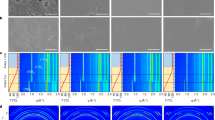
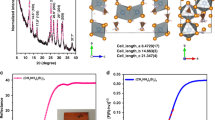
Abstract
The use of non-toxic or less-toxic solvents in the mass production of solution-processed perovskite solar cells is essential. However, halide perovskites are generally not completely soluble in most non-toxic solvents. Here we report the deposition of dense and uniform α-formamidinium lead triiodide (α-FAPbI3) films using perovskite precursor solutions dissolved in ethanol-based solvent. The process does not require an antisolvent dripping step. The combination of a Lewis base, such as dimethylacetamide (or dimethylsulfoxide), and an alkylammonium chloride (RNH3Cl) in ethanol results in the stable solvation of FAPbI3. The RNH3Cl added to the FAPbI3 precursor solution is removed during spin-coating and high-temperature annealing via iodoplumbate complexes, such as PbI2·RNH2 and PbI2·HCl, coordinated with dimethylacetamide (or dimethylsulfoxide). It is possible to form very dense and uniform α-FAPbI3 perovskite films with high crystallinity by combining several types of RNH3Cl. We obtain power conversion efficiencies of 24.3% using a TiO2 electrode, and of 25.1% with a SnO2 electrode.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
All data generated or analysed during this study are included in the published article and its Supplementary Information. Source data are provided with this paper.
References
Jeong, J. et al. Pseudo-halide anion engineering for α-FAPbI3 perovskite solar cells. Nature 592, 381–385 (2021).
Min, H. et al. Perovskite solar cells with atomically coherent interlayers on SnO2 electrodes. Nature 598, 444–450 (2021).
Kim, M. et al. Conformal quantum dot–SnO2 layers as electron transporters for efficient perovskite solar cells. Science 375, 302–306 (2022).
Yoo, J. J. et al. Efficient perovskite solar cells via improved carrier management. Nature 590, 587–593 (2021).
Kim, G., Min, H., Lee, K. S., Yoon, S. M. & Seok, S. I. Impact of strain relaxation on performance of α-formamidinium lead iodide perovskite solar cells. Science 370, 108–112 (2020).
Wang, M. et al. Systematic optimization of perovskite solar cells via green solvent systems. Chem. Eng. J. 387, 123966 (2020).
Cui, Y., Wang, S., Ding, L. & Hao, F. Green–solvent–processable perovskite solar cells. Adv. Energy Sustain. Res. 2, 2000047 (2021).
Vidal, R. et al. Assessing health and environmental impacts of solvents for producing perovskite solar cells. Nat. Sustain. 4, 277–285 (2021).
Hu, C. et al. Discovery of a new intermediate enables one-step deposition of high-quality perovskite films via solvent engineering. Sol. RRL 5, 2000712 (2021).
Küffner, J. et al. One-step blade coating of inverted double-cation perovskite solar cells from a green precursor solvent. ACS Appl. Energy Mater. 4, 11700–11710 (2021).
Zhang, Y. et al. The synergism of DMSO and diethyl ether for highly reproducible and efficient MA0.5FA0.5PbI3 perovskite solar cells. Adv. Energy Mater. 10, 2001300 (2020).
Radicchi, E., Mosconi, E., Elisei, F., Nunzi, F. & De Angelis, F. Understanding the solution chemistry of lead halide perovskites precursors. ACS Appl. Energy Mater. 2, 3400–3409 (2019).
Kim, M. et al. Methylammonium chloride induces intermediate phase stabilization for efficient perovskite solar cells. Joule 3, 2179–2192 (2019).
Zhang, Y. et al. Propylammonium chloride additive for efficient and stable FAPbI3 perovskite solar cells. Adv. Energy Mater. 11, 2102538 (2021).
Wang, X. et al. Perovskite solution aging: what happened and how to inhibit? Chem 6, 1369–1378 (2020).
Dong, Q. et al. Critical role of organoamines in the irreversible degradation of a metal halide perovskite precursor colloid: mechanism and inhibiting strategy. ACS Energy Lett. 7, 481–489 (2022).
Luongo, A., Brunetti, B., Vecchio Ciprioti, S., Ciccioli, A. & Latini, A. Thermodynamic and kinetic aspects of formamidinium lead iodide thermal decomposition. J. Phys. Chem. C 125, 21851–21861 (2021).
Xiang, W. et al. Intermediate phase engineering of halide perovskites for photovoltaics. Joule 6, 315–339 (2021).
Park, B.-W. et al. Stabilization of formamidinium lead triiodide α-phase with isopropylammonium chloride for perovskite solar cells. Nat. Energy 6, 419–428 (2021).
Min, H. et al. Efficient, stable solar cells by using inherent bandgap of α-phase formamidinium lead iodide. Science 366, 749–753 (2019).
Watthage, S. C. et al. Enhanced grain size, photoluminescence, and photoconversion efficiency with cadmium addition during the two-step growth of CH3NH3PbI3. ACS Appl. Mater. Interfaces 9, 2334–2341 (2017).
An, Q. et al. Small grains as recombination hot spots in perovskite solar cells. Matter 4, 1683–1701 (2021).
Acknowledgements
This work was supported by the Basic Science Research Program (NRF-2018R1A3B1052820) through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT and Future Planning (MSIP). This work was also supported by the Defense Challengeable Future Technology Program of the Agency for Defense Development, Republic of Korea, by a brand project (1.220026.01) of UNIST. Y.L. acknowledges financial support from the Creative-Challenge Research Program (NRF-2020R1I1A1A01072030). Finally, we thank UCRF (UNIST Central Research Facilities) for its support using the equipment and the beamline staff at Pohang Accelerator Laboratory.
Author information
Authors and Affiliations
Contributions
H.-S.Y. and S.I.S. conceived this work. H.-S.Y. and H.W.K. prepared ethanol-based perovskite precursor solutions and fabricated devices. Y.L., M.J.P., S.H., J.K., E.N. and J.P. characterized the films and devices. H.-S.Y., Y.L. and S.I.S. wrote the article. S.I.S. supervised the project. All authors discussed the results and commented on the article.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Energy thanks Shengzhong (Frank) Liu and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–11 and Tables 1–3.
Supplementary Video 1
What happens during coating?
Supplementary Video 2
What happens during drying?
Supplementary Video 3
What happens during spin coating of the precursor solution?
Supplementary Video 4
What happens during spin coating the surface treatment agent after annealing?
Source data
Source Data Fig. 3
Photovoltaic parameters of solar cells shown in Fig. 3a,b.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yun, HS., Kwon, H.W., Paik, M.J. et al. Ethanol-based green-solution processing of α-formamidinium lead triiodide perovskite layers. Nat Energy 7, 828–834 (2022). https://doi.org/10.1038/s41560-022-01086-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41560-022-01086-7
This article is cited by
-
Multifunctional sulfonium-based treatment for perovskite solar cells with less than 1% efficiency loss over 4,500-h operational stability tests
Nature Energy (2024)
-
Universal growth of perovskite thin monocrystals from high solute flux for sensitive self-driven X-ray detection
Nature Communications (2024)
-
Rigorous pH measurement in non-aqueous solution: measurement method and reference values in ethanol
Analytical and Bioanalytical Chemistry (2024)
-
Recent progress of eco-friendly manufacturing process of efficient perovskite solar cells
Nano Convergence (2023)
-
Controlled growth of perovskite layers with volatile alkylammonium chlorides
Nature (2023)