Abstract
Humans are spending an increasing amount of time in space, where exposure to conditions of microgravity causes 1–2% bone loss per month in astronauts. Through data collected from astronauts, as well as animal and cellular experiments conducted in space, it is evident that microgravity induces skeletal deconditioning in weight-bearing bones. This review identifies contentions in current literature describing the effect of microgravity on non-weight-bearing bones, different bone compartments, as well as the skeletal recovery process in human and animal spaceflight data. Experiments in space are not readily available, and experimental designs are often limited due to logistical and technical reasons. This review introduces a plethora of on-ground research that elucidate the intricate process of bone loss, utilising technology that simulates microgravity. Observations from these studies are largely congruent to data obtained from spaceflight experiments, while offering more insights behind the molecular mechanisms leading to microgravity-induced bone loss. These insights are discussed herein, as well as how that knowledge has contributed to studies of current therapeutic agents. This review also points out discrepancies in existing data, highlighting knowledge gaps in our current understanding. Further dissection of the exact mechanisms of microgravity-induced bone loss will enable the development of more effective preventative and therapeutic measures to protect against bone loss, both in space and possibly on ground.
Similar content being viewed by others
Introduction
Extended human spaceflight was once a distant fantasy; however, it is now almost a tangible reality. With NASA’s goal to send humans back to the moon by 2024, then onwards to Mars in the 2030s, it is now more critical than ever to understand the impacts of long-term space travel on human health1. Among the many technical, logistical and physiological challenges inherent to extended space exploration, the loss of gravitational force is a major prohibitive environmental factor that adversely affects the body of space travellers. The human body is intrinsically adapted to Earth’s gravity (~9.907 m/s2), thus exposure to conditions of reduced gravity, or microgravity (µG) can lead to a plethora of complications in normal bodily functions. µG decreases the effort required for movement, while causing mass fluid redistribution2. As a result, muscles in the arms and legs experience atrophy3, the cardiovascular system is compromised4, the immune system is suppressed5, and increased cranial pressure leads to vision problems and neurological impairments6. Exposure to µG also results in skeletal deconditioning, where significant reductions in bone mass increases the risk of fractures and osteoporosis, threatening the viability of long-duration missions and astronauts’ mobility upon return to Earth7,8.
A brief background to bone homeostasis
Before examining how bone is affected by microgravity, it is important to understand bone function in 1G. Under normal circumstances, bone remodeling is an adaptive and balanced process where bone resorption and formation are coupled to regulate homeostasis of bone tissue9. The overall process relies on osteoblasts and osteoclasts acting in concert to regulate bone formation and resorption, respectively. The inactive bone surface is lined with flat remnants of osteoblasts, where they serve as a membrane capable of detecting hormones and/or mechanical loading to initiate the bone remodeling process9,10,11,12,13 (Fig. 1). Once circulating osteoclast precursors are recruited to sites of bone remodeling, they differentiate into mature osteoclasts that secrete enzymes such as cathepsin K and metalloproteinase to digest the collagen-rich bone matrix14. This degradation process also releases calcium and embedded growth factors, such as bone morphogenic proteins (BMPs) and transforming growth factor-β (TGFβ), which contribute to bone formation9,13. Once the cavities beneath resorbing osteoclasts reach a certain size, osteoclasts undergo apoptosis to terminate bone resorption and prevent excess bone loss15.
Bone remodelling is a process where cycles of bone resorption and formation are separated by periods of quiescence. During quiescence, the relatively inactive bone surface is lined by flat remnants of osteoblasts. Events such as hormone detection and/or mechanical loading can activate the recruitment of circulating osteoclast precursor cells. These precursor cells fuse to form premature osteoclasts and migrate to the bone surface, while bone lining cells retract to enable preosteoclast binding. Once bound to the bone matrix to form a sealing zone in the isolated area, they differentiate into mature osteoclasts for bone resorption. Mature osteoclasts secrete protons to create an acidic environment that dissolves bone mineral, and proteolytic enzymes to digest the bone matrix. The resorption process results in the formation of cavities, also known as Howship’s lacunae, beneath active osteoclasts. Osteoclasts undergo apoptosis once these cavities reach a certain size, leading to the termination of bone resorption. The bone degradation process also releases embedded growth factors that reverses bone resorption by recruiting and stimulating the differentiation of mesenchymal stem cells (MSCs) into bone-forming osteoblast lineage cells. Once recruited to the lacunae, preosteoblasts secrete a variety of matrix proteins in the organic bone matrix, or the osteoid, which are then mineralised by mature osteoblasts. Bone formation is terminated upon completion of mineralisation. Osteoblasts either undergo apoptosis or differentiation into quiescent bone lining cells. Alternatively, osteoblasts can become embedded in the bone matrix to form osteocytes, which form a canalicular network of branched dendritic processes to communicate with bone lining cells, osteoblasts, and other osteocytes.
The newly liberated growth factors from bone degradation can recruit and stimulate the differentiation of mesenchymal stem cells (MSCs) to osteoblast lineage cells, including osteoprogenitors, osteoblasts and osteocytes16. Maturing osteoprogenitors and preosteoblasts secrete a variety of matrix proteins, such as type 1 collagen, as well as non-collagen proteins (osteocalcin, osteonectin, bone sialoprotein II and osteopontin) and proteoglycans, which are mineralised by mature osteoblasts17. In addition, preosteoblasts express alkaline phosphatase (ALP) for bone mineralisation, hence its expression and activity are key markers of osteoblast differentiation and maturation18,19. Osteoblasts either undergo apoptosis, differentiate into quiescent bone lining cells, or become embedded in the bone matrix to form osteocytes, which form a canalicular network of branched dendritic processes20,21. They communicate with bone lining cells, osteoblasts and other osteocytes, and are suggested to influence bone remodeling in response to mechanical loading17,22. Thus, with an understanding of bone homeostasis, what is currently known regarding the effect of microgravity on bone will be reviewed below.
