Abstract
Sleep disorders are common non-motor symptoms in patients with Parkinson’s disease (PD). The characteristics and impact of multiple comorbid sleep disorders remain to be elucidated. Our goal was to investigate the characteristics of various sleep disorder comorbidities, and their association with motor complications and the impact on the quality of life in PD patients. In this multicenter, observational, cross-sectional study, data concerning the clinical characteristics of complicated sleep disorders were collected from PD patients treated at 40 different hospitals in Shanghai. Sleep disorders were evaluated using the PD Sleep Scale-2, Epworth Sleepiness Scale, Rapid Eye Movement Sleep Behavior Disorder Questionnaire-Hong Kong, and the International Restless Legs Scale. Among the 1006 subjects evaluated, 77.53% exhibited signs of sleep disorders, and most had multiple sleep disorders (n = 502, 49.9%). A smaller percentage of patients with sleep disorders had a single disorder (n = 278, 27.6%). Furthermore, an increased number of sleep disorders, including nighttime problems, excessive daytime sleepiness, rapid eye movement sleep behavior disorder, and restless legs syndrome was a significant contributor to a poor quality of life (β = 4.33, CI: 3.33–5.33, P for trend <0.001), even when controlling for multiple factors. Moreover, motor complications partially mediated this relationship (indirect effect: β = 0.355, 95% boot CI: 0.134, 0.652).Our study showed that a large proportion of PD patients suffer from multiple comorbid sleep disorders, which greatly decreases the quality of life in PD patients and is partially mediated by motor complications.
Similar content being viewed by others
Introduction
Parkinson’s disease (PD) is a movement disorder. Sleep disorders, such as nighttime problems, excessive daytime sleepiness (EDS), rapid eye movement sleep behavior disorder (RBD), and restless legs syndrome (RLS) are commonly reported in patients with PD1,2,3. The pathophysiology of sleep disorders associated with dopaminergic and non-dopaminergic mechanisms during the course of PD and the sleep disorder type impart different effects on PD patients4. RLS is often associated with constipation in PD patients5. RBD is associated with longer disease duration, motor fluctuations, psychiatric comorbidities, and higher doses of levodopa in PD patients6,7. RBD can be a prodromal symptom of PD and can be used for diagnosis of PD at an early stage1,2,7,8. Furthermore, sleep disorders are observed throughout the course of PD. Nighttime problems and EDS significantly reduce the quality of life in patients with advanced PD, and require prompt recognition and intervention1,2,7. Clinical subtypes of sleep disorders may even constitute distinct phenotypes of PD.
Many previous studies have focused on the effects of a single type of sleep disorder on PD9,10,11,12,13. It is worth noting that PD patients may have multiple comorbid sleep disorders that require attention. However, few studies have investigated the clinical distribution and characteristics of complicated sleep disorders in PD patients. Furthermore, the association between an increased number of sleep disorders and the quality of life in PD patients, along with identification of the factors that influence this relationship, is largely unknown. Although an accurate sleep disorder diagnosis should be made using polysomnography, devising a screening tool using validated questionnaires that are reliable and diagnostically accurate would be a big step forward in early identification14,15,16. Therefore, large-scale studies on sleep disorders in patients with PD in China are needed to devise tools for faster identification of issues, which can then be better characterized using current methodologies (e.g., polysomnography).
Sleep disorders could be observed throughout the course of PD, while motor complications typically emerge only in advanced cases of disease. Any one, or a combination of sleep disorders and/or movement disorders negatively impacts quality of life. Whether motor complications influence or regulate the impact of sleep disorders on quality of life is unknown. Exploring the effects of motor complications on this relationship may provide important implications for early interventions designed to enrich and prolong quality of life.
In this multicenter, observational, cross-sectional study, we aimed to investigate the clinical characteristics of complicated sleep disorders in patients with PD, their association with motor complications and the overall impact on quality of life.
