Abstract
Females that are highly selective when choosing a mate run the risk of remaining unmated or delaying commencing reproduction. Therefore, low female choosiness would be beneficial when males are rare but it would be maladaptive if males become more frequent. How can females resolve this issue? Polyandry would allow mating-status-dependent choosiness, with virgin females selecting their first mate with little selectivity and becoming choosier thereafter. This plasticity in choosiness would ensure timely acquisition of sperm and enable females to increase offspring quality during later mating. Here, we show that Drosophila melanogaster females display such mating-status-dependent choosiness by becoming more selective once mated and identify the underlying neurohormonal mechanism. Mating releases juvenile hormone, which desensitizes Or47b olfactory neurons to a pheromone produced by males, resulting in increased preference for pheromone-rich males. Besides providing a mechanism to a long-standing evolutionary prediction, these data suggest that intersexual selection in D. melanogaster, and possibly in all polyandrous, sperm-storing species, is mainly the domain of mated females since virgin females are less selective. Juvenile hormone influences behaviour by changing cue responsiveness across insects; the neurohormonal modulation of olfactory neurons uncovered in D. melanogaster provides an explicit mechanism for how this hormone modulates behavioural plasticity.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
Raw sequencing reads are available at the National Center for Biotechnology Information Short Read Archive (https://www.ncbi.nlm.nih.gov/sra/SRX9940561); accession no. PRJNA694952. All other raw data are available at Dataverse-NL (https://doi.org/10.34894/454UBX).
Code availability
R scripts and the gene expression analysis code are available on Dataverse-NL.
References
Bateman, A. J. Intra-sexual selection in Drosophila. Heredity 2, 349–368 (1948).
Trivers, R. L. in Sexual Selection and the Descent of Man: The Darwinian Pivot (ed. Campbell, B) 136–179 (AldineTransaction, 1972).
De Jong, M. C. M. & Sabelis, M. W. Limits to runaway sexual selection: the wallflower paradox. J. Evol. Biol. 4, 637–655 (1991).
Martínez-Ruiz, C. & Knell, R. J. Sexual selection can both increase and decrease extinction probability: reconciling demographic and evolutionary factors. J. Anim. Ecol. 86, 117–127 (2017).
Jennisons, M. D. & Petrie, M. Why do females mate multiply? A review of the genetic benefits. Biol. Rev. Camb. Philos. Soc. 75, 21–64 (2000).
Kokko, H. & Mappes, J. Sexual selection when fertilization is not guaranteed. Evolution 59, 1876–1885 (2005).
Mautz, B. S. & Sakaluk, S. K. The effects of age and previous mating experience on pre- and post-copulatory mate choice in female house crickets (Acheta domesticus L.). J. Insect Behav. 21, 203–212 (2008).
Judge, K. A., Tran, K.-C. & Gwynne, D. T. The relative effects of mating status and age on the mating behaviour of female field crickets. Can. J. Zool. 88, 219–223 (2010).
King, B. H. & Miller, K. A. Mating status effects on sexual response of males and females in the parasitoid wasp Urolepis rufipes. J. Insect Behav. 31, 144–157 (2018).
Tanner, J. C., Garbe, L. M. & Zuk, M. When virginity matters: age and mating status affect female responsiveness in crickets. Anim. Behav. 147, 83–90 (2019).
Gromko, M. H. & Markow, T. A. Courtship and remating in field populations of Drosophila. Anim. Behav. 45, 253–262 (1993).
Civetta, A. & Ranz, J. M. Genetic factors influencing sperm competition. Front. Genet. 10, 820 (2019).
Behrman, E. L., Watson, S. S., O’Brien, K. R., Heschel, M. S. & Schmidt, P. S. Seasonal variation in life history traits in two Drosophila species. J. Evol. Biol. 28, 1691–1704 (2015).
Bateman, P. W., Gilson, L. N. & Ferguson, J. W. H. Male size and sequential mate preference in the cricket Gryllus bimaculatus. Anim. Behav. 61, 631–637 (2001).