The effect of microgravity on bone
Microgravity-induced bone loss in humans
The first observation of µG-induced bone loss was recorded in the mid-1970s, when Skylab crew members demonstrated the loss of 1–2% bone mass per month compared to pre-flight and ground controls23,24,25. Since then, despite the implementation of preventative exercises, bone loss in space has been one of the most frequently observed outcomes among astronauts (Table 1, row 1-7). The weightlessness experienced in microgravity reduces the loading on weight-bearing bones, resulting in adaptive changes that increase bone resorption and inhibit bone formation26. Indeed, bone mineral density (BMD) studies of astronauts demonstrate substantial decrease in the mass of weight-bearing bones such as the tibia, but not in non-weight-bearing bones like the distal radius8,27,28,29. Bone resorption is particularly exacerbated in the first 2 weeks of spaceflight, where urinary concentrations of resorption markers such as N-telopeptide and pyridinium crosslinks are increased26,30,31,32,33. On the other hand, urinary calcium levels are increased24,32,34, indicating reduced calcium absorption in astronauts32,34. As bone formation is reportedly unchanged or decreased30,32,33, this results in an overall negative calcium balance that contributes to bone loss in space. Notably, a recent study suggests that circulating biomarkers of bone turnover pre-flight can predict the severity of in-flight bone loss, where astronauts with elevated bone resorption and formation markers pre-mission experience greater losses in BMD and strength of their distal tibia during spaceflight29.
The severity of bone loss also increases with spaceflight duration, and the time required for recovery to pre-flight BMD levels is reportedly longer than the actual mission27,28. Another study evaluates the bone mass, microarchitecture and strength of 13 astronauts who spent 4–6 months aboard the International Space Station (ISS)35. This study monitored skeletal recovery of each astronaut for up to 12 months post-landing. Although the cortical bone thickness and density of the weight-bearing distal tibia eventually recover upon landing, the cortical porosity and trabecular bone fail to recover, leading to reduction in the ultimate load of the bone35. Congruent to previous findings8,36,37, the non-weight-bearing distal radius is preserved at landing35. Interestingly, this study suggests that the distal radius suffers progressive fragility 6 months after landing, which coincided with bone remodeling markers declining to below pre-flight levels between 6 and 12 months upon return35. The observation of progressive fragility in non-weight-bearing bones might have escaped other studies due to inadequate length of recovery monitoring. Future studies should increase follow-up duration to validate this phenomenon.
Microgravity-induced bone loss in animals
Despite the physiological relevance, relying solely on astronaut data to understand µG-induced bone loss is limiting for many reasons. One of which is the rarity of human spaceflight and the limited number of astronauts per mission, thus leading to the challenge of small sample sizes in these studies. The smaller physical sizes of animals such as rats, mice and fish allow for more compact storage in space missions, enabling larger sample sizes while maintaining some physiological relevance. Similar to observations in astronauts (Table 1), µG exposure for 8 days decreases overall bone volume and thickness by 6.3% and 11.9%, respectively, in 15 mice38. The negative effects of µG on trabecular bone mass are also reportedly discernible in weight-bearing bones such as tibia, femur and vertebrae39, complementing observations in human studies. Seven rats aboard the Soviet mission COSMOS 1667 for 7 days demonstrate a reduction in tibial trabecular bone volume by 47–55%, trabecular thickness by 20–24% and density by 40–43% compared to ground controls36. In addition, another study observes a 64% decrease in femoral trabecular bone volume and a 140% increase in bone resorption in 16 mice following 30 days of spaceflight compared to control mice on Earth40. Congruently, a larger sample size of 40 mice subjected to 22 days of µG exposure exhibit significant reductions in BMD of both left and right femur compared to ground controls, albeit to a smaller extent of 11% and 8% respectively41.
On the contrary, there is opposing evidence that suggests µG-induced bone loss may not be strictly related to its weight-bearing nature41. The aforementioned study also reports that BMD of the humerus, a weight-bearing bone, is not affected in spaceflight mice compared to ground controls despite changes in both femurs41. Similarly, 14 days of spaceflight reduces the BMD of femurs, but has no effect in the humerus of 12 rats compared to controls on Earth42. It should be noted that these rats are ovariectomized42, which amplifies phenotypes of bone loss as ovarian hormones are crucial regulators of skeletal growth43. The relationship between µG and the weight-bearing nature of bone loss remains unclear given the contradicting evidence presented. Nevertheless, these studies collectively highlight the damaging effects of µG on bone health during space travel, as well as the risk of premature osteoporosis from the uncertainties of post-flight skeletal recovery.
Limitations of studying bone loss in space
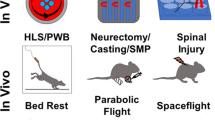
Spaceflight experiments are certainly the most physiologically relevant method of studying µG-induced bone loss, however, they come with significant limitations. Firstly, mission launches are infrequent, hence opportunities for experiments in space are limited. Secondly, experimental design must comply with cargo weight and space constraints in both the launch and space station. This often leads to small sample sizes and minimal, if any, biological replicates, which reduces statistical power and rigour of the research. Thirdly, the engineering components must be sound, ensuring that the experimental model survives the harsh launch conditions, the duration of data collection in orbit, and in some cases, the return to Earth. The strategies to overcome or bypass these restrictions ultimately feed into the final limitation – cost. Experiments requiring astronaut intervention incur even higher financial burdens, hence simplistic autonomous or remotely controlled experiments are favoured. In light of these limitations, many researchers elect to study µG-induced bone loss using simulated µG here on Earth.
The effect of simulated microgravity on bone
Current microgravity simulation technologies
To address the logistical problem of studying bone loss directly from humans in space, several ground-based methods have been developed since the 1970s to subject various models, such as cells, plants, animals and humans, to near-µG conditions. The following discussion will be limited to technologies relevant to research on µG-induced bone loss (Table 2); a wider scope of methods is thoroughly reviewed by Ferranti et al.44.
Horizontal bed rest/head-down tilt
Initially, horizontal bed rest (HBR) was used to mimic the inactivity of the human body in weightlessness. However, this method failed to recapitulate fluid redistribution towards the head as observed in inflight astronauts. As such, researchers experimented with tilting the subjects towards the head to encourage cephalad redistribution from the legs45,46,47. Head-down tilt (HDT) angles range from 4° to 15°, but a tilt angle of 6° which approximates to 0.1 G became the analogue for µG simulation in most human bed rest studies48. Comparisons between HBR/HDT and µG are thoroughly discussed by Hargens et al.49.
Water immersion
While HBR/HDT simulates microgravity by minimising G forces, water immersion achieves microgravity via neutral buoyancy. Neutral buoyancy describes the displacement of a medium which a mass is immersed in, resulting in the balance of gravitational force. During water immersion, the subject usually sits in water with a temperature of 34–35 °C. Such facilities have been utilised since the 1960s to prepare astronauts for spaceflight, as well as in experiments involving µG simulation50. However, immersing subjects in water for prolonged periods can lead to adverse cutaneous effects51. As such, the technique of dry immersion was introduced.