Results
Study population and the distribution of different types of sleep disorders
A total of 1006 patients with PD (mean age = 69.95 ± 8.41 years old, 577 males) were enrolled in the study. The mean PD disease duration was 5.54 ± 4.58 years. The mean modified Hoehn–Yahr (HY) stage was 2.17 ± 0.84 (stage 1–1.5, n = 296; stage 2–2.5, n = 471; and stage ≥3, n = 223). The Unified Parkinson’s Disease Rating Scale (UPDRS) parts I, II, III, and IV scores were 3.06 ± 2.82, 12.41 ± 8.44, 25.50 ± 15.51, and 3.20 ± 3.66, respectively. Of the total cohort, 920 patients (91.50%) were taking levodopa, 563 (56.00%) were taking dopamine agonists, 39 (3.90%) were taking a MAO-B inhibitor, and 173 (17.20%) were taking a COMT inhibitor. Complete demographic data for the entire cohort are shown in Table 1.
Regarding the sleep assessments, the prevalence of sleep disorders was 41.80% (421/1006) for nighttime problems, 25.20% (254/1006) for EDS, 46.90% (472/1006) for probable RBD (pRBD), and 49.80% (501/1006) for RLS. There was a significant overlap of various sleep disorders in patients with PD (Fig. 1). Accordingly, 226 (22.5%) of the patients had no sleep disorder, 278 (27.6%) had only one type of sleep disorder, and 502 (49.9%) had ≥2 sleep disorders (Fig. 1).
Factors associated with different types of sleep disorders
The characteristics of the patients with PD according to sleep disorder type (nighttime problems, EDS, pRBD, and RLS) are shown in Table 1. Compared with the patients without sleep disorders, the nighttime problems, EDS, pRBD, and RLS groups had longer disease durations, higher UPDRS scores (total, parts I, II, III, and IV) and non-motor symptom scale (NMSS) scores, worse cognitive function, and shorter total sleep times. A slight increase in age was associated with nighttime problems and EDS. We found no specific pattern of initial presentation of motor symptoms that was significantly related to the presence of nighttime problems, EDS, pRBD, or RLS.
Logistic regression analysis of nighttime problems, EDS, pRBD, and RLS using a likelihood ratio forward selection showed that UPDRS part IV (32–39) scores (OR = 1.186, CI: 1.118–1.259, P < 0.001) and NMSS scores (OR = 1.030, CI: 1.024–1.035, P < 0.001) were contributing factors to occurrence of nighttime problems. UPDRS part IV (32–39) scores (OR = 1.090, CI: 1.035–1.147, P = 0.001), NMSS scores (OR = 1.018, CI: 1.014–1.022, P < 0.001), and hallucinations (OR = 1.595, CI: 1.016–2.504, P = 0.043) were contributing factors for EDS. Age (OR = 0.983, CI: 0.968–0.999, P = 0.038), NMSS scores (OR = 1.014, CI: 1.010–1.018, P < 0.001, and hallucinations (OR = 2.367, CI: 1.513–3.703, P < 0.001) were contributing factors for pRBD. Age (OR = 0.979, CI: 0.963–0.997, P = 0.019), disease duration (OR = 1.045, CI: 1.010–1.081, P = 0.012), modified HY stage (OR = 0.723, CI: 0.571–0.914, P = 0.007), UPDRS parts II scores (OR = 1.062, CI: 1.036–1.088, P < 0.001), UPDRS parts IV (32–39) scores (OR = 1.182, CI: 1.115–1.252, P < 0.001), and dopamine agonist medication use (OR = 0.571, CI: 0.429–0.761, P < 0.001) were contributing factors for RLS (Supplementary Table 1).
