Abstract
Traditional fermented foods are of major importance with respect to the socio-economic growth, food security, nutrition, and health of African consumers. In several African countries, traditional fermentation processes provide a means of food preservation, improving the shelf life and adding to the nutrients in the food products. As with any fermented foods, the associated food microbiota is of great importance and interest. Recent studies on the microbiome of African fermented foods using high-throughput DNA sequencing techniques have revealed the presence of diverse microbial populations of fundamental, technological, and commercial interest that could be harnessed to further improve health, food safety, and quality. This review provides an overview of African fermented foods, their microbiota, and the health-promoting potential of these foods and microbes.
Similar content being viewed by others
Introduction
Fermented foods play an essential role in tackling issues of poverty, malnutrition, and hunger among African consumers1. These foods also enhance food security, sustainable development, and economic growth in Africa such as through the provision of employment opportunities, contribution to empowerment initiatives for unemployed women, opportunities for scaling up of traditional food processing techniques, and distribution of resultant products2. Fermented foods can be a rich source of nutrients, with fermentation bringing about a “pre-digestion” of food substrates to make the associated nutrients more bioavailable and, in some instances, removing allergens (including 2S albumin proteins, profilins, cupin, prolamine), antinutritional compounds (such as phytate, tannins, lectins, protease inhibitors, saponins, alkaloids, and oxalate) and toxins (such as cyanogenic glycosides, bacterial toxins, mycotoxins, biogenic amines)3. The associated microbes can have health-promoting properties either directly, with some probiotic strains from most commonly consumed and extensively investigated fermented foods in Africa or indirectly, through the production of health-promoting metabolites. Several fermented foods also contain prebiotic components4. Indigenous fermentation processes represent traditional, reliable, and affordable methods of preserving nutritional and sensory qualities of associated substrates while extending shelf life and enhancing safety. Fermentation of these foods in several African countries usually involves spontaneous activities of natural microbes found in the food or from a previous fermentation, such as through “back-slopping” to bring about the desirable changes5. Fermentation of certain foods facilitates wet milling, the aforementioned removal of toxic or anti-nutritive compounds, and improvement of organoleptic properties and extension of the shelf life of the food products. The preservative effects of fermented foods are particularly important in Africa considering the tropical climatic conditions across large regions of the continents. The climate makes the preservation of fresh farm products such as milk, fruits, and vegetables very challenging, resulting in reports of over 70% loss of some products yearly6,7. Post-harvest spoilage of fresh foods is usually enhanced by environmental pollution and contamination which results in physical rotting, esthetic spoiling of the environment, and bad odor, ultimately making the food unsuitable for consumption. Although the preferred approach for the preservation of fresh foods is refrigeration, inadequate power supplies and low living standards in certain regions of Africa limit this option8. Thus, in some instances, food fermentation is the only option for preserving foods, resulting in products with enhanced acceptability, digestibility, functionality, and nutritional quality.
Such enhancements are more controllable where there is a corresponding understanding of the microbiota of the fermented food. Thus, while, to date, there is a corresponding understanding of many of the functional pathways and metabolic potential of several fermenting genera including Leuconostoc, Lactobacillus, Pediococcus, Debaryomyces, Kluyveromyces, Saccharomyces, etc9,10,11,12,13,14, this is increasingly complemented by advances in shotgun metagenomic sequencing that, in addition to identifying the microorganisms present (including novel species and strains), can provide an insight into potential metabolic and functional pathways encoded15,16,17,18.
Commonly consumed fermented food products in Africa
Fermented foods represent a significant component of the diverse range of African diets, with individual foods being assigned, for example, as staple foods, condiments, or beverages. The foods can also be classified according to the raw materials used for their production. These include tuber-based products (e.g., “Fufu,” “Lafun,” and “Gari”); cereal-based products (e.g., “Ogi,” “Burukutu,” “Kunnu-zaki,” and “Pito”); legume-based products (e.g., “Iru” or “Dawadawa,” “Ogiri,” and “Ugba”); beverage-based products (e.g., “Emu,” “Oguro”), and dairy-based products (e.g., “Fura,” “Nunu,” “Wara-Kishi”) (Fig. 1). These respective categories are described in greater depth below.
Fermented tuber products
Tubers such as cassava (Manihot esculenta), yam (Dioscorea alata), and sweet potato (Ipomoea batatas) are common starchy foods. They are rich in carbohydrates and are generally cultivated for consumption in individual African households. Government policies in many African countries also support the production of cassava and fermented cassava products as an alternative to imported wheat and rice products19. Fermented cassava products commonly consumed across African countries include those produced from the tubers (fresh or dried), cassava leaves, milled paste or granulated fried products20. Tubers are usually peeled, washed, milled, and fermented for about 3–5 days into sour granulated flakes called “Gari” (Nigeria), steamed granulated flakes “Attieke” (Côte d’Ivoire) or milled into flour called “Lafun” or “Kokonte” (Nigeria and Ghana) to prepare meals such as “Amala,” “Eba,” and “Fufu” (Nigeria). Other fermented tuber products include “Agbelima” (Ghana), “Atangana,” “Kumkum,” “Myiodo” (Cameroon), “Maphumu,” and “Makaka” (Malawi)21,22,23. The hydrogen cyanide (HCN), linamarin, loutaustralin, and other undesirable compounds in cassava are eliminated during processing which includes peeling, grating, soaking, pressing, and frying/cooking24. The cyanogenic glycosides are hydrolyzed by beta-glucosidases and HCN evaporates prior to consumption of the products25. Fermentation further eliminates the cryogenic glycosides through microbial activities (such as that by lactic acid bacteria (LAB) and yeasts) generating metabolites, including organic acids and volatile compounds, which contribute to the distinct organoleptic properties of the fermented products26,27,28,29. Several LABs have been isolated from African fermented tuber food products and found to produce antimicrobial agents, vitamins, and other compounds of interest. Microorganisms isolated from fermented tuber products include representatives of the species: Lactobacillus acidophilus, Levilactobacillus brevis, Limosilactobacillus fermentum, Ligilactobacillus salivarius, Limosilactobacillus reuteri, Lacticaseibacillus casei, Enterococcus faecium, Bacillus subtilis, Geotrichum candidum, Mucor racemosus, Saccharomyces cerevisae, Neurospora sitophila, and Rhizopus oryzae30,31,32.