Dry immersion
In keeping with the neutral buoyancy concept of water immersion, subjects are immersed in a bath to neck level, but are “kept dry by the use of a waterproof, highly elastic cloth”52. While some localised pressure can be experienced at the seat and/or feet during the conventional water immersion, the buoyant force from the air between the elastic cloth and skin in dry immersion effectively prevents any localised surface pressures. Furthermore, dry immersion enables longer experiments for up to 56 days, while reproducing µG-induced physiological effects similar to HDT experiments53. The history, utilisation and effects of dry immersion is extensively reviewed by Tomilovskaya et al.53.
Rodent HLU
The hindlimb unloading (HLU) model is the most common method to simulate spaceflight conditions in rodents, where rats or mice are suspended by their tails, or the use of surgical pins or body harnesses54. This causes the removal of mechanical loading from the hindlimbs, as well as head-ward fluid shifts comparable to the human HDT model55. Despite the hindlimb suspension, it should be noted that internal organs remain affected by gravity, thus HLU presents as a limited model for µG.
There are 2 common models of replicating µG in research involving small biological samples like cell cultures: free fall state and clinorotation. When cells reach terminal velocity during a state of free fall, they are unable to respond to gravitational force. As such, the cells are in a state of functional weightlessness. The Free Fall Machine (FFM) involves dropping a sample in a long vacuum tube, resulting in a free fall for 900 milliseconds (ms) before being propelled back to the top by a current of air at ~2–20 times normal gravity for 80 ms56. This theory relies on the assumption that cells do not have sufficient time to react to the small hyper-gravity windows between free falls56, which was later disproved57,58.
Clinorotation models µG by rotating a sample with enough speed to disable adaptation of gravity vector, but slow enough to prevent generation of centrifugal shear forces. Depending on the number of rotation axes, clinostats can be classified into 2 major classes:
1D/2D Clinostat – RWV
1D clinostats rotate the specimen along its vertical axis, while 2D clinostats rotate the sample on a plane perpendicular to the rotation axis. These clinostats come in varying rotation speed and vessel body sizes. Rotating wall vessels (RWVs) are 2D clinostats with a larger body (5–20 cm in diameter) and a slower rotation speed (10–20 rpm). The culture media rotates at the same angular velocity as the rotating vessel wall, creating laminar fluid flow that minimises shear stress. The rotation frequency also prevents particle sedimentation, such that the cells remain at the centre of the vessel in near zero gravity.
3D Clinostat – RPM
Random positioning machines (RPMs) consist of a sample area mounted at the centre of two frames that rotate independently with randomised speed and direction. The sample is therefore constantly reoriented randomly such that the gravity vector is averaged to near-zero. However, this only applies to the intersection of the two rotational axes. Accelerative forces from the rotation become stronger the further samples stray from centre, hence attention to sample positioning is critical on the RPM. Moreover, it has been shown that the RPM can induce stress responses in gravity-sensitive cell systems from its small vibrations and shear forces59,60. This can lead to cell detachment, and promote the formation of multicellular spheroid structures from increased intercellular interaction due to gravitational unloading61. Although, these effects were also observed in other clinostat types during multiple comparisons of 2D and 3D clinostats62,63,64. As such, studies involving either type of clinostat are widely accepted as models of microgravity.
Human bone loss induced by simulated microgravity
So far, bone loss experiments under simulated-µG largely confirm the data collected in space. Bone resorption symptomatic of long-duration space missions is widely reproduced in bed rest studies on Earth65,66,67,68,69,70,71 (Table 3). Following 4, 14, or 30 days of 6° HDT, bone resorption is increased in test subjects as reflected by an increase in bone resorption markers, as well as an overall calcium imbalance72,73. Interestingly, bone resorption levels fail to be rescued despite increasing dietary calcium intake74. As previously mentioned, ALP is responsible for bone mineralisation. Consistent with the reportedly unchanged or decreased bone formation in space travellers30,32,33, levels of ALP remain constant72 or even decreased73 in HDT test subjects. Together, overall bone loss and alterations in biomarkers of bone turnover induced by simulated µG appears consistent with spaceflight data.
On the other hand, there are some discrepancies in current literature describing how simulated- and true- µG affects different bone types and their compartments. One on-ground study subjected participants to a 60-day bed rest with 6° HDT, simulating the inactivity and fluid shift experienced in µG. The cortical area, thickness and density of the distal tibia in test subjects is decreased75, supporting the theory that µG negatively affects load-bearing bones more than non-weight-bearing bones8,27,28,29. In contrast to existing spaceflight data that indicates µG has little/no effect on non-weight-bearing bones8,27,28,29, HDT causes an increase in cortical area, thickness and bone density of the distal radius in subjects, despite a reduction in the trabecular area75. It is possible that an increased use of hands during the HDT study could be responsible for alterations in bone loading patterns, and is reflected in bone growth of the non-load-bearing distal radius. However, a meta-analysis of homogenous bone-related datasets from Gemini, Apollo, Soyuz, Skylab, Salyut, STS, Mir and ISS missions suggests a trend of underreporting positive bone density changes in the upper-limb and thorax region76. Taking into account data from both spaceflight and simulated-µG, the relationship between µG and the weight-bearing nature of bone or its compartments remains unclear. This calls for further clarification in future investigations.
Some advantages of studying bone-loss recovery in simulated-µG over spaceflight observations are that ground-based alternatives offer more accessible measurement timepoints, testing options, and longer post-µG-exposure monitoring. As such, they provide further insights into the limited understanding of the recovery process post-landing obtained from crewmembers alone. Consistent with post-landing data regarding the distal tibia, its cortical compartment generally recovers by 1 year following exposure to simulated-µG75,77,78. The recovery time of the tibia cortical thickness appears to be shorter in females (90 days)78 compared to males (180 days)75, despite both cohorts having been subjected to 60 days of 6° HDT. A combination of bed rest and unilateral lower limb suspension (ULLS) studies indicate that both cortical and trabecular compartments of the tibia suffer initial deterioration for the first month post-reambulation, with the cortical compartment to a larger extent77. However, unlike the cortical area of the tibia, the trabecular compartment does not recover even after 1–2 years post-ambulation75,77,78. Although the trabecular compartment of the tibia appears to begin recovering 3-6 months post-HDT75,79, this is followed by a reversal in BMD to −2% from 6 to 12 months in females78 and −1% from 3 to 24 months in males75. It is unlikely that these observations are related to age or biological sex, as biological sex of the two cohorts are balanced (N = 24 males and N = 24 females) with a similar age bracket (20- to 45-year-old males and 25- to 40-year-old females). It should be noted that similar to spaceflight data, recovery of non-weight-bearing bones such as the distal radius following exposure to simulated-µG is relatively underreported compared to weight-bearing-bones such as the hip and the distal tibia. Existing datasets can be reanalysed for radial bone data, and future studies can also direct more focus on the recovery of non-weight-bearing bones.
