Abstract
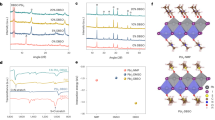
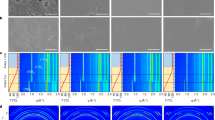
Formamidinium lead triiodide (FAPbI3) perovskite solar cells (PSCs) are mainly fabricated by sequentially coating lead iodide and formamidinium iodide, or by coating a solution in which all components are dissolved in one solvent (one-pot process). The PSCs produced by both processes exhibited similar efficiencies; however, their long-term stabilities were notably different. We concluded that the major reason for this behaviour is the stabilization of the α-FAPbI3 phase by isopropylammonium cations produced by the chemical reaction between isopropyl alcohol, used as solvent, and methylammonium chloride, added during the process. On this basis, we fabricated PSCs by adding isopropylammonium chloride to the perovskite precursor solution for the one-pot process and achieved a certified power conversion efficiency of 23.9%. Long-term operational current density–voltage measurements (one sweep every 84 min under 1-Sun irradiation in nitrogen atmosphere) showed that the as-fabricated device with an initial efficiency of approximately 20% recorded an efficiency of about 23% after 1,000 h that gradually degraded to about 22% after an additional 1,000 h.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
All data generated or analysed during this study are included in the published article and its Supplementary Information. Source data are provided with this paper.
Change history
06 May 2021
A Correction to this paper has been published: https://doi.org/10.1038/s41560-021-00844-3
References
Saliba, M. et al. Cesium-containing triple cation perovskite solar cells: improved stability, reproducibility and high efficiency. Energy Environ. Sci. 9, 1989–1997 (2016).
Min, H. et al. Efficient, stable solar cells by using inherent bandgap of α-phase formamidinium lead iodide. Science 366, 749–753 (2019).
Kim, M. et al. Methylammonium chloride induces intermediate phase stabilization for efficient perovskite solar cells. Joule 3, 2179–2192 (2019).
Jeon, N. J. et al. Compositional engineering of perovskite materials for high-performance solar cells. Nature 517, 476–480 (2015).
Kim, G. et al. Impact of strain relaxation on performance of α-formamidinium lead iodide perovskite solar cells. Science 370, 108–112 (2020).
Bai, S. et al. Planar perovskite solar cells with long-term stability using ionic liquid additives. Nature 571, 245–250 (2019).
Yang, W. S. et al. Iodide management in formamidinium-lead-halide–based perovskite layers for efficient solar cells. Science 356, 1376–1379 (2017).
Burschka, J. et al. Sequential deposition as a route to high-performance perovskite-sensitized solar cells. Nature 499, 316–319 (2013).
Wang, X. et al. Improving efficiency of planar hybrid CH3NH3PbI3−xClx perovskite solar cells by isopropanol solvent treatment. Org. Electron. 24, 205–211 (2015).
Li, Z. et al. Extrinsic ion migration in perovskite solar cells. Energy Environ. Sci. 10, 1234–1242 (2017).
Cao, J. et al. Identifying the molecular structures of intermediates for optimizing the fabrication of high-quality perovskite films. J. Am. Chem. Soc. 138, 9919–9926 (2016).
Petrov, A. A. et al. Crystal structure of DMF-intermediate phases uncovers the link between CH3NH3PbI3 morphology and precursor stoichiometry. J. Phys. Chem. C. 121, 20739–20743 (2017).
Gratia, P. et al. The many faces of mixed ion perovskites: unraveling and understanding the crystallization process. ACS Energy Lett. 2, 2686–2693 (2017).
Prasanna, R. et al. Design of low bandgap tin–lead halide perovskite solar cells to achieve thermal, atmospheric and operational stability. Nat. Energy 4, 939–947 (2019).
Kim, J. et al. Unveiling the relationship between the perovskite precursor solution and the resulting device performance. J. Am. Chem. Soc. 142, 6251–6260 (2020).
Jain, V., Biesinger, M. C. & Linford, M. R. The Gaussian-Lorentzian sum, product, and convolution (Voigt) functions in the context of peak fitting X-ray photoelectron spectroscopy (XPS) narrow scans. Appl. Surf. Sci. 447, 548–553 (2018).
Roghabadi, F. A., Ahmadi, V. & Aghmiuni, K. O. Organic–inorganic halide perovskite formation: in situ dissociation of cation halide and metal halide complexes during crystal formation. J. Phys. Chem. C. 121, 13532–13538 (2017).
Jacobsson, T. J. et al. Unreacted PbI2 as a double-edged sword for enhancing the performance of perovskite solar cells. J. Am. Chem. Soc. 138, 10331–10343 (2016).
Inamura, K., Inoue, Y., Ikeda, S. & Kishi, K. X-ray photoelectron spectroscopic study for the adsorption and the decomposition of alkylamines on nickel. Surf. Sci. 155, 173–186 (1985).
Lee, M. V. et al. Transamidation of dimethylformamide during alkylammonium lead triiodide film formation for perovskite solar cells. J. Mater. Res. 32, 45–55 (2017).
Appiagyei, B., Bhatia, S., Keeney, G. L., Dolmetsch, T. & Jackson, J. E. Electroactivated alkylation of amines with alcohols via both direct and indirect borrowing hydrogen mechanisms. Green Chem. 22, 860–869 (2020).
Bhän, S. et al. The catalytic amination of alcohols. ChemCatChem 3, 1853–1864 (2011).
Kelefiotis-Stratidakis, P., Tyrikos-Ergas, T. & Pavlidis, I. V. The challenge of using isopropylamine as an amine donor in transaminase catalysed reactions. Org. Biomol. Chem. 17, 1634–1642 (2019).