Clinical characteristics and factors associated with different numbers of sleep disorders
PD patients were categorized into the following five groups according to the number of sleep disorders ranked 0–4 as follows: zero sleep disorders (n = 226, 22.50%), one type of sleep disorder (n = 278, 27.60%), two types of sleep disorders (n = 235, 23.40%), three types of sleep disorders (n = 168, 16.70%), and four types of sleep disorders (n = 99, 9.80%) (Table 2). The clinical characteristics of patients according to the number of sleep disorders are shown in Table 2. Among all PD patients, those with a greater number of sleep disorders were more likely to have a longer disease duration, higher modified HY stage, greater UPDRS score (total, III and IV), worse cognitive function, and a greater number of non-motor symptoms. They were also more likely to be currently on L-dopa medication and to have a higher levodopa equivalent daily doses (LEDDs). However, they were less likely to be taking dopamine agonists. Compared to patients who suffered only one type of sleep disorder, those with multiple sleep disorders had a worse quality of life and more severe motor complications. These associations were slightly attenuated after controlling for disease duration, disease progression, motor symptoms, non-motor symptoms, and dopaminergic medications (Table 3). The multivariable regression coefficients (95% confidence intervals (95% CIs)) of quality of life in reference to no sleep disorders were 1.79 (−1.17 to 4.76) for one type of sleep disorders, 3.89 (0.71–7.08) for two types of sleep disorders, 14.63 (10.96–18.31) for three types of sleep disorders, and 16.04 (11.43–20.65) for four types of sleep disorders; this trend was statistically significant (P for trend <0.001). Furthermore, we found the same trend in the severity of motor complications and the number of sleep disorders (P for trend <0.001). The multivariable regression coefficients (95% CIs) for quality of life in reference to no sleep disorders were 7.88 (1.29–14.47) for the nighttime problems + EDS + RLS group, 10.48 (1.72–19.24) for the nighttime problems + EDS + pRBD group, 14.67 (9.93–19.40) for the nighttime problems + pRBD + RLS group, and 12.56 (2.83–22.30) for the EDS + pRBD + RLS group (Table 3).
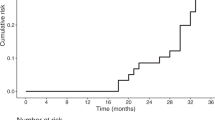
We further stratified the analyses by motor complication status. Positive associations between an increased number of sleep disorders and quality of life were found both among patients with and without motor complications (β = 4.467, 3.312; P for trend <0.001, <0.001; Fig. 2). However, the interaction with motor complication status was not statistically significant (P for interaction = 0.759).
a Stratification analyses of positive associations between an increased number of sleep disorders and quality of life by motor complications status. Number of sleep disorders (0–4; nighttime problems, EDS, pRBD, and RLS). b Motor complications as a mediator between an increased number of sleep disorders and quality of life. All associations were adjusted for sex, age, disease duration, modified HY stage, UPDRS part III, NMSS, MMSE, L-dopa medication status, dopamine agonist medication status, and levodopa equivalent daily dose.
The mediation analyses of the associations between multiple comorbid sleep disorders and quality of life
When the motor complications score was entered as a mediator of the number of sleep disorders and PDQ-39, the total effect was significant (β = 4.351, CI: 3.370–5.332, P < 0.001), the indirect effect was significant (β = 0.355, 95% boot CI: 0.134, 0.652), and the direct effect remained significant (β = 3.996, CI: 3.001–4.991, P < 0.001). Therefore, we can infer that motor complications partially mediated the effect of multiple comorbid sleep disorders on quality of life (Fig. 2).
Discussion
In this large-scale, multicenter, cross-sectional study, we found that PD patients have a significantly higher prevalence of sleep disorders. Our results showed that the prevalence of sleep disorders (77.5%) in patients with PD is higher than previously reported in China7,17. In addition, there were 502 patients (49.9%) with multiple comorbid sleep disorders, while only 278 patients (27.6%) had only one type of sleep disorder. The prevalence of nighttime problems with pRBD and RLS was 9.5%, which is significantly higher than the prevalence of any other combination of three of sleep disorders. Nighttime problems combined with RLS (7.5%), and nighttime problems combined with pRBD (6.4%) were significantly more prevalent than any other combination of two disorders. In contrast, EDS alone, EDS combined with nighttime problems, EDS combined with RLS, or EDS combined with pRBD are not common. These results reveal that the proportion of patients with multiple comorbid sleep disorders is much greater than the proportion with only one sleep disorder. In addition to one specific type of sleep disorder, multiple comorbidities should also be considered when assessing patients with PD who have sleep disorder complaints. Comprehensive evaluation and therapy will achieve a better curative effect.