Using animal and cell models to understand microgravity-induced bone loss
The establishment of cellular and animal models have greatly improved our understanding of the molecular mechanisms behind µG-induced bone loss. The following sections review our knowledge to date, comparing cell and animal data between spaceflight (Table 4) and simulated-µG (Table 5) experiments.
Osteoblasts
One of the most reported observations is changes in osteoblast cell morphology, which is reflected in actin-related cellular structures. Exposure to µG on a 4-day spaceflight causes collapse of the actin cytoskeleton in quiescent osteoblasts, with some cells exhibiting a spindle shape80, and some becoming rounder and covered with microvilli81. In addition, µG-exposure results in altered nuclei morphology; one study reports elongated nuclei with a 30% size reduction in spaceflight osteoblasts82, and another observes more condensed and fragmented nuclei compared to ground controls83. A symptom of altered actin cytoskeleton is the reduction in number of stress fibres and smaller stress fibre area in spaceflight osteoblasts82,84, indicating that µG impairs actin polymerisation. Focal adhesions are another mechanosensitive structure composed of polymerised actin, which appears to be destabilised upon exposure to µG. Consistent with the decrease in stress fibre formation, multiple studies observe reductions in the number of focal contacts and focal adhesion area in osteoblasts following spaceflight80,83,84. Moreover, focal contacts established during spaceflight appear less mature than those formed in ground control osteoblasts83, confirming that µG negatively impacts osteoblast adhesion.
Since cytoskeletal integrity is critical in signal transduction and expression of genes that regulate cell cycle, the loss of both actin cytoskeletal structure and cell adhesion can deter osteoblast proliferation in space. Indeed, µG delays cell cycle initiation in quiescent osteoblasts compared to ground-based analogues82,85. Human bone marrow stem cells (hBMSCs) aboard the ISS for 14 days encounter cell cycle arrest despite initial osteoblastic differentiation, and the phenotype of terminal differentiation to osteocyte is inhibited86. In support of this, spaceflight osteoblasts also demonstrate reduced osteocalcin and type I collagen expression, indicating reduced differentiation and matrix development87. However, it should be noted that despite the impairment of osteoblastic proliferation and differentiation under µG, genome-wide and Next Generation Sequencing analysis does not suggest apoptosis or cell senescence86. The authors hypothesised that BMSCs or immature osteoblasts respond to µG by reverting to a quiescent state, in line with the observations of increased BMSC differentiation potential seen in µG-exposed mice following reloading on Earth gravity88,89.
Congruent to spaceflight observations, most of the current literature consistently suggest that simulated-µG inhibits the proliferation and differentiation of MSC towards osteoblasts, despite using different methods of µG simulation (Table 5). This is indicated by the suppressed gene expression of osteoblast differentiation markers such as bone morphogenic protein (BMP) and Runt-related transcription factor 2 (Runx2)18,90,91,92,93,94,95,96,97,98,99,100,101, in addition to the lowered expression of other osteoblastogenesis-related genes18,97,99,101,102,103. The marker for osteoblast maturation from MSC progenitors – ALP, is also expressed at lower levels in preosteoblasts and osteoblasts exposed to various forms of simulated-µG95,96,97,99,104. Subsequently, enzymatic activity of ALP is reduced in these cells18,99,103. Indeed, MSCs extracted from femurs of rats subjected to HLU for 28 days show reduced Runx2 and ALP mRNA expression, as well as decreased ALP activity105. However, the authors did not mention whether these changes in biomarker expressions correlated to any physical BMD changes in the rats.
The reduced expression and activity of ALP in simulated-µG-cells correlates well with decreased secretion of matrix proteins such as type 1 collagen and osteocalcin95,96,97,99, which could lead to reduced production of mineralisation crystals93,94. It is suggested that simulated-µG prevents extracellular calcium from entering osteoblasts, thus reducing intracellular free calcium levels which impairs calcium deposition and bone formation102,106. Furthermore, simulated-µG has been shown to cause cell cycle arrest at G2 and even induce apoptosis in osteoblasts100,102. HLU-induced bone loss in mice is consistently accompanied by a reduction in the number of viable osteoblasts and osteocytes in mice107. It is of note that some studies observed morphological changes in osteoblasts treated with simulated-µG, where actin cytoskeleton disruptions cause “bulging” or “spheroidal” morphologies94,106,108. However, while Hu et al. have reported simulated-µG-induced inhibition on preosteoblast differentiation, no alterations in cell morphology is observed96. Of note, studies that report morphological changes subjected bone cells to extended periods of simulated-µG (up to 20 days)94,106,108, compared to the 7-day-exposure period by Hu et al.96. One study attributes the inhibition of osteogenesis in simulated-µG to the obliteration of primary cilia on osteoblasts cultured on the RPM93, suggesting that primary cilia play a sensory role in bone metabolism109,110. Since the actin cytoskeleton plays a critical role in osteogenic differentiation111 and cell cycle processes112, it is possible these phenomena could be symptoms of µG-induced disruptions. Collectively, these observations provide strong evidence that impaired differentiation, maturation and proliferation of osteoblasts could be responsible for the µG-induced reduction in bone formation93,113.
Conversely, a smaller fraction of the literature suggests an opposing argument, that simulated-µG in fact promotes proliferative and differentiation capabilities in MSCs114, and does not directly induce osteoblast cell death104. RPM-induced µG causes MSCs to express increased levels of Runx2 and Osterix (Osx)115, which are transcription factors essential in osteoblastic differentiation. Runx2 gene expression also remains unaltered in osteoblasts cultured on the RPM compared to 1 G controls104. Furthermore, osteoblasts exposed to simulated-µG show elevated mRNA expression of SOX9108, which is a transcription factor characteristic of commitment to the osteoblastic lineage from MSC differentiation. Consequently, simulated-µG reportedly increases bone matrix protein production in MSCs and osteoblasts/osteoblast-like cells, such as osteopontin and osteocalcin gene expression, and type 1 collagen secretion104,108,115. It should be noted that immortalised osteoblast/osteoblast-like cell lines are used in some of these studies, and the effects of inherent mutations must be considered. Nonetheless, further investigation is required to either confirm or dispute these observations in future studies.