Fermented cereal products
Cereal fermented porridges or beverages with live and active microorganisms are much more common in Sub-Sahara Africa than in other places of the world. The food products form major components of staple foods, used as a part of weaning diets for infants and essential functional foods for children. The foods are produced from millet (Panicum miliaceum), wheat (Triticum aestivum), maize (Zea mays), rice (Oryza sativa), and sorghum (Sorghum bicolor) and, as with other fermented foods, they undergo biochemical, and nutritional changes during the fermentation process33,34. The fermented cereal products can be in the form of a paste or slurry as in the case for “Ogi” (Nigeria), “Akasa” (Ghana), “Uji” (Kenya), “Abreh” (Sudan), “Mahewu” (Zimbabwe and South Africa) including fermented drinks like “Burukutu” (Nigeria) and “Kunnu” (Nigeria)35,36. The cereal-based fermented foods are reported as good sources of beneficial microorganisms, micronutrients and are regarded as health-promoting foods37,38,39. Fermented cereal products also contain LAB that contributes extensively to nutritional properties as well as to food safety, shelf-life quality, and organoleptic properties through the production of organic acids, bacteriocins, and volatile compounds. These LABs include Lactiplantibacillus plantarum, Limosilactobacillus fermentum, Levilactobacillus brevis, Lactococcus lactis, Leuconostoc species, and Pedicoccus species, while the other bacteria represented include Streptococcus and Corynebacterium species. Yeasts such as Saccharomyces cerevisiae, Geotrichum fermentum, Candida tropicalis, Rhodotorula graminis, and molds such as Aspergillus species, Fusarium subglutinans, Rhizopus nigricans, and Penicillum citrinum have been reported to be present at the early stage of the fermentation of these products (Table 1)36,37,40.
Fermented legume products
African fermented legume products are important sources of proteins, which are made more bioavailable following microbial digestion and detoxification, thereby contributing to countering malnutrition. These foods are often produced as condiments but have not been successfully commercialized on a large scale due to their short shelf-life and challenges with achieving packaging standards for the finished products and, so, continue to be produced using traditional methods41,42. The fermented condiments of plant-based proteinaceous foods include; “Iru” or “Dawadawa,” “Tempeh,” “Soumbala,” and “Netetu” from African locust beans, (Parkia biglobosa) (Nigeria, Ghana, Burkina Faso, Senegal), “Ugba” from African oil beans (Pentaclethra macrophylla) (Nigeria, Benin), “Ogiri” from either of castor oil beans (Ricinus Communis), Melon seeds (Citrullus vulgaris) (Nigeria), Fluted pumpkin seeds (Telfairia occidentalis) (Nigeria), or Bambara groundnut seeds (Vigna subterranean) (Nigeria), “Okpiye” from Prosopis Africana seeds (Prosopis africana), “Owoh” from African yam beans (Sphenostylis stenocarpa) (Nigeria) and “Siljo” from Faba beans (Vicia faba) flour mixed with safflower (Carthamus tinctorius) (Ethiopia). Fermented condiments are produced by the alkaline fermentation of legume seeds and are associated with abundance of Bacillus subtilis, Bacillus megaterium, Bacillus circulans, Bacillus licheniformis, and Bacillus coagulans43,44,45. Other bacteria identified previously included strains of Lactiplantibacillus plantarum, Lb. acidophilus, Lb. delbrueckii subsp. bulgaricus, Yarrowia lipolytica, Saccharomyces cerevisiae, Saccharomyces rouxii, Rhodotorula glutinis, Staphylococcus aureus, Escherichia coli, Streptococcus natalensis, Proteus species, Alcaligenes species, Micrococcus, Corynebacterium, Enterococcus faecium, and Pseudomonas species46,47.
Fermented dairy products
Fermented dairy products are often produced through the fermentation of milk. Dairy products such as “Nunu” (Nigeria, Ghana), “Wara” (Nigeria, Togo), “Fene” (Côte d’Ivoire, Mali), “Suusac” (Kenya, Somalia), “Pendidam” (Cameroon), “Gariss” (Sudan), “Nyamie” (Ghana), “Leben/lben” (Tunisia) and “Kulenaoto” (Kenya) are important fermented dairy products of considerable nutritional importance providing macronutrients, micronutrients, and health-promoting microorganisms. Such dairy fermented foods are normally introduced by backslopping of previously fermented dairy products to serve as a starter culture for a new fermentation. Fermented dairy products are widely consumed by Africans due to the health-promoting benefits linked to the microbial contents of the foods and the presence of specific micronutrients48. Various researchers have documented the dominance of LABs in African fermented milk, including strains of Lb. delbrueckii subsp. bulgaricus, Lb. acidophilus, Lactococcus lactis subsp. lactis, Lactococcus lactis subsp. cremoris, Limosilactobacillus fermentum, Ligilactobacillus salivarius, Lactiplantibacillus plantarum, Enterococcus, and Streptococcus species (Table 1)48,49,50,51. Other microorganisms including Staphylococcus species, Streptococcus infantarius subsp. infantarius, Trichosporon species, Saccharomyces cerevisae, Rhodotorula mucilaginosa, and Geotrichum penicillatum have also been identified. Notably, several researchers have reported that the presence in dairy products of strains corresponding to many of the taxa listed above, introduced as starter cultures or as part of the resident microbiota, is associated with improved organoleptic, nutritional safe, and shelf-life qualities48,52.
