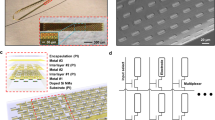
Abstract
Electrophysiology is the most used approach for the collection of functional data in basic and translational neuroscience, but it is typically limited to either intracellular or extracellular recordings. The integration of multiple physiological modalities for the routine acquisition of multimodal data with microelectrodes could be useful for biomedical applications, yet this has been challenging owing to incompatibilities of fabrication methods. Here, we present a suite of glass pipettes with integrated microelectrodes for the simultaneous acquisition of multimodal intracellular and extracellular information in vivo, electrochemistry assessments, and optogenetic perturbations of neural activity. We used the integrated devices to acquire multimodal signals from the CA1 region of the hippocampus in mice and rats, and show that these data can serve as ground-truth validation for the performance of spike-sorting algorithms. The microdevices are applicable for basic and translational neurobiology, and for the development of next-generation brain–machine interfaces.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$99.00 per year
only $8.25 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
The authors declare that all data supporting the findings of this study are available within the paper and its Supplementary information. The raw data acquired in this study are available from the corresponding author on reasonable request.
Code availability
The custom routines for Matlab used in this work are available from the corresponding author.
References
Eccles, J. C. The synapse: from electrical to chemical transmission. Ann. Rev. Neurosci. 5, 325–339 (1982).
Magee, J. C. Dendritic integration of excitatory synaptic input. Nat. Rev. Neurosci. 1, 181–190 (2000).
Schmidt-Hieber, C. & Nolan, M. F. Synaptic integrative mechanisms for spatial cognition. Nat. Neurosci. 20, 1483–1492 (2017).
Harvey, C. D., Collman, F., Dombeck, D. A. & Tank, D. W. Intracellular dynamics of hippocampal place cells during virtual navigation. Nature 461, 941–946 (2009).
Lee, D., Lin, B. J. & Lee, A. K. Hippocampal place fields emerge upon single-cell manipulation of excitability during behavior. Science 337, 849–853 (2012).
Long, M. A., Jin, D. Z. & Fee, M. S. Support for a synaptic chain model of neuronal sequence generation. Nature 468, 394–399 (2010).
Tan, A. Y., Chen, Y., Scholl, B., Seidemann, E. & Priebe, N. J. Sensory stimulation shifts visual cortex from synchronous to asynchronous states. Nature 509, 226–229 (2014).
Petersen, C. C. H. Whole-cell recording of neuronal membrane potential during behavior. Neuron 95, 1266–1281 (2017).
Poulet, J. F. A. & Petersen, C. C. H. Internal brain state regulates membrane potential synchrony in barrel cortex of behaving mice. Nature 454, 881–U836 (2008).
Yuste, R. From the neuron doctrine to neural networks. Nat. Rev. Neurosci. 16, 487–497 (2015).
Buzsaki, G., Anastassiou, C. A. & Koch, C. The origin of extracellular fields and currents—EEG, ECoG, LFP and spikes. Nat. Rev. Neurosci. 13, 407–420 (2012).
Einevoll, G. T., Kayser, C., Logothetis, N. K. & Panzeri, S. Modelling and analysis of local field potentials for studying the function of cortical circuits. Nat. Rev. Neurosci. 14, 770–785 (2013).
Mazzoni, A., Logothetis, N. K. & Panzeri, S. in Principles of Neural Coding (eds Quiroga, R. D. & Panzeri, S.) 411–429 (CRC Press, 2013).
Buzsáki, G. Large-scale recording of neuronal ensembles. Nat. Neurosci. 7, 446–451 (2004).
Lewicki, M. S. A review of methods for spike sorting: the detection and classification of neural action potentials. Network 9, R53–R78 (1998).
Anastassiou, C. A., Perin, R., Buzsaki, G., Markram, H. & Koch, C. Cell type- and activity-dependent extracellular correlates of intracellular spiking. J. Neurophysiol. 114, 608–623 (2015).
Chorev, E. & Brecht, M. In vivo dual intra- and extracellular recordings suggest bidirectional coupling between CA1 pyramidal neurons. J. Neurophysiol 108, 1584–1593 (2012).
Harris, K. D., Henze, D. A., Csicsvari, J., Hirase, H. & Buzsaki, G. Accuracy of tetrode spike separation as determined by simultaneous intracellular and extracellular measurements. J. Neurophysiol. 84, 401–414 (2000).