At the same time, we found that nighttime problems, EDS, pRBD, and RLS are all accompanied by cognitive decline, which was confirmed by other studies17,18,19. Compared with participants without RLS, those with RLS have a higher rate of a family history of PD. However, nighttime problems, EDS, and pRBD have no correlation with a family history of PD, which was consistent with other studies20. RLS results in fewer sleep interruptions, while nighttime problems, EDS, and pRBD are typically accompanied by frequent sleep interruptions. As a clinical symptom, the prevalence of hallucinations among the different types of sleep disorders (nighttime problems, EDS, pRBD, and RLS) is described in Table 1. Our results indicated that hallucinations were more likely to occur in PD patients with EDS and RLS than those without. It is worth noting that dopamine agonists were less frequently prescribed or used in the PD group with EDS and RLS than in the PD group without EDS and RLS. Therefore, it is difficult to infer whether the appearance of hallucinations is related to the use of dopamine agonists. According to the UPDRS part I and NMSS scores, PD patients with sleep disorders (nighttime problems, EDS, pRBD, or RLS) usually have a greater number of more severe non-motor symptoms. Patients with nighttime problems, pRBD, and RLS have an inferior quality of life compared to patients with any other combination of three comorbid sleep conditions. Therefore, nighttime problems are more likely to coincide with RLS and pRBD, and these three types of sleep disorders may play some role in pathogenesis because they have similar clinical characteristics and have a more significant impact on quality of life. Alternatively, it is possible that nighttime problems may be the result of RLS and pRBD. In contrast, EDS is relatively independent, and its pathogenesis may be different from the other disorders.
Our study revealed that patients with a greater number of sleep disorders were less likely to be taking dopamine agonists (P = 0.026). The dopamine agonists used in the China are pramipexole and piribedil. We performed a subgroup analysis, and there was no negative correlation between the number of sleep disorders and the use of pramipexole or piribedil. Therefore, dopamine agonist is not likely the cause of sleep disorders, and our results represent statistically significant correlations. Due to the limitations of observational cross-sectional studies, we are unable to make further inferences. Future longitudinal studies would enhance our interpretations. Furthermore, the analyses revealed that the number of sleep disorders was an independent and significant contributor to quality of life in PD patients, even after we adjusted for multiple factors. Our findings demonstrated that an increased number of sleep disorders had a significant negative impact on quality of life.
Through regression analysis, we found that motor complications were related to nighttime problems, EDS, and RLS, while both sleep disorders and motor complications were necessary for significant deteriorations in quality of life. These findings suggested that there may be interactions between sleep disorders and motor complications. Notably, rather than examining the direct relationship between motor complications and quality of life in PD, we investigated the interaction effect between motor complications and multiple comorbid sleep disorders. Our findings show there was no significant interactive effect between motor complications and multiple sleep disorder comorbidities on quality of life in PD patients (P for interaction = 0.759). Nevertheless, the impact of motor complications on quality of life shows a mediating effect according to our study. Motor complications act as a mediator of the association between multiple comorbid sleep disorders and quality of life. Controlling for the severity of motor complications resulted in a decrease in the strength of the correlation between multiple sleep disorders and quality of life. Motor complications had a statistically significant indirect effect on the relationship between multiple sleep disorders and quality of life. Although the size of the indirect effect was moderate, despite worse sleep quality, a patient may experience a further improvement in quality of life when his or her motor complications are treated, and motor function is better maintained.
The strengths of this study include the systematic assessment of sleep disorders in a relatively sizeable multicenter sample of patients with PD. There are two implications for the present findings. First, it may be valuable for clinicians to consider how multiple comorbid sleep disorders relate to quality of life in people with PD. Further assessment could provide a clearer understanding of these relationships, enabling the design and implementation of comprehensive therapeutic approaches. Second, the present findings highlight the complex mediating effect between multiple comorbid sleep disorders and motor complications. Previous research has demonstrated that PD patients with motor complications are more likely to suffer from a lower quality of life21. Revealing the impact of motor complications and impaired sleep problems may help clinicians better manage symptoms to improve the quality of life.
Nonetheless, this study also has several limitations. First, our study was a cross-sectional study and did not include premorbid or de novo PD patients. Therefore, the causality between multiple comorbid sleep disorders, motor complications, and quality of life cannot be confirmed. It is crucial to perform prospective studies to investigate causality. Second, an increasing number of sleep disorder screening tools have been developed for use in research and clinical practice. Therefore, cross-comparison between different cohorts is often limited by the types of screening tool used. Finally, assessments of motor complications were limited to the UPDRS IV rather than the scale for wearing off and dyskinesia. Hence, the detailed characteristics of motor complications in patients could not be identified.