African fermented foods as a “storehouse” for potential health-promoting microorganisms
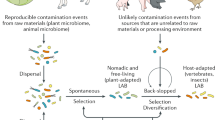
As noted above, there is a wide range of locally produced fermented foods and beverages across Africa. This results from a wide variety of fermentation types (lactic acid, alcohol, alkaline fermentation), substrates, microorganisms, and methods. Although food fermentation in Africa mostly occurs through spontaneous fermentation, there are also instances where the microorganisms were introduced by backslopping from previously fermented foods into new fermentations53. African fermented foods have been reported to be sources of many potentially nutritionally beneficial and/or health-promoting components, including microorganisms. Unsurprisingly, from a microbial perspective, there has been a particular focus on members of the lactic acid bacteria such as members of the former genus Lactobacillus54 (e.g., from fermented condiments “Iru,” “Ugba,” and “Ogiri” and fermented cereal-based foods “Ogi”), Lactococcus species (e.g., from fermented milk “Nunu” and yoghurt “Wara”) Pediococcus species and Weissella species (e.g., from fermented cereal-based foods “Ogi” and “Kunun”)55,56. We use the word “potentially” with care, as many indications of health benefits do not match the criteria required to use the term probiotic57. Further investigations are needed (including clinical trials) before the term probiotic can be used in the context of these microorganisms.
Despite this, the data that is available in relation to the health-promoting properties of specific African fermented foods and their associated microorganisms provides cause for optimism, with potential future applications including preventing the colonization of pathogens, treatment of infectious diseases, improved lactose tolerance, immune system modulations, improved barrier function, interference with quorum sensing signaling and the production of antimicrobial substances58. There are, however, limited reports on clinical randomized trials on the application of African fermented foods to improve general health with positive outcomes on conditions such as acute diarrhea in infants59,60,61. This could be associated with the microbiota in controlling enteric pathogens by inhibiting attachment of the pathogens’ pili to gut mucosa, through the release of organic acids and antimicrobial peptides, by competing for limited nutrients as well as the neutralization of exotoxins released by pathogens62,63.
The gut microbiome is now increasingly becoming a target with a view to improving human health including, in its most extreme form, the use of fecal microbiome transplants for the treatment of Clostridioides difficile infection64. However, from a practical “every day,” perspective, dietary modulation of the human microbiome is a more appealing alternative. Fermented foods were a particularly popular option in this regard, with the potential for modulation of the host microbiome (or host cell activity) by components of the foods (i.e., microbes, metabolites, digested components of the original substrate). Studies showing consumption of fermented foods is both correlated with, and responsible for, improved health outcomes65. Despite this, fermented foods and associated microbes remain surprisingly relatively underexplored, particularly in respect to potential health benefits and modulation of the human microbiome66. African fermented foods are especially underexplored in this respect67. Fundamentally, African fermented food research provides researchers with novel insights into underexplored foods (and associated microbiota), but applying the research can also be beneficial for empowering local non-scientific producers of the food with prospects for commercialization and health-promoting benefits68,69,70.
Health-promoting potentials of bioactive components in African fermented foods
The cereal-based African fermented foods (rice, millet, maize, or sorghum) are rich in bioactive substances released during fermentation but also contain anti-nutritional factors including polyphenols, tannins, and phytic acid, which reduce the digestibility and bioavailability of carbohydrates and proteins. These foods can also serve as a source of prebiotics, which are “substrates that are selectively utilized by host microorganisms conferring health benefits”, which include non-digestible dietary fractions, such as oligosaccharides, polysaccharides, resistant starches, polyphenols, and resistant proteins71. Microbial hydrolysis of certain prebiotics has also been reported to reduce anti-nutritional factors and thus improve the nutritional profile of the food products. Fermented cereal foods (such as “Ogi,” “Mahewu,” “Incwancwa,” “Togwa,” “Borde,” or “shamita”) made from maize were previously reported to be a very rich source of vitamin B complex including folic acid, pyridoxine, pantothenic acid, thiamine, and riboflavin while fermented sorghum-based foods (such as “Munkoyo,” “Uji,” “Ogi,” “Kunu”) are rich in amino acids such as alanine, aspartic acid, glutamic acid, leucine, phenylalanine proline, and valine with low tryptophan and lysine. Some studies have also reported the presence of trace amounts of β-carotene72,73.
Microbial profiling techniques and the microbiota of African fermented foods
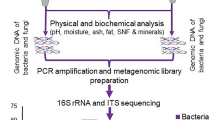
In recent years, high-throughput sequencing (HTS) techniques have been used to an increasing extent to study the microbiome of fermented foods from across the globe, including gaining insights into microbial dynamics, food safety, and putative health benefits74. Despite the increasingly widespread use of these approaches, the study of fermented foods in Africa still relies heavily on the use of classical culture-based methods combined with some polymerase chain reaction (PCR)-based techniques. Other population-based analyses such as those involving DGGE (denaturing gradient gel electrophoresis) have also been used in the past75 but do not facilitate the easy identification of the taxa present. In addition, the use of DGGE provides a dependable and considerable profile of diverse bacteria communities in cereal-based fermented foods (Amasi) commonly consumed in south Africa76 and PCR-DGGE fingerprints of microbial successional changes during fermentation of cereal-legume weaning foods in Nigeria77. Currently, Rep-PCR fingerprinting, 16S and 23S rRNA gene sequencing techniques, shotgun metagenomics sequencing, and whole-genome sequencing are infrequently used to study the microbial ecology of African fermented foods, and the microbial species/strains that are present therein. This infrequent use reflects the lack of access to the appropriate infrastructure and the high cost of applying HTS techniques to study the microbiomes and genomic diversity of strains in Africa fermented foods78,79.