Osteoclasts
Mechanosensitive osteoclasts also contribute to space-related bone loss by disrupting normal bone homeostasis. Although much less studied than osteoblasts, osteoclasts have demonstrated increased resorptive activity in response to µG compared to controls on Earth83,116,117,118. Mature osteoclasts cultured on ivory or bovine bone slices demonstrate an increase in number of resorption pits formed following 5 or 7 days of spaceflight, respectively, compared to their relative ground controls83,116. Indeed, µG induced a dramatic increase in the expression of bone resorption-related genes, as well as elevated collagen telopeptide production116, which is correlated with bone resorption119. The increase in resorptive activity is reflected in the faster differentiation and maturation of spaceflight osteoclasts. Expression of genes involved in osteoclast differentiation, such as integrin β3, cathepsin K, MMP‐9 and calcitonin receptor are significantly upregulated following µG exposure in comparison to controls on Earth116,117,120. Collectively, these observations in spaceflight experiments indicate that osteoclasts play a critical role in µG-induced bone loss.
There are limited reports of osteoclast behaviour in simulated-µG, however, current literature is in consensus that simulated-µG promotes osteoclastogenesis and osteoclast function121,122,123,124,125,126,127. Osteoclastogenesis can be influenced by secretion of osteoprotegerin (OPG) and receptor activator of nuclear factor κB ligand (RANKL) from osteoblasts and osteocytes. RANKL – an osteoclastogenesis promotor, binds receptor activator of nuclear factor κB (RANK) receptors on the surface of osteoclasts for differentiation/maturation, while OPG acts as a decoy receptor that also binds RANKL – thus serving as a negative regulator of osteoclastogenesis128,129. RPM-facilitated µG decreases osteoblast production of OPG, thereby increasing RANKL/OPG ratios, which enhances osteoclastogenesis121. Supporting this observation, RANKL-stimulated osteoclastogenesis is increased in mice despite 28 days of HLU126. Autophagosome production reportedly enhances osteoclast differentiation, and RWV treatment enhances expression of autophagic genes123. This in turn promotes differentiation of osteoclasts, while inhibition of autophagy by 3-methyladenine conversely prevents osteoclastogenesis despite simulated-µG-exposure123. The positive effect of simulated-µG on osteoclastogenesis is also evidenced by the upregulation of cytokines, growth factors, proteases, signalling proteins and transcription factors such as c-Jun, MITF and CREB in osteoclasts cultured in RWV compared to 1 G controls122. Mice subjected to HLU displays increased osteoclast number, elevated osteoclast surfaces in hindlimb and vertebral sites, and ultimately reduced spinal BMD and strength127,130. µG-stimulated osteoclastogenesis and osteoclast activity are consistently observed in both on-ground and in-space experiments, highlighting the opportunity of these processes as a therapeutic target against bone loss.
Osteocytes
Osteocytes reside in the cavities of the mineralised bone matrix, also known as lacunae (Fig. 1), where they synthesise proteins such as collagen and glycosaminoglycans which contributes to bone mineralisation131. µG-exposure from spaceflight is found to impede the differentiation of osteoblasts into osteocytes131. This causes underdevelopment in the Golgi complexes of osteocytes responsible for the secretion of matrix proteins, leading to retardation in bone matrix mineralisation131. Furthermore, osteocytes are reported to undergo apoptosis as early as three days into µG-exposure from spaceflight, leaving an increased number of empty lacunae40,131, or lacunae with reduced volume and altered shape40. On top of reduced bone formation/mineralisation, this may trigger further bone resorption to cause deterioration of bone microstructure and loss of bone mass40.
Congruent to spaceflight observations, HLU rats demonstrated an increase in apoptotic osteocytes by 66% compared controls132, while HLU mice displayed enhanced osteocyte apoptosis in their spine127. The death of osteocytes is associated with increased osteoclast activity133,134. Osteocytes can promote osteoclast-mediated bone loss by secreting proteins such as sclerostin – an inhibitor of bone formation, and RANKL – a promotor of osteoclastogenesis. Simulated µG exposure increases RANKL and sclerostin gene expression in osteocytes cultured in RWV135, suggesting that osteocytes play a role in µG-induced bone resorption. The mechano-sensitive influence of osteocytes on osteoclasts can be demonstrated even within the same animal in a rodent HLU model. Osteocyte proteins (including sclerostin) is elevated in the unloaded femur compared to the weight-bearing forelimb of the same HLU rat, which coincides with decreased osteocyte number and increased osteoclast activity of the unloaded hindlimb compared to the loaded forelimb136.
The elevated sclerostin secretion by osteocytes also negatively regulates oasteoblast-mediated bone formation by antagonising BMP/Wnt signalling137,138,139. Simulated µG has been shown to depress the Wnt signalling pathway and downregulate cell cycle related genes such as Cyclin D1 in osteocyte-like cells, which could be partially restored by the administration of antibodies against sclerostin140. Indeed, the ablation of sclerostin or RANKL appears to be protective over unloading-induced bone loss in HLU mice models107,141. Interestingly, osteocyte-deficient mice demonstrated resistance to HLU-induced bone loss, where bone loss and microstructural deterioration is prevented despite unloading142. Thus, osteocytes appear to play a vital role in promoting osteoclastogenesis and bone resorption upon mechanical unloading or µG-exposure. Future studies should leverage the unique ability of osteocytes to influence both osteoblasts and osteoclasts when exploring therapeutic interventions against bone loss.
Despite being the most abundant type of bone cells, osteocytes represent the least studied cell type due to their inaccessibility from being embedded in the bone matrix. The current literature landscape also points to a general lack of methods for osteocyte isolation and culture. In addition, isolated osteocytes may not be physiologically relevant to osteocytes in vivo, as their native environment is three dimensional and complex. As such, there is a need to establish more robust protocols for osteocyte culture and characterisation.