Henze, D. A. et al. Intracellular features predicted by extracellular recordings in the hippocampus in vivo. J. Neurophysiol. 84, 390–400 (2000).
Andrásfalvy, B. K. et al. Quantum dot-based multiphoton fluorescent pipettes for targeted neuronal electrophysiology. Nat. Methods 11, 1237–1241 (2014).
Canales, A. et al. Multifunctional fibers for simultaneous optical, electrical and chemical interrogation of neural circuits in vivo. Nat. Biotechnol. 33, 277–284 (2015).
LeChasseur, Y. et al. A microprobe for parallel optical and electrical recordings from single neurons in vivo. Nat. Methods 8, 319–325 (2011).
Katz, Y., Yizhar, O., Staiger, J. & Lampl, I. Optopatcher—an electrode holder for simultaneous intracellular patch-clamp recording and optical manipulation. J. Neurosci. Methods 214, 113–117 (2013).
Wise, K. D. et al. Microelectrodes, microelectronics, and implantable neural microsystems. Proc. IEEE 96, 1184–1202 (2008).
Jun, J. J. et al. Fully integrated silicon probes for high-density recording of neural activity. Nature 551, 232–236 (2017).
O’Keefe, J. & Recce, M. L. Phase relationship between hippocampal place units and the EEG theta rhythm. Hippocampus 3, 317–330 (1993).
Wilson, M. A. & McNaughton, B. L. Dynamics of the hippocampal ensemble code for space. Science 261, 1055–1058 (1993).
Felix, S. H. et al. Insertion of flexible neural probes using rigid stiffeners attached with biodissolvable adhesive. J. Vis. Exp. 79, e50609 (2013).
Fu, T. M. et al. Stable long-term chronic brain mapping at the single-neuron level. Nat. Methods 13, 875–882 (2016).
Stieglitz, T., Beutel, H., Schuettler, M. & Meyer, J. U. Micromachined, polyimide-based devices for flexible neural interfaces. Biomed. Microdevices 2, 283–294 (2000).
Robinson, D. A. The electrical properties of metal microelectrodes. Proc. IEEE 56, 1065–1071 (1968).
Madisen, L. et al. A toolbox of Cre-dependent optogenetic transgenic mice for light-induced activation and silencing. Nat. Neurosci. 15, 793–802 (2012).
Robinson, D. L., Venton, B. J., Heien, M. L. A. V. & Wightman, R. M. Detecting subsecond dopamine release with fast-scan cyclic voltammetry in vivo. Clin. Chem. 49, 1763–1773 (2003).
Hamid, A. A. et al. Mesolimbic dopamine signals the value of work. Nat. Neurosci. 19, 117–126 (2016).
Lebedev, M. A. & Nicolelis, M. A. Brain-machine interfaces: past, present and future. Trends Neurosci. 29, 536–546 (2006).
Bittner, K. C. et al. Conjunctive input processing drives feature selectivity in hippocampal CA1 neurons. Nat. Neurosci. 18, 1133–1142 (2015).
Bittner, K. C., Milstein, A. D., Grienberger, C., Romani, S. & Magee, J. C. Behavioral time scale synaptic plasticity underlies CA1 place fields. Science 357, 1033–1036 (2017).
Izhikevich, E. M., Desai, N. S., Walcott, E. C. & Hoppensteadt, F. C. Bursts as a unit of neural information: selective communication via resonance. Trends Neurosci. 26, 161–167 (2003).
Li, C. Y. T., Poo, M. M. & Dan, Y. Burst spiking of a single cortical neuron modifies global brain state. Science 324, 643–646 (2009).
Lisman, J. E. Bursts as a unit of neural information: making unreliable synapses reliable. Trends Neurosci. 20, 38–43 (1997).
Rey, H. G., Pedreira, C. & Quiroga, R. Q. Past, present and future of spike sorting techniques. Brain Res. Bull. 119, 106–117 (2015).
Neto, J. P. et al. Validating silicon polytrodes with paired juxtacellular recordings: method and dataset. J. Neurophysiol. 116, 892–903 (2016).
Wild, J., Prekopcsak, Z., Sieger, T., Novak, D. & Jech, R. Performance comparison of extracellular spike sorting algorithms for single-channel recordings. J. Neurosci. Methods 203, 369–376 (2012).
Quiroga, R. Q., Nadasdy, Z. & Ben-Shaul, Y. Unsupervised spike detection and sorting with wavelets and superparamagnetic clustering. Neural Comput. 16, 1661–1687 (2004).