Lynch, K. S., Rand, A. S., Ryan, M. J. & Wilczynski, W. Plasticity in female mate choice associated with changing reproductive states. Anim. Behav. 69, 689–699 (2005).
Billeter, J.-C. & Wolfner, M. F. Chemical cues that guide female reproduction in Drosophila melanogaster. J. Chem. Ecol.44, 750–769 (2018).
Liu, H. & Kubli, E. Sex-peptide is the molecular basis of the sperm effect in Drosophila melanogaster. Proc. Natl Acad. Sci. USA 100, 9929–9933 (2003).
Yang, C.-H.et al. Control of the postmating behavioral switch in Drosophila females by internal sensory neurons. Neuron 61, 519–526 (2009).
Häsemeyer, M., Yapici, N., Heberlein, U. & Dickson, B. J. Sensory neurons in the Drosophila genital tract regulate female reproductive behavior. Neuron 61, 511–518 (2009).
Rezával, C. et al. Neural circuitry underlying Drosophila female postmating behavioral responses. Curr. Biol. 22, 1155–1165 (2012).
Moshitzky, P. et al. Sex-peptide activates juvenile hormone biosynthesis in the Drosophila melanogaster corpus allatum. Arch. Insect Biochem. Physiol. 32, 363–374 (1996).
Reiff, T. et al. Endocrine remodelling of the adult intestine sustains reproduction in Drosophila. eLife 4, e06930 (2015).
Sugime, Y. et al. Upregulation of juvenile hormone titers in female Drosophila melanogaster through mating experiences and host food occupied by eggs and larvae. Zoolog. Sci. 34, 52–57 (2017).
Santos, C. G., Humann, F. C. & Hartfelder, K. Juvenile hormone signaling in insect oogenesis. Curr. Opin. Insect Sci. 31, 43–48 (2019).
Grillet, M., Dartevelle, L. & Ferveur, J.-F. A Drosophila male pheromone affects female sexual receptivity. Proc. Biol. Sci. 273, 315–323 (2006).
Billeter, J.-C., Atallah, J., Krupp, J. J., Millar, J. G. & Levine, J. D. Specialized cells tag sexual and species identity in Drosophila melanogaster. Nature 461, 987–991 (2009).
Jallon, J. M. A few chemical words exchanged by Drosophila during courtship and mating. Behav. Genet. 14, 441–478 (1984).
van der Goes van Naters, W. & Carlson, J. R. Receptors and neurons for fly odors in Drosophila. Curr. Biol. 17, 606–612 (2007).
Kurtovic, A., Widmer, A. & Dickson, B. J. A single class of olfactory neurons mediates behavioural responses to a Drosophila sex pheromone. Nature 446, 542–546 (2007).
Billeter, J.-C. & Levine, J. D. Neurogenetics: sex and the female brain. Curr. Biol. 24, R812–R814 (2014).
Dweck, H. K. M. et al. Pheromones mediating copulation and attraction in Drosophila. Proc. Natl Acad. Sci. USA 112, E2829–E2835 (2015).
Lin, H.-H. et al. Hormonal modulation of pheromone detection enhances male courtship success. Neuron 90, 1272–1285 (2016).
Pitts, S., Pelser, E., Meeks, J. & Smith, D. Odorant responses and courtship behaviors influenced by at4 neurons in Drosophila. PLoS ONE 11, e0162761 (2016).
Larsson, M. C. et al. Or83b encodes a broadly expressed odorant receptor essential for Drosophila olfaction. Neuron 43, 703–714 (2004).
Steck, K. et al. Internal amino acid state modulates yeast taste neurons to support protein homeostasis in Drosophila. eLife 7, e31625 (2018).
Inagaki, H. K., Panse, K. M. & Anderson, D. J. Independent, reciprocal neuromodulatory control of sweet and bitter taste sensitivity during starvation in Drosophila. Neuron 84, 806–820 (2014).