Frequently, the methods used to analyze fermented foods in Africa precluded the characterization of non-culturable, or difficult to culture microbes, and thus specific components of microbial populations in these food ecosystems may have been overlooked. Although some recent studies have tried to address this problem by developing modified DNA extraction protocols for subsequent genomic and metagenomic sequencing of selected African fermented foods such as “Dengue” (Burkina-Faso), “Lafun” (Nigeria), “Ogi” (Nigeria), “Masau” (Zimbabwe), “Obusera” (Uganda), “Fura” (Ghana), the full potential of HTS has yet to be widely applied to the study of African fermented food microbiomes80,81,82,83.
Potential application of HTS techniques for commonly consumed African fermented foods
Advances in microbial analysis of the food ecosystem have involved the use of HTS techniques such as whole-genome sequencing, amplicon bases meta-taxonomic approaches (such as 16S rRNA sequencing), shotgun metagenomics, and (meta) transcriptomics, and rely on downstream bioinformatics analysis84. In Africa, whole-genome sequencing was initially used for the analysis and surveillance of foodborne pathogens. This process supplements other pre-existing methods such as culture-based isolation, bio-typing, serotyping, genotyping, as well as virulence and antimicrobial gene detection for food-associated pathogenic microorganisms85,86. As discussed, this technique could also be harnessed to assess food isolates from the perspective of their value as a starter or adjunct cultures by identifying genotypic traits underpinning features such as the production of bacteriocins or other antimicrobials of relevance from most consumed African fermented foods for improved food preservation and shelf-life quality as listed in Table 2. These tools could be applied to screen for pathways associated with improving the nutritional quality of the food and/or imparting other health-promoting characteristics.
The 16S rRNA gene amplification combined HTS allows for genus level assessment of the microbiota and technological improvement is constantly reducing sequencing costs, resulting in affordability of HTS in diverse research applications. Shotgun metagenomic sequencing facilitates deeper insights into the microbiome, allowing strain-level identification, functional annotation including carbohydrate pathways and bioactive molecule production (such as bacteriocins), and the assembly of high-quality genomes in the form of metagenome-assembled genomes (MAGs)18,87. In the latter case, the calculation of Average Nucleotide Identity scores of newly assembled MAGs with existing genome databases facilitate taxonomic assignment at species and even at strain level88.
Several HTS sequencing platforms, such as those developed by Illumina, Ion Torrent, PacBio, BGI, and Oxford Nanopore technologies and protocols could reveal microbial taxonomy, diversity, functional potential, and/or gene expression patterns with different results. The Illumina sequencing platforms have been widely employed for the generation of extremely high quality, short reads of up to 2×300 bp. Conversely, MinION platform based on single-molecule sequencing technology generates considerably longer read lengths but with lower accuracy and read numbers than short-read HTS platforms. Short read sequencing is not capable of completely closing bacterial genomes from mixed communities while using long-read data with a high error rate has other associated drawbacks, such as, for example, an inability to reliably identify single-nucleotide polymorphisms. However, by combining both approaches for whole-genome sequencing, fully assembled chromosomes and plasmids can be recovered with low error rates89. As DNA sequencing technologies continue to improve, the use of existing techniques inevitably becomes more cost-effective, facilitating the more widespread use of these techniques globally. Indeed, access to HTS-based analyses is slowly becoming more accessible to African researchers interested in fermented foods and, as noted, it is hoped that this can contribute greatly towards the development of nutrient-based and fortified African fermented food90. As previously noted, the emergences of these innovations provide many possibilities for future research relating to commonly consumed African fermented foods shown in Fig. 2, and in particular, the development of microbial cultures for optimized food fermentations. Limitations of sequencing-based analyses still remain, such as live and dead cell differentiation (particularly important for spontaneously fermented foods), absolute count of microbes present (as opposed to relative abundance), and with some methods, limited taxonomic information and ambiguous assignment. The propidium monoazide-based sequencing methods are getting better at resolving live/dead cell differentiation, while metatranscriptomics can add a further layer of information in terms of which taxa are metabolically active. Flow cytometry and quantitative PCR can also be employed to facilitate insights into counts91,92,93,94,95,96,97,98. As noted above, combining long and short read data can facilitate the complete assembly of genomes including plasmids, mitigating some of the issues related to ambiguous assignment and false positives. However, sequencing-based analyses (particularly functional attributes) need validation by culture-based experiments.
Microbial diversity profiling of African fermented foods ecosystem
For community-based investigations, amplicon sequencing of the hypervariable (V1–V4 or V4–V5) regions of the 16S rRNA amplicon gene and shotgun metagenomics, sequencing have improved insights into African fermented food products17. While fermented food products around the world are increasingly being studied using shotgun metagenomic techniques, very few studies have explored the use of shotgun sequencing in African fermented foods, with some exceptions being “Nunu” (Ghana), “Kokonte” (Ghana/Togo) and “Wagashi” (Benin)17. Leech et al. analyzed 58 fermented foods including “Nunu” (Ghana) and “Wagashi” (Benin) through shotgun metagenomic sequencing, which provided key insights into their microbial ecology and functional capabilities for health-promoting attributes18. Diaz et al. also investigated 40 African fermented foods using 16S rRNA metagenomic sequencing and found members of the genera Lactobacillus, Leuconostoc, Weissella, Bacillus, Zygomonas, Streptococcus, Zymomonas, Gemmella, Brevibacillus, Jeotgalicoccus, Vagococcus, Atopstipes, and others belonging to the family Carnobacteriaceae79. While these studies have highlighted the merits of applying HTS technologies to the study of African fermented food communities, there is a need for a broader-scale application.
