylene conversion on polyamine-incorporated Cu electrodes. Nat. Catal. 4, 20–27 (2021).
Zhang, W. et al. Atypical oxygen-bearing copper boosts ethylene selectivity toward electrocatalytic CO2 reduction. J. Am. Chem. Soc. 142, 11417–11427 (2020).
Gunathunge, C. M. et al. Spectroscopic observation of reversible surface reconstruction of copper electrodes under CO2 reduction. J. Phys. Chem. C. 121, 12337–12344 (2017).
Gunathunge, C. M., Li, J., Li, X., Hong, J. J. & Waegele, M. M. Revealing the predominant surface facets of rough Cu electrodes under electrochemical conditions. ACS Catal. 10, 6908–6923 (2020).
Gunathunge, C. M., Ovalle, V. J. & Waegele, M. M. Probing promoting effects of alkali cations on the reduction of CO at the aqueous electrolyte/copper interface. Phys. Chem. Chem. Phys. 19, 30166–30172 (2017).
An, H. et al. Sub‐second time‐resolved surface‐enhanced Raman spectroscopy reveals dynamic CO intermediates during electrochemical CO2 reduction on copper. Angew. Chem. Int. Ed. 60, 16576–16584 (2021).
Heyes, J., Dunwell, M. & Xu, B. CO2 reduction on Cu at low overpotentials with surface-enhanced in situ spectroscopy. J. Phys. Chem. C. 120, 17334–17341 (2016).
Akemann, W. & Otto, A. Vibrational modes of CO adsorbed on disordered copper films. J. Raman Spectrosc. 22, 797–803 (1991).
Li, J. et al. Silica-copper catalyst interfaces enable carbon-carbon coupling towards ethylene electrosynthesis. Nat. Commun. 12, 2808 (2021).
Verma, S. et al. Insights into the low overpotential electroreduction of CO2 to CO on a supported gold catalyst in an alkaline flow electrolyzer. ACS Energy Lett. 3, 193–198 (2017).
Xiao, H., Cheng, T., Goddard, W. A. III & Sundararaman, R. Mechanistic explanation of the pH dependence and onset potentials for hydrocarbon products from electrochemical reduction of CO on Cu (111). J. Am. Chem. Soc. 138, 483–486 (2016).
Yadegari, H. et al. Glycerol oxidation pairs with carbon monoxide reduction for low-voltage generation of C2 and C3 product streams. ACS Energy Lett. 6, 3538–3544 (2021).
Ozden, A. et al. Cascade CO2 electroreduction enables efficient carbonate-free production of ethylene. Joule 5, 706–719 (2021).
Voiry, D. et al. High-quality graphene via microwave reduction of solution-exfoliated graphene oxide. Science 353, 1413–1416 (2016).
Lee, W. H. et al. Highly selective and stackable electrode design for gaseous CO2 electroreduction to ethylene in a zero-gap configuration. Nano Energy 84, 105859 (2021).
Ripatti, D. S., Veltman, T. R. & Kanan, M. W. Carbon monoxide gas diffusion electrolysis that produces concentrated C2 products with high single-pass conversion. Joule 3, 240–256 (2019).
Rabinowitz, J. A. & Kanan, M. W. The future of low-temperature carbon dioxide electrolysis depends on solving one basic problem. Nat. Commun. 11, 5231 (2020).
Gabardo, C. M. et al. Continuous carbon dioxide electroreduction to concentrated multi-carbon products using a membrane electrode assembly. Joule 3, 2777–2791 (2019).
Paier, J., Hirschl, R., Marsman, M. & Kresse, G. The Perdew–Burke–Ernzerhof exchange-correlation functional applied to the G2-1 test set using a plane-wave basis set. J. Chem. Phys. 122, 234102 (2005).
Grimme, S., Ehrlich, S. & Goerigk, L. Effect of the damping function in dispersion corrected density functional theory. J. Comput. Chem. 32, 1456–1465 (2011).
Henkelman, G., Arnaldsson, A. & Jónsson, H. A fast and robust algorithm for Bader decomposition of charge density. Comp. Mater. Sci. 36, 354–360 (2006).