In summary, our large-scale, cross-sectional study indicates a close relationship between multiple comorbid sleep disorders and reduced quality of life in PD patients. In addition, motor complications may at least in part mediate the detrimental effects on quality of life caused by multiple comorbid sleep disorders. Therefore, complete and comprehensive assessments of all types of sleep disorders and motor complications in PD patients is imperative to improve the quality of life.
Methods
Study design and participants
We performed a multicenter, observational, outpatient-based, cross-sectional study entitled, “Nocturnal symptoms and quality of life in patients with Parkinson’s disease in Shanghai” (SHAPD, clinical trail.gov ID: NCT04023201). From June to November of 2019, the study recruited 1006 PD patients from the clinics of 40 hospitals in Shanghai. Patients had been diagnosed with PD according to the Movement Disorder Society (MDS) PD Criteria22. Patients with secondary Parkinsonism, stroke, brain tumor, or an alternative cause for parkinsonism symptoms were excluded. Written informed consent was obtained from all participants, and the study was performed with the approval of the Ethics Committee of Xinhua Hospital affiliated to the Shanghai Jiao Tong University School of Medicine and the Research Ethics Committee of each site in the SHAPD study group.
Clinical assessment
All patients received standardized assessments. The assessments included an evaluation of demographic and clinical characteristics, disease duration, family history of PD, and medication history for PD-related complications. Total LEDD was calculated according to a previously suggested conversion formulae23. There were in total 45 examiners from all 40 study sites who attended training and calibration sessions to prevent inter-rater variability before recruitment. All PD subjects were on medication for their condition at the time of evaluation. The motor and non-motor symptoms of PD were measured with the following scales: the UPDRS, the modified HY stage, Mini-Mental State Examination (MMSE), and the NMSS. Motor fluctuation scores (UPDRS questions 36–39) and dyskinesia scores (32–34) were summed to assess the severity of motor complications related to dopaminergic therapy.
The following instruments were administered by qualified examiners. Nocturnal sleep impairments were assessed using the PD Sleep Scale-2, an instrument that was appraised as “recommended” by the MDS Sleep Scale Task Force16. Patients who report complaints of nighttime problems are considered to be suffering from nighttime problems if they score 15 or more24. EDS was screened using the Epworth Sleepiness Scale. Patients are considered to be suffering from EDS if they score 10 or more25. RBD was screened using the Rapid Eye Movement Sleep Behavior Disorder Questionnaire-Hong Kong. Patients are considered to be suffering from probable RBD (pRBD) if they score 19 or more26. An RLS diagnosis was based on questionnaire-based screening. In agreement with the International RLS Study Group criteria for epidemiology studies27, participants were considered to have RLS if they reported unpleasant leg sensations combined with an urge to move their legs, occurring mainly at rest, improving with movement, and worsening in the evening or night. The International Restless Legs Scale was used to assess RLS severity28.
Statistical analysis
Summary statistics (means, SDs, and frequencies) were computed for all variables of interest. Between-group differences were determined by t-tests (continuous variables) or χ2 tests (categorical variables). We performed logistic regression analysis for nighttime problems, EDS, pRBD, or RLS as dependent variables to analyze risk factors that could be independently associated with each sleep disorder. The linear trends in clinical characteristics, according to an increased number of sleep disorders, were tested by linear regression analysis for continuous variables and logistic regression analysis for proportional variables. Unstandardized regression coefficients (βs) and 95% CIs were calculated for motor complications and the quality of life associated with the number of sleep disorders using multivariable-adjusted models. Linear regression models were adjusted for sex, age, disease duration, PD severity, and dopaminergic therapy. We performed tests for linear trends by entering the number of sleep disorders (0–4) as a continuous variable in the models.