Conclusion
Fermented foods are a key component of the African diet and the associated microbes and microbial communities are being studied in ever-greater depth. The further harnessing of traditional culture-based and HTS techniques has the potential to characterize and optimize the microbiome of African fermented foods, from commercial to nutritional and health-promoting aspects. HTS can also be used in isolation, or in combination with cultivation, to identify virulence, antimicrobial resistance determinants, and provide genotypic characterization. Further access to HTS platforms and the democratization of sequencing have the potential to be of considerable value with respect to the further development of these foods.
Data availability
The authors confirm that all data are available within the article.
References
Fagunwa, O. E. & Olanbiwoninu, A. A. Accelerating the sustainable development goals through microbiology: some efforts and opportunities. Access Microbiol. 1, acmi000112 (2020).
Akinsemolu, A. A. The role of microorganisms in achieving the sustainable development goals. J. Clean. Prod. 182, 139–155 (2018).
Samtiya, M., Aluko, R. E., Puniya, A. K. & Dhewa, T. Enhancing micronutrients bioavailability through fermentation of plant-based foods: a concise review. Fermentation 7, 63–76 (2021).
Şanlier, N., Gökcen, B. B. & Sezgin, A. C. Health benefits of fermented foods. Crit. Rev. Food Sci. Nutr. 59, 506–527 (2019).
Montemurro, M., Pontonio, E., Gobbetti, M. & Rizzello, C. G. Investigation of the nutritional, functional, and technological effects of the sourdough fermentation of sprouted flours. Int. J. Food Microbiol. 302, 47–58 (2019).
Jideani, A. I., Anyasi, T. A., Mchau, G. R., Udoro, E. O. & Onipe, O. O. Processing and preservation of fresh-cut fruit and vegetable products. Postharvest Handl. 1, 47–73 (2017).
Obafemi, Y. D., Ajayi, A. A., Olasehinde, G. I., Atolagbe, O. M. & Onibokun, E. A. Screening and partial purification of amylase from Aspergillus niger isolated from deteriorated tomato (Lycopersicon esculentum mill.) fruits. Afr. J. Clin. Exp. Microbiol. 19, 47–57 (2018).
Poore, J. & Nemecek, T. Reducing food’s environmental impacts through producers and consumers. Science 360, 987–992 (2018).
Bihon, W. et al. Extending the shelf-life of vegetables using low-cost evaporative cooling systems in Mali. Acta Hortic. 1275, 419–426 (2018).
Obafemi, Y. D., Ajayi, A. A., Taiwo, O. S., Olorunsola, S. J. & Isibor, P. O. Isolation of polygalacturonase-producing bacterial strain from tomatoes (Lycopersicon esculentum Mill.). Int. J. Microbiol. 1, 7505606 (2019).
Muyonga, J. H., Nansereko, S., Steenkamp, I., Manley, M. & Okoth, J. K. in Exploring the Nutrition and Health Benefits of Functional Foods 320–346 (IGI Global, 2017).
Okoye, J. I. & Oni, K. Promotion of indigenous food preservation and processing knowledge and the challenge of food security in Africa. J. Food Secur. 5, 75–87 (2017).
Adebiyi, J. A., Kayitesi, E., Adebo, O. A., Changwa, R. & Njobeh, P. B. Food fermentation and mycotoxin detoxification: an African perspective. Food Control 1, 106731 (2019).
Walsh, A. M., Crispie, F., Claesson, M. J. & Cotter, P. D. Translating omics to food microbiology. Annu. Rev. Food Sci. Technol. 8, 113–134 (2017).
Olasupo, N. A. & Okorie, P. C. African fermented food condiments: Microbiology impacts on their nutritional values. In (Ed.), Frontiers and new trends in the science of fermented food and beverages. Vol 1, 1–20 (IntechOpen, 2019).
Tamang, J. P. et al. Fermented foods in a global age: East meets West. Compr. Rev. Food Sci. Food Saf. 19, 184–217 (2020).
Walsh, A. M. et al. Strain-level metagenomic analysis of the fermented dairy beverage nunu highlights potential food safety risks. Appl. Environ. Microbiol. 83, e01144-17 (2017).
Leech, J. et al. Fermented-food metagenomics reveals substrate-associated differences in taxonomy and health-associated and antibiotic resistance determinants. MSystems 5, e00522-20 (2020).
Kaur, K. & Ahluwalia, P. Cassava as potential crop for the food and fermentation industry: a review. Int. J. Food Ferment. Technol. 7, 1–12 (2017).
Ahaotu, N. N. et al. Influence of soy fortification on microbial diversity during cassava fermentation and subsequent physicochemical characteristics of Gari. Food Microbiol. 66, 165–172 (2017).
Awoyale, W., Abass, A. B., Ndavi, M., Maziya-Dixon, B. & Sulyok, M. Assessment of the potential industrial applications of commercial dried cassava products in Nigeria. J. Food Meas. Charact. 11, 598–609 (2017).
Abass, A. B. et al. Can food technology innovation change the status of a food security crop? A review of cassava transformation into “bread” in Africa. Food Rev. Int. 34, 87–102 (2018).
Braide, W., Azuwike, C. O. & Adeleye, S. A. The role of microorganisms in the production of some indigenous fermented foods in Nigeria. Int. J. Adv. Res. 5, 86–94 (2018).
Babatuyi, C. Y., Boboye, B. E., Fagbemi, T. N. & Enujiugha, V. N. Cyanide, haematology and histopathology profiles of albino rats fed with ‘Fufu’-based diets produced from mixed starter cultures. Heliyon 6, e04391 (2020). (2020).