Li, S. et al. Amination of isopropanol to isopropylamine over a highly basic and active Ni/LaAlSiO catalyst. J. Catal. 350, 141–148 (2017).
Turner, W. D. & Howald, A. M. Methyl amines from methyl alcohol and ammonium cloride. J. Am. Chem. Soc. 42, 2663–2665 (1920).
Madenwald, F. A., Henke, C. O. & Brown, O. W. Catalytic activity of lead. J. Phys. Chem. 31, 862–866 (1927).
Xue, C., Li, J., Lee, J. P., Zhang, P. & Wu, J. Continuous amination of aryl/heteroaryl halides using aqueous ammonia in a Teflon AF-2400 tube-in-tube micro-flow reactor. React. Chem. Eng. 4, 346–350 (2019).
Singh, A., Gupta, S. & Khurana, J. M. Zinc chloride mediated nucleophilic substitution: amination and thioetherification of alcohols at room temperature. Org. Prep. Proced. Int. 52, 110–119 (2020).
Ferreira, A. C. et al. Elastic softness of hybrid lead halide perovskites. Phys. Rev. Lett. 121, 085502 (2018).
Chen, Y. et al. Strain engineering and epitaxial stabilization of halide perovskites. Nature 577, 209–215 (2020).
Jung, M., Shin, T. J., Seo, J., Kim, G. & Seok, S. I. Structural features and their functions in surfactant-armoured methylammonium lead iodide perovskites for highly efficient and stable solar cells. Energy Environ. Sci. 11, 2188–2197 (2018).
Tan, H. et al. Efficient and stable solution-processed planar perovskite solar cells via contact passivation. Science 355, 722–726 (2017).
Lee, J.-W. et al.2D perovskite stabilized phase-pure formamidinium perovskite solar cells. Nat. Commun. 9, 3021 (2018).
Li, Y. et al. Mixed cation FAxPEA1–xPbI3 with enhanced phase and ambient stability toward high-performance perovskite solar cells. Adv. Energy Mater. 7, 1601307 (2017).
Nemnes, G. A. et al. How measurement protocols influence the dynamic J-V characteristics of perovskite solar cells: theory and experiment. Sol. Energy 173, 976–983 (2018).
Bastos, J. P. et al. Light-induced degradation of perovskite solar cells: the influence of 4-tert-butyl pyridine and gold. Adv. Energy Mater. 8, 1800554 (2018).
Blöchl, P. E. Projector augmented-wave method. Phys. Rev. B 50, 17953 (1994).
Kresse, G. & Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 59, 1758 (1999).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865 (1996).
Tkatchenko, A. & Scheffler, M. Accurate molecular van der waals interactions from ground-state electron density and free-atom reference data. Phys. Rev. Lett. 102, 073005 (2009).
Chen, T. et al. Entropy-driven structural transition and kinetic trapping in formamidinium lead iodide perovskite. Sci. Adv. 2, e1601650 (2016).
Acknowledgements
This work was supported by the Basic Science Research Program (NRF-2018R1A3B1052820) through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning (MSIP). B.-w.P. acknowledges financial support from the Creative-Challenge Research Program (NRF-2020R1I1A1A01063868). T.J.S. acknowledges financial support under CAP-18-05-KAERI. This work was also supported by a brand project (1.210037.01) of UNIST. Experiments at the PLS-II 6D and 10A2 beamlines were supported in part by MSIT and POSTECH. We thank S. Y. Lee, J. H. Park, I. Choi, G. A. Lee, J. H. Lee, H. J. Mun, D. H. Lee and S.-P. Han of UCRF for their support in powder XRD, FIB sampling, HR-TEM, FESEM, GI-WAXD, ToF-SIMS and NMR analyses.
Author information
Authors and Affiliations
Contributions
B.-w.P., H.W.K. and S.I.S. conceived this work. B.-w.P. and H.W.K. prepared the perovskite thin layers and solar cells and interpreted all data from the analyses. Y.L. and D.Y.L. fabricated the materials and devices for the perovskite solar cells. G.K. and K.-j.K. conducted the XPS investigation. M.G.K. performed the chemical analysis. Y.K.K. carried out HR-TEM analysis. J.I. calculated the formation energy of iPAmHCl–FAPbI3. T.J.S. guided the XPS and GI-WAXD investigations and contributed to their interpretation. B.-w.P. and S.I.S. wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Energy thanks Caterina Ducati and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–25, Tables 1–3 and references.
Source data
Source Data Fig. 5
Photovoltaic parameters of the solar cells shown in the Fig. 5b.
Rights and permissions
About this article
Cite this article
Park, Bw., Kwon, H.W., Lee, Y. et al. Stabilization of formamidinium lead triiodide α-phase with isopropylammonium chloride for perovskite solar cells. Nat Energy 6, 419–428 (2021). https://doi.org/10.1038/s41560-021-00802-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41560-021-00802-z
This article is cited by
-
All-perovskite-based unassisted photoelectrochemical water splitting system for efficient, stable and scalable solar hydrogen production
Nature Energy (2024)
-
Multifunctional sulfonium-based treatment for perovskite solar cells with less than 1% efficiency loss over 4,500-h operational stability tests
Nature Energy (2024)
-
Toward stabilization of formamidinium lead iodide perovskites by defect control and composition engineering
Nature Communications (2024)
-
Multifunctional dual-anion compensation of amphoteric glycine hydrochloride enabled highly stable perovskite solar cells with prolonged carrier lifetime
Nano Research (2024)
-
Controlled growth of perovskite layers with volatile alkylammonium chlorides
Nature (2023)