Molecular therapies against microgravity-induced bone loss
Research using cell and animal models of µG-induced bone loss has revealed various possible therapeutic targets as described above, while providing accessible and ethical platforms for the testing of molecular therapeutic agents. Multiple agents have been identified to prevent µG-induced bone loss by promoting osteoblast differentiation, maturation and activity. Rapamycin treatment of human blood-derived stem cells (hBDSCs) aboard the ISS for 3 days induces earlier differentiation towards osteogenic lineage cells compared to analogous ground controls in 1G143. hBDSCs exposed to µG exhibit reduced expression of embryonic markers Sox2, Oct3/4, Nanog and E-cadherin to a larger extent compared to ground controls, indicating that rapamycin induces an earlier loss of pluripotency in space than on Earth143. Furthermore, expression of differentiation-related transcription factors in µG-exposed hBDSCs are altered in favour towards osteogenesis143. Downregulation of the transcription factors Otx2 and Snail in µG inhibits osteogenic differentiation; while GATA4 and SOX17, which promote differentiation towards osteogenesis are upregulated143. In another study, IL-6 neutralisation prevented the reduction in Runx2 and ALP mRNA expression, as well as ALP activity induced by simulated-µG treatment103. Similarly, IL-6-neutralisation successfully alleviated HLU-induced bone loss in mice tibia103. Irisin administration has also been shown to prevent µG-induced downregulation of transcription factors and proteins critical to osteoblast differentiation and activity120. Similar to IL6-neutralisation, irisin treatment elevated Runx2 and ALP expression, and ALP activity, as well as increased osteoblast numbers and bone formation in mice144,145,146. These observations suggest that the administration of rapamycin, irisin and IL-6 neutralisation can prevent µG-induced downregulation of osteoblast differentiation, maturation and mineralisation activity.
Irisin has been demonstrated to prevent bone loss induced by HLU in mice147. As previously discussed, osteocytes can secrete sclerostin to promote osteoclast-mediated bone loss. The number of sclerostin-positive osteocytes is higher in the hindlimb of HLU mice compared to controls130. Upon irisin treatment, the sclerostin-positive population was significantly lowered, indicating a negative effect on osteoclast activity as an increase in bone formation rate was observed130. Irisin has also been demonstrated to downregulate osteoclastogenesis. Its administration reduced RANKL-induced osteoclastogenesis146, and decreased osteoclast-covered bone surfaces in HLU mice compared to ambulatory controls130. Analogously, melatonin treatment upregulated osteoclast inhibitor calcitonin and downregulated RANKL in goldfish scales117. However, it is unknown whether this translates into suppression of osteoclast activity in this study. Nevertheless, these molecular compounds all serve as promising therapeutic agents against µG-induced bone loss. However, further animal studies will be required prior to phase 1 human clinical safety trials.
Conclusion
Through the combination of spaceflight and simulation data on humans, animals and cellular models, we are continuously improving our knowledge of µG-induced bone loss. Our current understanding suggests µG affects weight-bearing bones to a larger degree than non-weight-bearing bones. However, this relationship warrants further investigation, especially with the underreporting of changes in non-weight-bearing bones in astronauts. Moreover, there are inherent biases in HDT/HBR studies, where the use of non-weight-bearing bones, such as the distal radius, may be increased in subjects. Together, these observations might mask the true effect of µG on non-weight-bearing bones. Future studies can also clarify the discrepancies in the effect of µG on different bone compartments, as well as how µG influences recovery processes.
The use of cellular models has shed light into the molecular mechanisms behind µG-induced bone loss, which in turn provides potential targets for therapeutic intervention. Although bone loss stems from both reduced bone formation by osteoblasts and elevated resorption by osteoclasts, the current literature landscape is largely focused on osteoblasts. Preliminary observations of increased osteoclast activity in µG present promising therapeutic targets, as such, the role of osteoclasts in bone loss requires further elucidation. In addition, as osteocytes are capable of influencing both osteoblasts and osteoclasts, this unique position should be leveraged for potential therapeutic options. Thus, the role of osteocytes in balancing bone resorption and formation under µG also deserves further clarification. Collectively, these efforts should enable the development of more effective preventative and therapeutic measures against µG-induced bone loss, thus paving the way for safer journeys as we venture further away from Earth.
Future directions
-
Extend monitoring of astronauts post-mission for at least 12 months to investigate the possibility of progressive fragility, particularly in non-weight-bearing bones such as the distal radius.
-
Clarify the relationship between µG and the weight-bearing nature of bone loss, particularly whether µG affects non-weight-bearing bones.
-
Clarify how µG affects different bone compartments in humans and animals.
-
Investigate how the bone-loss recovery process differs between weight-bearing and non-weight-bearing bones, as well as between different bone compartments.
-
Address discrepancies in the literature that points to µG promoting osteogenic proliferation and differentiation.
-
Establish robust differentiation, isolation and culturing protocols for osteoclasts and osteocytes, as well as characterisation and validation of existing osteoclast/osteocyte-like cell lines.
-
Dissect the mechanisms behind how osteoclasts and osteocytes contribute to µG-induced bone loss to identify possible therapeutic targets.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
No datasets were analyzed or generated in the writing of this review.
Code availability
No codes were used in the writing of this review.
References
Dunbar, B. Moon to Mars Overview | NASA. https://www.nasa.gov/topics/moon-to-mars/overview (2020).
Nelson, E. S., Mulugeta, L. & Myers, J. G. Microgravity-induced fluid shift and ophthalmic changes. Life 4, 621–665 (2014).
LeBlanc, A. et al. Muscle volume, MRI relaxation times (T2), and body composition after spaceflight. J. Appl. Physiol. 89, 2158–2164 (2000).
Meck, J. V., Reyes, C. J., Perez, S. A., Goldberger, A. L. & Ziegler, M. G. Marked exacerbation of orthostatic intolerance after long- vs. short-duration spaceflight in veteran astronauts. Psychosom. Med. 63, 865–873 (2001).
Stowe, R. P. et al. Leukocyte subsets and neutrophil function after short‐term spaceflight. J. Leukoc. Biol. 65, 179–186 (1999).
Mader, T. H. et al. Optic disc edema, globe flattening, choroidal folds, and hyperopic shifts observed in astronauts after long-duration space flight. Ophthalmology 118, 2058–2069 (2011).
Clément, G. Fundamentals of Space Medicine. https://doi.org/10.1007/978-1-4419-9905-4 (2011).
Vico, L. et al. Effects of long-term microgravity exposure on cancellous and cortical weight-bearing bones of cosmonauts. Lancet 355, 1607–1611 (2000).
Kenkre, J. & Bassett, J. The bone remodelling cycle. Ann. Clin. Biochem. 55, 308–327 (2018).
Matic, I. et al. Quiescent bone lining cells are a major source of osteoblasts during adulthood. Stem Cells 34, 2930–2942 (2016).
Turner, R. T. et al. Acute exposure to high dose γ-radiation results in transient activation of bone lining cells. Bone 57, 164–173 (2013).