Obi, C. N., Okezie, O. & Ukaegbu, T. Fermentation reduces cyanide content during the production of cassava flours from sweet and bitter cassava tuber varieties. Asian Food Sci. J. 11, 1–10 (2019).
Oyeyinka, S. A., Adeloye, A. A., Olaomo, O. O. & Kayitesi, E. Effect of fermentation time on physicochemical properties of starch extracted from cassava root. Food Biosci. 33, 100485 (2020).
Indrastuti, Y. E., Estiasih, T., Christanti, R. A., Pulungan, M. H. & Zubaedah, E. Microbial and some chemical constituent changes of high cyanide cassava during simulant spontaneous submerged and solid-state fermentation of “Gadungan pohung”. Int. Food Res. J. 25, 487–498 (2018).
Hawashi, M., Widjaja, T. & Gunawan, S. Solid-State Fermentation of Cassava Products for Degradation of Anti-Nutritional Value and Enrichment of Nutritional Value. In (Ed.), New Advances on Fermentation Processes. Vol 1, 1–19 (IntechOpen, 2019).
Femi, A., Fayemi, S., Olukanni, O., Ogunbiyi, T. & Oluniyi, P. Molecular characterization of lactic acid organisms isolated from spontaneous fermentation of cassava-fufu and gari. Asian Food Sci. J. 8, 1–10 (2019).
Anyogu, A., Awamaria, B., Sutherland, J. P. & Ouoba, L. I. Molecular characterization and antimicrobial activity of bacteria associated with submerged lactic acid cassava fermentation. Food Control 39, 119–127 (2014).
Behera, S. S., El Sheikha, A. F., Hammami, R. & Kumar, A. Traditionally fermented pickles: How the microbial diversity associated with their nutritional and health benefits? J. Funct. Foods 70, 103971 (2020).
Ogbo, F. A., Page, A., Idoko, J., Claudio, F. & Agho, K. E. Trends in complementary feeding indicators in Nigeria, 2003-2013. BMJ Open 5, e008467 (2015).
Liptáková, D., Matejčeková, Z. & Valík, L. Lactic acid bacteria and fermentation of cereals and pseudocereals. Ferment. Process 10, 65459 (2017).
Assohoun-Djeni, N. M. C. et al. Biodiversity, dynamics, and antimicrobial activity of lactic acid bacteria involved in the fermentation of maize flour for Doklu production in Côte d’Ivoire. Food Control 62, 397–404 (2016).
Obinna-Echem, P. C. Effect of processing method on pasting, morphological and sensory properties of akamu-a Nigerian fermented maize product. Am. J. Food Technol. 5, 101–108 (2017).
Ezekiel, C. N. et al. High-throughput sequence analyses of bacterial communities and multi-mycotoxin profiling during processing of different formulations of Kunu, a traditional fermented beverage. Front. Microbiol. 9, 3282 (2019).
Achi, O. K. & Ukwuru, M. Cereal-based fermented foods of Africa as functional foods. Int. J. Microbiol. 2, 71–83 (2015).
Fuller, S., Beck, E., Salman, H. & Tapsell, L. New horizons for the study of dietary fiber and health: a review. Plant Foods Hum. Nutr. 71, 1–12 (2016).
Okafor, U. I., Omemu, A. M., Obadina, A. O., Bankole, M. O. & Adeyeye, S. A. Nutritional composition and antinutritional properties of maize ogi co-fermented with pigeon pea. Food Sci. Nutr. 6, 424–439 (2018).
Adesulu-Dahunsi, A. T. et al. Extracellular polysaccharide from Weissella confusa OF126: production, optimization, and characterization. Int. J. Biol. Macromol. 111, 514–525 (2018).
Olanbiwoninu, A. A. & Odunfa, S. A. Microbial interaction in selected fermented vegetable condiments in Nigeria. Front. Microbiol. 25, 439–445 (2018).
Olasupo, N. A., Okorie, C. P. & Oguntoyinbo, F. A. The biotechnology of ugba, a Nigerian traditional fermented food condiment. Front. Microbiol. 7, 1153 (2016).
Enujiugha, V. N. & Badejo, A. A. Probiotic potentials of cereal-based beverages. Crit. Rev. Food Sci. Nutr. 57, 790–804 (2017).
Ibeabuchi, J. C., Ojukwu, M. & Ijeoma, O. Comparative study of proximate and sensory qualities of Iru powder, ogiri powder and iru-ogiri blend. Natl Products Indian J. 9, 288–293 (2013).
Adeyemo, S. M., Agun, T. F. & Ogunlusi, E. D. Antimicrobial activity of lactic acid bacteria isolated from ‘pupuru’: an African fermented staple against food borne-pathogens. Int. J. Biotechnol. 3, 1–5 (2018).
Okorie, C. P. & Olasupo, N. A. Controlled fermentation and preservation of UGBA–an indigenous Nigerian fermented food. SpringerPlus 2, 470 (2013).
Wedajo Lemi, B. Microbiology of Ethiopian traditionally fermented beverages and condiments. Int. J. Microbiol. 1, 1478536 (2020).
Agyei, D., Owusu-Kwarteng, J., Akabanda, F. & Akomea-Frempong, S. Indigenous African fermented dairy products: processing technology, microbiology, and health benefits. Crit. Rev. Food Sci. Nutr. 60, 991–1006 (2020).
Doutoum, A. A. et al. Identification of the lactic microorganism responsible for milk coagulation in Abeche (Chad). Afr. J. Food Sci. 7, 103–106 (2013).
Marsh, A. J., Hill, C., Ross, R. P. & Cotter, P. D. Fermented beverages with health-promoting potential: past and future perspectives. Trends Food Sci. Technol. 38, 113–124 (2014).