Hussain, A., Üçpunar, H. K., Zhang, M., Loschek, L. F. & Grunwald Kadow, I. C. Neuropeptides modulate female chemosensory processing upon mating in Drosophila. PLoS Biol. 14, e1002455 (2016).
Root, C. M., Ko, K. I., Jafari, A. & Wang, J. W. Presynaptic facilitation by neuropeptide signaling mediates odor-driven food search. Cell 145, 133–144 (2011).
Miura, K., Oda, M., Makita, S. & Chinzei, Y. Characterization of the Drosophila methoprene-tolerant gene product. Juvenile hormone binding and ligand-dependent gene regulation. FEBS J. 272, 1169–1178 (2005).
Willis, D. K., Wang, J., Lindholm, J. R., Orth, A. & Goodman, W. G. Microarray analysis of juvenile hormone response in Drosophila melanogaster S2 cells. J. Insect Sci. 10, 66 (2010).
Yamamoto, R., Bai, H., Dolezal, A. G., Amdam, G. & Tatar, M. Juvenile hormone regulation of Drosophila aging. BMC Biol. 11, 85 (2013).
Ng, R. et al. Amplification of Drosophila olfactory responses by a DEG/ENaC channel. Neuron 104, 947–959.e5 (2019).
Sethi, S. et al. Social context enhances hormonal modulation of pheromone detection in Drosophila. Curr. Biol. 29, 3887–3898.e4 (2019).
Ryner, L. C. et al. Control of male sexual behavior and sexual orientation in Drosophila by the fruitless gene. Cell 87, 1079–1089 (1996).
Younus, F. et al. Identification of candidate odorant degrading gene/enzyme systems in the antennal transcriptome of Drosophila melanogaster. Insect Biochem. Mol. Biol. 53, 30–43 (2014).
Chertemps, T. et al. A carboxylesterase, esterase-6, modulates sensory physiological and behavioral response dynamics to pheromone in Drosophila. BMC Biol. 10, 56 (2012).
Anton, S. & Gadenne, C. Effect of juvenile hormone on the central nervous processing of sex pheromone in an insect. Proc. Natl Acad. Sci. USA 96, 5764–5767 (1999).
Guo, W. et al. Juvenile hormone suppresses aggregation behavior through influencing antennal gene expression in locusts. PLoS Genet. 16, e1008762 (2020).
Scheiner, R. et al. Learning, gustatory responsiveness and tyramine differences across nurse and forager honeybees. J. Exp. Biol. 220, 1443–1450 (2017).
Huang, Z. Y. et al. Hormonal regulation of behavioural development in the honey bee is based on changes in the rate of juvenile hormone biosynthesis. J. Insect Physiol. 37, 733–741 (1991).
Pankiw, T. & Page, R. E.Jr. Effect of pheromones, hormones, and handling on sucrose response thresholds of honey bees (Apis mellifera L.). J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 189, 675–684 (2003).
Jaycox, E. R., Skowronek, W. & Guynn, G. Behavioral changes in worker honey bees (Apis mellifera) induced by injections of a juvenile hormone mimic. Ann. Entomol. Soc. Am. 67, 529–534 (1974).
Schenkel, M. A., Pen, I., Beukeboom, L. W. & Billeter, J. C. Making sense of intralocus and interlocus sexual conflict. Ecol. Evol. 8, 13035–13050 (2018).
Billeter, J.-C. et al. Isoform-specific control of male neuronal differentiation and behavior in Drosophila by the fruitless gene. Curr. Biol. 16, 1063–1076 (2006).
Billeter, J.-C., Rideout, E. J., Dornan, A. J. & Goodwin, S. F. Control of male sexual behavior in Drosophila by the sex determination pathway. Curr. Biol. 16, 766–776 (2006).
Guo, H., Kunwar, K. & Smith, D. Odorant receptor sensitivity modulation in Drosophila. J. Neurosci. 37, 9465–9473 (2017).
Wilson, T. G., DeMoor, S. & Lei, J. Juvenile hormone involvement in Drosophila melanogaster male reproduction as suggested by the methoprene-tolerant27 mutant phenotype. Insect Biochem. Mol. Biol. 33, 1167–1175 (2003).