Henkelman, G., Uberuaga, B. P. & Jónsson, H. A climbing image nudged elastic band method for finding saddle points and minimum energy paths. J. Chem. Phys. 113, 9901–9904 (2000).
Neugebauer, J. & Scheffler, M. Adsorbate–substrate and adsorbate–adsorbate interactions of Na and K adlayers on Al (111). Phys. Rev. B 46, 16067 (1992).
Acknowledgements
Support from the European Research Council is gratefully acknowledged, as is support from the European Union’s Horizon 2020 research and innovation programme (grant agreement number 804320) to D.V. This work was also financially supported by the Special Fund Project of Jiangsu Province for Scientific and Technological Innovation in Carbon Peaking and Carbon Neutrality (grant agreement number BK20220023), as support to D.R. We thank the National Facility ELECMI ICTS (‘Division de Microscopia Electronica’, Universidad de Cadiz, DME-UCA) for the use of TEM instrumentation. We also acknowledge funding from the European Union’s Horizon 2020 research and innovation programme (grant agreement 823717-ESTEEM3) and the Spanish Ministerio de Economia y Competitividad (PID2019-107578GA-I00), the Ministerio de Ciencia e Innovación MCIN/AEI/10.13039/501100011033 and the European Union ‘NextGenerationEU’/PRTR (RYC2021-033764-I, CPP2021-008986), as support to L.L. We also acknowledge funding from the French National Agency (ANR, JCJC programme, MONOMEANR-20-CE08-0009), as support to C.S. We acknowledge M. Rüscher, F. T. Haase and L. Bai for their help on synchrotron tests. We also acknowledge SOLEIL for provision of synchrotron radiation facilities and we would like to thank A. Zitolo for assistance in using beamline SAMBA within the proposal 20200732. XAS experiments were also performed at CLAESS beamline at ALBA synchrotron with the collaboration of ALBA staff. P. Montels and D. Valenza are acknowledged for their technical assistance with the MEA cells.
Author information
Authors and Affiliations
Contributions
D.V. conceived the idea and designed the experiments. D.R. performed the DFT calculations and discussed the results with D.V. and H.W. H.W. designed the experiments with D.V., prepared the electrodes and performed the electrochemical measurements. H.W., D.R. and D.V. analysed the data and wrote the manuscript. L.H. and V.F. performed the XPS and Auger measurements and analysed the data with H.W. J.T. and H.W. performed the ex situ and operando XAS measurements and analysed the data. L.L. performed HR-STEM, HR-TEM and EELS on the Cu-X catalysts and discussed the results with H.W., and D.V. and S.Y. performed DFT data collections and discussed the results with H.W. E.P. carried out the liquid NMR spectroscopy measurements. K.Q. and Y.Z. assisted H.W. with the electrochemical investigations and the gas chromatography analyses. W.W., J. Li and J. Liu assisted H.W. with the physical characterization of the samples and the operando Raman measurements. C.S. and P.M. discussed the electrocatalytic performance with H.W. and D.V. B.R.C. discussed the XAS data with J.T., H.W. and D.V. All authors discussed the results and assisted during manuscript preparation.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Energy thanks Tao Cheng and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–80, Tables 1–32, Methods, Notes 1–6 and Refs. 1–33.
Supplementary Data 1
Source data for Supplementary Figs. 31, 35, 51, 52a–c, 54, 65 and 72b,c.
Supplementary Data 2
The optimized computational models, the energy in the reaction.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Statistical source data.
Source Data Fig. 6
Statistical source data.
Source Data Fig. 7
Statistical source data.
Source Data Fig. 8
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wu, H., Huang, L., Timoshenko, J. et al. Selective and energy-efficient electrosynthesis of ethylene from CO2 by tuning the valence of Cu catalysts through aryl diazonium functionalization. Nat Energy 9, 422–433 (2024). https://doi.org/10.1038/s41560-024-01461-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41560-024-01461-6
This article is cited by
-
An organic approach
Nature Energy (2024)