Mediation analyses were conducted with SPSS mediation modeling software and PROCESS29 to evaluate whether the severity of motor complications mediated the associations between multiple comorbid sleep disorders and the quality of life. The analysis considered the total effect of multiple sleep disorders (number of sleep disorders) on the quality of life (PDQ-39) and the indirect effect mediated by the motor complications score. A bootstrap estimation approach with 5000 samples was used to measure the indirect effect30 with 95% CIs. The indirect effect was considered significant when the 95% CIs did not contain zero31.
Statistical significance was set at P < 0.05. Statistical computations were performed by SPSS Statistics 26.0 (IBM Corp., Armonk, New York, USA), and OriginPro 2019b software (OriginLab Corp., Northampton, Massachusetts, USA) was used to generate the figure.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
The data are available from the corresponding author upon reasonable request.
Code availability
Analytic code can be made available upon request to the corresponding author.
References
Liu, C.-F. et al. Management recommendations on sleep disturbance of patients with Parkinson’s disease. Chin. Med. J. 131, 2976–2985 (2018).
Stefani, A. & Högl, B. Sleep in Parkinson’s disease. Neuropsychopharmacology 45, 121–128 (2019).
Matar, E. & Lewis, S. J. REM sleep behaviour disorder: not just a bad dream. Med. J. Aust. 207, 262–268 (2017).
Gan-Or, Z., Alcalay, R. N., Rouleau, G. A. & Postuma, R. B. Sleep disorders and Parkinson disease; lessons from genetics. Sleep. Med. Rev. 41, 101–112 (2018).
Iwaki, H., Hughes, K., Gao, X., Schwarzschild, M. & Ascherio, A. The association between restless legs syndrome and premotor symptoms of Parkinson’s disease. J. Neurol. Sci. 394, 41–44 (2018).
Sixel-Döring, F., Trautmann, E., Mollenhauer, B. & Trenkwalder, C. Associated factors for REM sleep behavior disorder in Parkinson disease. Neurology 77, 1048–1054 (2011).
Suzuki, K. et al. Impact of sleep-related symptoms on clinical motor subtypes and disability in Parkinson’s disease: a multicentre cross-sectional study. J. Neurol. Neurosurg. Psychiatry 88, 953 (2017).
Postuma, R. B. et al. Risk and predictors of dementia and parkinsonism in idiopathic REM sleep behaviour disorder: a multicentre study. Brain 142, 744–759 (2019).
Zhu, K., Hilten, J. Jvan & Marinus, J. The course of insomnia in Parkinson’s disease. Parkinsonism Relat. Disord. 33, 51–57 (2016).
Jozwiak, N. et al. REM sleep behavior disorder and cognitive impairment in Parkinson’s disease. Sleep 40, zsx101 (2017).
Zhang, J., Xu, C.-Y. & Liu, J. Meta-analysis on the prevalence of REM sleep behavior disorder symptoms in Parkinson’s disease. BMC Neurol. 17, 23 (2017).
Bargiotas, P., Lachenmayer, M. L., Schreier, D. R., Mathis, J. & Bassetti, C. L. Sleepiness and sleepiness perception in patients with parkinson’s disease: a clinical and electrophysiological study. Sleep 42, zsz004 (2019).
Yang, X. et al. Prevalence of restless legs syndrome in Parkinson’s disease: a systematic review and meta-analysis of observational studies. Sleep. Med. 43, 40–46 (2018).
Kurtis, M. M., Balestrino, R., Rodriguez-Blazquez, C., Forjaz, M. J. & Martinez-Martin, P. A review of scales to evaluate sleep disturbances in movement disorders. Front. Neurol. 9, 369 (2018).
Rodríguez-Blázquez, C., Forjaz, M. J., Kurtis, M. M., Balestrino, R. & Martinez-Martin, P. Rating scales for movement disorders with sleep disturbances: a narrative review. Front. Neurol. 9, 435 (2018).
Högl, B. et al. Scales to assess sleep impairment in Parkinson’s disease: critique and recommendations. Mov. Disord. 25, 2704–2716 (2010).
Yan, Y.-Y., Lei, K., Li, Y.-Y., Liu, X.-F. & Chang, Y. The correlation between possible RBD and cognitive function in Parkinson’s disease patients in China. Ann. Clin. Transl. Neurol. 6, 848–853 (2019).