Ng, R., Lin, H.-H., Wang, J. W. & Su, C.-Y. Electrophysiological recording from Drosophila trichoid sensilla in response to odorants of low volatility. J. Vis. Exp.(125), 56147 (2017).
Wang, J. W., Wong, A. M., Flores, J., Vosshall, L. B. & Axel, R. Two-photon calcium imaging reveals an odor-evoked map of activity in the fly brain. Cell 112, 271–282 (2003).
Kaissling, K. E. & Thorson, J. Insect olfactory sensilla: structure, chemical and electrical aspect of the functional organization. In Proc. of the Workshop on Neurotransmitter and Hormone Receptors in Insects 261–282 (1980).
Dobin, A. et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29, 15–21 (2013).
Robinson, M. D., McCarthy, D. J. & Smyth, G. K. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26, 139–140 (2010).
Yapici, N., Kim, Y.-J., Ribeiro, C. & Dickson, B. J. A receptor that mediates the post-mating switch in Drosophila reproductive behaviour. Nature 451, 33–37 (2008).
Benton, R., Sachse, S., Michnick, S. W. & Vosshall, L. B. Atypical membrane topology and heteromeric function of Drosophila odorant receptors in vivo. PLoS Biol. 4, e20 (2006).
Fishilevich, E. & Vosshall, L. B. Genetic and functional subdivision of the Drosophila antennal lobe. Curr. Biol. 15, 1548–1553 (2005).
Acknowledgements
We thank J. Wang for critical advice on the experiments and manuscript and M. Maan, T. Verschut and M. Laturney for feedback on the manuscript. This work was supported in part by a grant from the Dutch organization for scientific research (no. ALWOP.611) to J.-C.B., a fellowship by the German Academic Exchange Service (no. 57386478) and a fellowship of the German National Academy of Sciences Leopoldina (no. LPDS 2018-11) to P.K. and grants from the National Institutes of Health to C.-Y.S. (nos. R01DC016466, R01DC015519 and R21DC108912).
Author information
Authors and Affiliations
Contributions
P.K., C.-Y.S. and J.-C.B. designed the study. P.K. and J.-C.B. performed the behavioural experiments, analytical chemistry, RNA-seq and data analysis. Y.Z. performed the single-sensillum recordings. J.A.G. provided the reagents. P.K., Y.Z., C.-Y.S. and J.-C.B. wrote the manuscript with feedback from all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Ecology & Evolution thanks Mariana Wolfner, Jen Perry and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
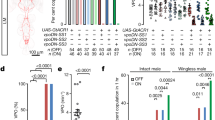
Extended Data Fig. 1 Mating latency is influenced by an interaction between female mating status and male genotype.
Canton-S virgin females were offered a single male (Nl or Tai) for mating. After mating, females were isolated for 24h and offered a new single male (Nl or Tai) for remating. Virgin and mated female mating latency was recorded. PLots display (Mean ± s.e.m). A generalized linear mixed model including mating latency as a response variable, male genotype (Nl vs. Tai), female mating status (virgin, mated) and their interaction as explanatory factors and female ID as a random factor revealed that mating latency is influenced by an interaction between male genotype and mating status (GLMM: p = 0.038). The interaction was then explored using posthoc tests.
Extended Data Fig. 2 Expression of transcripts potentially modulating neuronal sensitivity.
Normalized and log transformed read counts of antennal transcriptomes separated by mating status. Isoforms marked with (*) were differentially expressed between virgin and mated females (Mean ± s.e.m). *: p<0.05; **:p<0.01; ***:p<0.001.
Supplementary information
Rights and permissions
About this article
Cite this article
Kohlmeier, P., Zhang, Y., Gorter, J.A. et al. Mating increases Drosophila melanogaster females’ choosiness by reducing olfactory sensitivity to a male pheromone. Nat Ecol Evol 5, 1165–1173 (2021). https://doi.org/10.1038/s41559-021-01482-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41559-021-01482-4