Nyambane, B., Thari, W. M., Wangoh, J. & Njage, P. M. Lactic acid bacteria and yeasts involved in the fermentation of amabere amaruranu, a Kenyan fermented milk. Food Sci. Nutr. 2, 692–699 (2014).
Jans, C. et al. African fermented dairy products–overview of predominant technologically important microorganisms focusing on African Streptococcus infantarius variants and potential future applications for enhanced food safety and security. Int. J. Food Microbiol. 250, 27–36 (2017).
Mokoena, M. P., Mutanda, T. & Olaniran, A. O. Perspectives on the probiotic potential of lactic acid bacteria from African traditional fermented foods and beverages. Food Nutr. Res. 60, 29630 (2016).
Zheng, J. et al. A taxonomic note on the genus Lactobacillus: Description of 23 novel genera, emended description of the genus Lactobacillus Beijerinck 1901, and union of Lactobacillaceae and Leuconostocaceae. Int. J. Syst. Evol. 70, 2782–2858 (2020).
Adesulu-Dahunsi, A. T., Jeyaram, K. & Sanni, A. I. Probiotic and technological properties of exopolysaccharide producing lactic acid bacteria isolated from cereal-based Nigerian fermented food products. Food Control 92, 225–231 (2018).
Setta, M. C., Matemu, A. & Mbega, E. R. Potential of probiotics from fermented cereal-based beverages in improving health of poor people in Africa. J. Food Sci. Technol. 57, 3935–3946 (2020).
Marco, M. L. et al. The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on fermented foods. Nat. Gastroenterol. Hepatol. 18, 196–208 (2021).
Heeba, S., & Nisha, P. in Nutrition, Food and Diet in Ageing and Longevity 179–192 (Springer, 2021).
Pedone, C. A., Arnaud, C. C., Postaire, E. R., Bouley, C. F. & Reinert, P. Multicentric study of the effect of milk fermented by Lactobacillus casei on the incidence of diarrhoea. Int J. Clin. Pract. 54, 568–571 (2000).
Van Niel, C. W., Feudtner, C., Garrison, M. M. & Christakis, D. A. Lactobacillus therapy for acute infectious diarrhea in children: a meta-analysis. Pediatrics 109, 678–684 (2002).
Fabiano, V. et al. Term infant formulas influencing gut microbiota: an overview. Nutrients 13, 4200 (2021).
Adesulu-Dahunsi, A. T., Dahunsi, S. O. & Olayanju, A. Synergistic microbial interactions between lactic acid bacteria and yeasts during production of Nigerian indigenous fermented foods and beverages. Food Control 110, 106963 (2020).
Adebo, O. A. et al. in Innovations in Technologies for Fermented Food and Beverage Industries: Food Microbiol. Food Safety (eds Panda, S. & Shetty, P.) 71–87 (Springer, 2018).
Shogbesan, O. et al. A systematic review of the efficacy and safety of fecal microbiota transplant for Clostridium difficile infection in immunocompromised patients. Can. J. Gastroenterol. Hepatol. 1394379, 1–10 (2018).
Shiferaw Terefe, N. & Augustin, M. A. Fermentation for tailoring the technological and health related functionality of food products. Crit. Rev. Food Sci. Nutr. 60, 2887–2913 (2020).
Marco, M. L. et al. Health benefits of fermented foods: microbiota and beyond. Curr. Opin. Biotechnol. 44, 94–102 (2017).
Sharifi-Rad, J. et al. Probiotics: versatile bioactive components in promoting human health. Medicina 56, 433 (2020).
Westerik, N., Kort, R., Sybesma, W. & Reid, G. Lactobacillus rhamnosus probiotic food as a tool for empowerment across the value chain in. Afr. Front. Microb. 9, 1501 (2018).
Anyogu, A. et al. Microorganisms and food safety risks associated with indigenous fermented foods from Africa. Food Control 129, 108227 (2021).
Homayoni, R. A., Vaghef, M. E., Alipoor, B. & Vaghef, M. L. The comparison of food and supplement as probiotic delivery vehicles. Crit. Rev. Food Sci. Nutr. 56, 896–909 (2016).
Gibson, G. et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 14, 491–502 (2017).
Mashau, M. E., Maliwichi, L. L. & Jideani, A. I. Non-alcoholic fermentation of maize (Zea mays) in Sub-Saharan Africa. Fermentation 7, 158–174 (2021).
Adebo, O. A. African sorghum-based fermented foods: past, current and future prospects. Nutrients 12, 1111–1136 (2020).
Tsafrakidou, P., Michaelidou, A. M. & Biliaderis, G. C. Fermented cereal-based products: nutritional aspects, possible impact on gut microbiota and health implications. Foods 9, 734 (2020).
Mayo, P. B. et al. Impact of next generation sequencing techniques in food microbiology. Curr. Genomics 15, 293–309 (2014).
Wakil, S. M., Onilude, A. A., Adetutu, E. M. & Ball, A. S. PCR-DGGE fingerprints of microbial successional changes during fermentation of cereal-legume weaning foods. Afr. J. Biotechnol. 7, 4643–4652 (2008).
Osvik, R. D. et al. Bacterial diversity of amasi, a South African fermented milk product, determined by clone library and denaturing gradient gel electrophoresis analysis. Afr. J. Microbiol. Res. 7, 4146–4148 (2013).
Muthee, D., Kilemba, G. & Masinde, J. The role of indigenous knowledge systems in enhancing agricultural productivity in Kenya. East. Afr. J. Contemp. Res. 1, 34–35 (2019).
Diaz, M. et al. Comparison of the microbial composition of African fermented foods using amplicon sequencing. Sci. Rep. 9, 1–8 (2019).
Cissé, H. et al. Molecular characterization of Bacillus, lactic acid bacteria and yeast as potential probiotic isolated from fermented food. Sci. Afr. 6, e00175 (2019).