Pagano, G. et al. REM behavior disorder predicts motor progression and cognitive decline in Parkinson disease. Neurology 91, e894–e905 (2018).
Kamble, N. et al. Impaired sleep quality and cognition in patients of Parkinson’s disease with REM sleep behavior disorder: a comparative study. Sleep. Med. 62, 1–5 (2019).
Jiménez-Jiménez, F. J., Alonso-Navarro, H., García-Martín, E. & Agúndez, J. A. G. Genetics of restless legs syndrome: an update. Sleep. Med. Rev. 39, 108–121 (2018).
Chapuis, S., Ouchchane, L., Metz, O., Gerbaud, L. & Durif, F. Impact of the motor complications of Parkinson’s disease on the quality of life. Mov. Disord. 20, 224–230 (2004).
Postuma, R. B. et al. MDS clinical diagnostic criteria for Parkinson’s disease: MDS-PD Clinical Diagnostic Criteria. Mov. Disord. 30, 1591–1601 (2015).
Tomlinson, C. L. et al. Systematic review of levodopa dose equivalency reporting in Parkinson’s disease. Mov. Disord. 25, 2649–2653 (2010).
Suzuki, K. et al. Evaluation of cutoff scores for the Parkinson’s disease sleep scale-2. Acta Neurol. Scand. 131, 426–430 (2014).
Arnulf, I. et al. Parkinson’s disease and sleepiness: an integral part of PD. Neurology 58, 1019–1024 (2002).
Li, S. X. et al. Validation of a new REM sleep behavior disorder questionnaire (RBDQ-HK). Sleep. Med. 11, 43–48 (2009).
Allen, R. P. et al. Restless legs syndrome: diagnostic criteria, special considerations, and epidemiology. Sleep. Med. 4, 101–119 (2003).
Group, T. I. R. L. S. S. Validation of the International Restless Legs Syndrome Study Group rating scale for restless legs syndrome. Sleep. Med. 4, 121–132 (2003).
Hayes, A. F. Introduction to Mediation, Moderation, and Conditional Process Analysis: A Regression-based Approach (Guilford publications, 2017).
Shrout, P. E. & Bolger, N. Mediation in experimental and nonexperimental studies: new procedures and recommendations. Psychol. Methods 7, 422–445 (2002).
Preacher, K. J. & Hayes, A. F. SPSS and SAS procedures for estimating indirect effects in simple mediation models. Behav. Res. Methods Instrum. Comput. 36, 717–731 (2004).
Acknowledgements
We wish to thank all physicians and patients for participating in this study. This study was supported by the National Key R&D Program of China (2017YFC1310300, 2016YFC1306601, 2017YFC1306002, and 2018YFC1314700), and the Shanghai Health and Family Planning Commission Foundation (201940021).
Author information
Authors and Affiliations
Contributions
Y.Z. and J.h.Z.: drafting and critical revision of the manuscript. D.y.H., W.C., C.x.Y., L.r.J., Y.h.W., L.j.J., L.L., X.p.W., C.d.W., and X.Z.: enrolling patients, critical revision of the manuscript. X.Z.: analysis and interpretation of data. W.t.L. and Z.g.L.: critical revision of the manuscript and study supervision.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhang, Y., Zhao, J.h., Huang, D.y. et al. Multiple comorbid sleep disorders adversely affect quality of life in Parkinson’s disease patients. npj Parkinsons Dis. 6, 25 (2020). https://doi.org/10.1038/s41531-020-00126-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41531-020-00126-x
This article is cited by
-
Safety and efficacy of melatonin, clonazepam, and trazodone in patients with Parkinson’s disease and sleep disorders: a randomized, double-blind trial
Neurological Sciences (2022)
-
Prevalence and profile of nocturnal disturbances in Chinese patients with advanced-stage Parkinson’s disease: a cross-sectional epidemiology study
BMC Neurology (2021)
-
“Hot cross bun” is a potential imaging marker for the severity of cerebellar ataxia in MSA-C
npj Parkinson's Disease (2021)
-
Sleep Disorders and Cognitive Dysfunctions in Parkinson’s Disease: A Meta-Analytic Study
Neuropsychology Review (2021)