Teti, A. Bone development: overview of bone cells and signaling. Curr. Osteoporos. Rep. 9, 264 (2011).
Katsimbri, P. The biology of normal bone remodelling. Eur. J. Cancer Care 26, e12740 (2017).
Delaissé, J. et al. Matrix metalloproteinases (MMP) and cathepsin K contribute differently to osteoclastic activities. Microsc. Res. Tech. 61, 504–513 (2003).
Everts, V. et al. The bone lining cell: its role in cleaning howship’s lacunae and initiating bone formation. J. Bone Min. Res. 17, 77–90 (2002).
Grigoriadis, A. E., Heersche, J. N. & Aubin, J. E. Differentiation of muscle, fat, cartilage, and bone from progenitor cells present in a bone-derived clonal cell population: effect of dexamethasone. J. Cell Biol. 106, 2139–2151 (1988).
Capulli, M., Paone, R. & Rucci, N. Osteoblast and osteocyte: games without frontiers. Arch. Biochem. Biophys. 561, 3–12 (2014).
Patel, M. J. et al. Identification of mechanosensitive genes in osteoblasts by comparative microarray studies using the rotating wall vessel and the random positioning machine. J. Cell. Biochem. 101, 587–599 (2007).
Roeder, E., Brya, G., Matthews & Kalajzic, I. Visual reporters for study of the osteoblast lineage. Bone 92, 189–195 (2016).
Dudley, H. R. & Spiro, D. The fine structure of bone cells. J. Cell Biol. 11, 627–649 (1961).
Kamioka, H., Honjo, T. & Takano-Yamamoto, T. A three-dimensional distribution of osteocyte processes revealed by the combination of confocal laser scanning microscopy and differential interference contrast microscopy. Bone 28, 145–149 (2001).
Gaël, Y., Rochefort & Benhamou, C. Osteocytes are not only mechanoreceptive cells. Int. J. Numer. Method Biomed. Eng. 29, 1082–1088 (2013).
Tilton, F. E., Degioanni, J. J. & Schneider, V. S. Long-term follow-up of Skylab bone demineralization. Aviat. Space Environ. Med. 51, 1209–1213 (1980).
Whedon, G. D. et al. Effect of weightlessness on mineral metabolism; Metabolic studies on skylab orbital space flights. Calcif. Tissue Res. 21, 423–430 (1976).
Rambaut, P. C. & Johnston, R. S. Prolonged weightlessness and calcium loss in man. Acta Astronaut. 6, 1113–1122 (1979).
Caillot-Augusseau, A. et al. Bone formation and resorption biological markers in cosmonauts during and after a 180-day space flight (Euromir 95). Clin. Chem. 44, 578–585 (1998).
Collet, P. H. et al. Effects of 1- and 6-month spaceflight on bone mass and biochemistry in two humans. Bone 20, 547–551 (1997).
Sibonga, J. D. et al. Recovery of spaceflight-induced bone loss: bone mineral density after long-duration missions as fitted with an exponential function. Bone 41, 973–978 (2007).
Gabel, L. et al. Pre-flight exercise and bone metabolism predict unloading-induced bone loss due to spaceflight. Brit. J. Sport Med. https://doi.org/10.1136/bjsports-2020-103602 (2021).
Caillot-Augusseau, A. et al. Space flight is associated with rapid decreases of undercarboxylated osteocalcin and increases of markers of bone resorption without changes in their circadian variation: observations in two cosmonauts. Clin. Chem. 46, 1136–1143 (2000).
Smith, S. M. et al. Collagen cross-link excretion during space flight and bed Rest1. J. Clin. Endocrinol. Metab. 83, 3584–3591 (1998).
Smith, S. M. et al. Bone markers, calcium metabolism, and calcium kinetics during extended‐duration space flight on the mir space station. J. Bone Miner. Res. 20, 208–218 (2005).
Smith, S. M., Zwart, S. R., Block, G., Rice, B. L. & Davis-Street, J. E. The nutritional status of astronauts is altered after long-term space flight aboard the international space station. J. Nutr. 135, 437–443 (2005).
Zittermann, A. et al. Microgravity inhibits intestinal calcium absorption as shown by a stable strontium test. Eur. J. Clin. Invest. 30, 1036–1043 (2000).
Vico, L. et al. Cortical and trabecular bone microstructure did not recover at weight‐bearing skeletal sites and progressively deteriorated at non‐weight‐bearing sites during the year following international space station missions. J. Bone Miner. Res. 32, 2010–2021 (2017).
Vico, L. et al. Trabecular bone remodeling after seven days of weightlessness exposure (BIOCOSMOS 1667). Am. J. Physiol. 255, R243–R247 (1988).
Sibonga, J. D., Spector, E. R., Johnston, S. L. & Tarver, W. J. Evaluating bone loss in ISS astronauts. Aerosp. Med. Hum. Perform. 86, 38–44 (2015).
Blaber, E. A. et al. Microgravity induces pelvic bone loss through osteoclastic activity, osteocytic osteolysis, and osteoblastic cell cycle inhibition by CDKN1a/p21. PLoS ONE 8, e61372 (2013).
Maupin, K. A. et al. Skeletal adaptations in young male mice after 4 weeks aboard the International Space Station. Npj Microgravity 5, 21 (2019).
Gerbaix, M. et al. One-month spaceflight compromises the bone microstructure, tissue-level mechanical properties, osteocyte survival and lacunae volume in mature mice skeletons. Sci. Rep. 7, 2659 (2017).
Lee, S.-J. et al. Targeting myostatin/activin A protects against skeletal muscle and bone loss during spaceflight. Proc. Natl Acad. Sci. USA 117, 23942–23951 (2020).
Keune, J. A., Branscum, A. J., Iwaniec, U. T. & Turner, R. T. Effects of spaceflight on bone microarchitecture in the axial and appendicular skeleton in growing ovariectomized rats. Sci. Rep. 5, 18671 (2015).
Strollo, F. et al. The pituitary-testicular axis in microgravity: analogies with the aging male syndrome. J. Endocrinol. Invest. 28, 78–83 (2005).
Ferranti, F., Marta, D., Bianco & Pacelli, C. Advantages and limitations of current microgravity platforms for space biology research. Appl. Sci. 11, 68 (2020).
Kakurin, L., Lobachik, V., Mikhailov, V. & Senkevich, Y. A. Antiorthostatic hypokinesia as a method of weightlessness simulation. Aviat. Space Environ. Med. 47, 1083–1086 (1976).