Owusu-Kwarteng, J., Tano-Debrah, K., Akabanda, F., Nielsen, D. S. & Jespersen, L. Partial characterization of bacteriocins produced by Lactobacillus reuteri 2-20B and Pediococcus acidilactici 0-11A isolated from Fura, a Millet-based fermented food in Ghana. J. Food Res. 2, 50 (2013).
Angelov, A. I. et al. Molecular identification of yeasts and lactic acid bacteria involved in the production of Beninese fermented food degue. Open Biotechnol. J. 11, 94–104 (2017).
van Rijswijck, I. M., Derks, M. F., Abee, T., de Ridder, D. & Smid, E. J. Genome sequences of Cyberlindnera fabianii 65, Pichia kudriavzevii 129, and Saccharomyces cerevisiae 131 isolated from fermented masau fruits in Zimbabwe. Genome Announc. 5, e00064-17 (2017).
Verce, M., De Vuyst, L. & Weckx, S. Shotgun metagenomics of a water kefir fermentation ecosystem reveals a novel Oenococcus species. Front. Microbiol. 10, 479 (2019).
Fadiji, A. E. & Babalola, O. O. Metagenomics methods for the study of plant-associated microbial communities: a review. J. Microbiol. Methods 120, 105860 (2020).
Ferrocino, I. & Cocolin, L. Current perspectives in food-based studies exploiting multi-omics approaches. Curr. Opin. Food Sci. 13, 10–15 (2017).
Jain, C., Rodriguez-R, L. M., Phillippy, A. M., Konstantinidis, K. T. & Aluru, S. High throughput ANI analysis of 90K prokaryotic genomes reveals clear species boundaries. Nat. Commun. 9, 1–8 (2018).
Berbers, B. et al. Combining short and long read sequencing to characterize antimicrobial resistance genes on plasmids applied to an unauthorized genetically modified Bacillus. Sci. Rep. 10, 1–13 (2020).
Jagadeesan, B. et al. The use of next generation sequencing for improving food safety: Translation into practice. Food Microbiol. 79, 96–115 (2019).
Isibor, P. O. et al. Significance of African diets in biotherapeutic modulation of the gut microbiome. Bioinform. Biol. Insights 15, 11779322211012697 (2021).
Anagnostopoulos, D. A., Kamilari, E. & Tsaltas, D. Evolution of bacterial communities, physicochemical changes and sensorial attributes of natural whole and cracked picual table olives during spontaneous and inoculated fermentation. Front. Microbiol. 11, 1128 (2020).
Daliri, E. B. M., Ofosu, F. K., Chelliah, R., Lee, B. H. & Oh, D. H. Challenges and perspective in integrated multi-omics in gut microbiota studies. Biomolecules 11, 300–310 (2021).
Bücher, C., Burtscher, J. & Domig, K. J. Propionic acid bacteria in the food industry: an update on essential traits and detection methods. Compr. Rev. Food Sci. Food Saf. 20, 4299–4323 (2021).
Emerson, J. B. et al. Schrödinger’s microbes: tools for distinguishing the living from the dead in microbial ecosystems. Microbiome 5, 1–23 (2017).
Zhu, Y. et al. Propidium monoazide pretreatment on a 3D-printed microfluidic device for efficient PCR determination of ‘live versus dead’ microbial cells. Environ. Sci. Water Res. Technol. 4, 56–963 (2018).
Li, J., Zhu, Y., Wu, X. & Hoffmann, M. R. Rapid detection methods for bacterial pathogens in ambient waters at the point of sample collection: a brief review. Clin. Infect. Dis. 71, 84–90 (2020).
Priyadarshini, B. M., Dikshit, V. & Zhang, Y. 3D-printed bioreactors for in vitro modeling and analysis. Int. J. Bioprinting 6, 267 (2020).
Wang, Y. et al. Whole microbial community viability is not quantitatively reflected by propidium monoazide sequencing approach. Microbiome 9, 1–13 (2021).
Acknowledgements
The authors acknowledge the provision of fellowship support for the study by Nutricia Research Foundation, Netherlands (NRF 2020-T1). Other costs and technical supports were provided by Teagasc Food Research Centre, Moorepark, Co Cork, Ireland.
Author information
Authors and Affiliations
Contributions
Manuscript preparation and editing: Y.D.O., P.A.A.; manuscript reviewing: P.A.A., J.L., S.U.O., K.O.A., P.D.C.; advisory: P.A.A., S.U.O., K.O.A.; supervision: P.D.C. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing non-financial Interests. With respect to potential competing financial interests, P.D.C. is a co-founder and Chief Technical Officer of SeqBiome Limited, Ireland. However, this role did not influence the composition of the manuscript.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Obafemi, Y.D., Oranusi, S.U., Ajanaku, K.O. et al. African fermented foods: overview, emerging benefits, and novel approaches to microbiome profiling. npj Sci Food 6, 15 (2022). https://doi.org/10.1038/s41538-022-00130-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41538-022-00130-w
This article is cited by
-
Untargeted metabolomics-based network pharmacology reveals fermented brown rice towards anti-obesity efficacy
npj Science of Food (2024)
-
Fermented foods and gastrointestinal health: underlying mechanisms
Nature Reviews Gastroenterology & Hepatology (2024)
-
Traditional fermented foods of Nigeria: microbiological safety and health benefits
Journal of Food Measurement and Characterization (2024)
-
Microbiological properties, anti-nutritional and nutritional composition of spontaneously and starter culture fermented malted acha flour
Proceedings of the Indian National Science Academy (2024)
-
Traditional Fermented Products: Potential Origin for Probiotic Strains
Current Food Science and Technology Reports (2024)