Kadir, S. N., Goodman, D. F. & Harris, K. D. High-dimensional cluster analysis with the masked EM algorithm. Neural Comput. 26, 2379–2394 (2014).
Anastassiou, C. A., Perin, R., Markram, H. & Koch, C. Ephaptic coupling of cortical neurons. Nat. Neurosci. 14, 217–223 (2011).
Holt, G. R. & Koch, C. Electrical interactions via the extracellular potential near cell bodies. J. Comput. Neurosci. 6, 169–184 (1999).
Barbic, M., Moreno, A., Harris, T. D. & Kay, M. W. Detachable glass microelectrodes for recording action potentials in active moving organs. Am. J. Physiol. Heart Circ. Physiol. 312, H1248–H1259 (2017).
Lee, A. K., Epsztein, J. & Brecht, M. Head-anchored whole-cell recordings in freely moving rats. Nat. Protoc. 4, 385–392 (2009).
Long, M. A. & Lee, A. K. Intracellular recording in behaving animals. Curr. Opin. Neurobiol. 22, 34–44 (2012).
Margrie, T. W., Brecht, M. & Sakmann, B. In vivo, low-resistance, whole-cell recordings from neurons in the anaesthetized and awake mammalian brain. Pflügers Arch. 444, 491–498 (2002).
Vreeland, R. F. et al. Biocompatible PEDOT: Nafion composite electrode coatings for selective detection of neurotransmitters in vivo. Anal. Chem. 87, 2600–2607 (2015).
Atta, N. F., Galal, A. & Ahmed, R. A. Poly(3,4-ethylene-dioxythiophene) electrode for the selective determination of dopamine in presence of sodium dodecyl sulfate. Bioelectrochemistry 80, 132–141 (2011).
Tang, H., Lin, P., Chan, H. L. W. & Yan, F. Highly sensitive dopamine biosensors based on organic electrochemical transistors. Biosens. Bioelectron. 26, 4559–4563 (2011).
Hunt, D. L., Linaro, D., Si, B., Romani, S. & Spruston, N. A novel pyramidal cell type promotes sharp-wave synchronization in the hippocampus. Nat. Neurosci. 21, 985–995 (2018).
Cui, X. Y. & Martin, D. C. Electrochemical deposition and characterization of poly(3,4-ethylenedioxythiophene) on neural microelectrode arrays. Sens. Actuators B 89, 92–102 (2003).
Acknowledgements
We would like thank A. Pais, D. Lee, V. Reddy, H. Esmailbeigi, B. Bowers, B. Biddle, J. Macklin, R. Patel, W. Sun, B. Barbarits, J. Venton, E. Privman, P. Ahamad and L. Coddington for their valuable contributions to this study. We would also like to thank J. Markara and B. Andrasfalvy for helpful discussions. This work was supported by the Howard Hughes Medical Institute.
Author information
Authors and Affiliations
Contributions
D.L.H., A.K.L., T.D.H. and M.B. conceived the project. T.D.H. supervised the project. M.B. developed and fabricated all multimodal devices. A.K.L. and M.B. aquired data with the Patch-Tritrode. D.L.H. and C.L. analysed the Patch-Tritrode data. D.L.H. and M.B. aquired data with the Patch-Silvertrode. D.L.H. analysed the Patch-Silvertrode data. R.D.S. and M.B. aquired Patch-Carbontrode data. D.L.H. and R.D.S. analysed the Patch-Carbontrode data. D.L.H. and M.B. wrote the manuscript with input from all authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary figures.
Rights and permissions
About this article
Cite this article
Hunt, D.L., Lai, C., Smith, R.D. et al. Multimodal in vivo brain electrophysiology with integrated glass microelectrodes. Nat Biomed Eng 3, 741–753 (2019). https://doi.org/10.1038/s41551-019-0373-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41551-019-0373-8
This article is cited by
-
Multifunctional microelectronic fibers enable wireless modulation of gut and brain neural circuits
Nature Biotechnology (2023)
-
Energy-efficient firing modes of chay neuron model in different bursting kinetics
Science China Technological Sciences (2022)
-
SHYBRID: A Graphical Tool for Generating Hybrid Ground-Truth Spiking Data for Evaluating Spike Sorting Performance
Neuroinformatics (2021)
-
Flexible, diamond-based microelectrodes fabricated using the diamond growth side for neural sensing
Microsystems & Nanoengineering (2020)
-
Pipette-integrated microelectrodes
Nature Biomedical Engineering (2019)