Krupina, T. N., Fyodorov, B. M., Filatova, L. M., Tsyganova, N. I. & Matsnev, E. Effect of antiorthostatic bed rest on the human body. Life Sci. Space Res. 14, 285–287 (1976).
Lamb, L. E. & Roman, J. The head-down tilt and adaptability for aerospace flight. Aerosp. Med. 32, 473–486 (1961).
Katkovskiĭ, B. S., Georgievskiĭ, V. S., Machinskiĭ, G. V., Mikhaĭlov, V. M. & Pometov, I. D. Some physiological effects caused by 30 days of bed rest in different body positions. Kosm. Biol. Aviakosm. Med. 14, 55–58 (1980).
Hargens, A. R. & Vico, L. Long-duration bed rest as an analog to microgravity. J. Appl. Physiol. 120, 891–903 (2016).
Duddy, J. H. The simulation of weightlessness using water immersion techniques: an annotated bibliography. Hum. Factors 11, 507–539 (1969).
Tsai, T. & Maibach, H. I. How irritant is water? An overview. Contact Dermatitis 41, 311–314 (1999).
Shulzhenko, E. B., Tigranyan, R. A., Panfilov, V. E. & Bzhalava, I. I. Life sciences and space research: proceedings of the open meeting of the working group on space biology of the twenty-second plenary meeting. Gravitational Biology Primates 175–179 (1980).
Tomilovskaya, E., Shigueva, T., Sayenko, D., Rukavishnikov, I. & Kozlovskaya, I. Dry immersion as a ground-based model of microgravity physiological effects. Front. Physiol. 10, 284 (2019).
Tesch, P. A., Lundberg, T. R. & Fernandez-Gonzalo, R. Unilateral lower limb suspension: from subject selection to “omic” responses. J. Appl Physiol. 120, 1207–1214 (2016).
Horneck, G., Klaus, D. M. & Mancinelli, R. L. Space microbiology. Microbiol. Mol. Biol. Rev. 74, 121–156 (2010).
Mesland, D. A., Anton, A. H., Willemsen, H. & van den, E. H. The Free Fall Machine–a ground-based facility for microgravity research in life sciences. Microgravity Sci. Technol. 9, 10–14 (1996).
Schwarzenberg, M. et al. Signal transduction in T lymphocytes — a comparison of the data from space, the free fall machine and the random positioning machine. Adv. Space Res. 24, 793–800 (1999).
Skagen, E. B. & Inersen, T.-H. Effect of simulated and real weightlessness on early regeneration stages of Brassica napus protoplasts. In Vitro Cell. Dev. Biol. Plant 36, 312–318 (2000).
Brungs, S., Hauslage, J. & Hemmersbach, R. Validation of random positioning versus clinorotation using a macrophage model system. Microgravity Sci. Technol. 31, 223–230 (2019).
Krause, L., Braun, M., Hauslage, J. & Hemmersbach, R. Analysis of statoliths displacement in chara rhizoids for validating the microgravity-simulation quality of clinorotation modes. Microgravity Sci. Technol. 30, 229–236 (2018).
Unsworth, B. R. & Lelkes, P. I. Growing tissues in microgravity. Nat. Med. 4, 901–907 (1998).
Svejgaard, B. et al. Common effects on cancer cells exerted by a random positioning machine and a 2D clinostat. PLoS ONE 10, e0135157 (2015).
van Loon, J. J. W. A. Some history and use of the random positioning machine, RPM, in gravity related research. Adv. Space Res. Ser. 39, 1161–1165 (2007).
Wuest, S. L., Richard, S., Kopp, S., Grimm, D. & Egli, M. Simulated microgravity: critical review on the use of random positioning machines for mammalian cell culture. Biomed. Res. Int. 2015, 1–8 (2015).
Baecker, N. et al. Bone resorption is induced on the second day of bed rest: results of a controlled crossover trial. J. Appl. Physiol. 95, 977–982 (2003).
Heer, M., Baecker, N., Mika, C., Boese, A. & Gerzer, R. Immobilization induces a very rapid increase in osteoclast activity. Acta Astronaut. 57, 31–36 (2005).
LeBlanc, A. D., Spector, E. R., Evans, H. J. & Sibonga, J. D. Skeletal responses to space flight and the bed rest analog: a review. J. Musculoskelet. Neuronal Interact. 7, 33–47 (2007).
Traon, A. P.-L., Heer, M., Narici, M. V., Rittweger, J. & Vernikos, J. From space to Earth: advances in human physiology from 20 years of bed rest studies (1986–2006). Eur. J. Appl Physiol. 101, 143–194 (2007).
Shackelford, L. C. et al. Resistance exercise as a countermeasure to disuse-induced bone loss. J. Appl Physiol. 97, 119–129 (2004).
Smith, S. M. et al. Evaluation of treadmill exercise in a lower body negative pressure chamber as a countermeasure for weightlessness‐induced bone loss: a bed rest study with identical twins. J. Bone Miner. Res. 18, 2223–2230 (2003).
Watanabe, Y. et al. Intravenous pamidronate prevents femoral bone loss and renal stone formation during 90‐day bed rest. J. Bone Miner. Res 19, 1771–1778 (2004).
Morgan, J. L. L. et al. Bone metabolism and nutritional status during 30-day head-down-tilt bed rest. J. Appl. Physiol. 113, 1519–1529 (2012).
Smith, S. M. et al. Effects of artificial gravity during bed rest on bone metabolism in humans. J. Appl. Physiol. 107, 47–53 (2009).
Baecker, N., Frings-Meuthen, P., Smith, S. M. & Heer, M. Short-term high dietary calcium intake during bedrest has no effect on markers of bone turnover in healthy men. Nutrition 26, 522–527 (2010).
Belavy, D. L., Beller, G., Ritter, Z. & Felsenberg, D. Bone structure and density via HR-pQCT in 60d bed-rest, 2-years recovery with and without countermeasures. J. Musculoskelet. Neuronal Interact. 11, 215–226 (2011).
Stavnichuk, M., Mikolajewicz, N., Corlett, T., Morris, M. & Komarova, S. V. A systematic review and meta-analysis of bone loss in space travelers. Npj Microgravity 6, 13 (2020).
Cervinka, T., Sievänen, H., Hyttinen, J. & Rittweger, J. Bone loss patterns in cortical, subcortical, and trabecular compartments during simulated microgravity. J. Appl. Physiol. 117, 80–88 (2014).
Armbrecht, G. et al. Trabecular and cortical bone density and architecture in women after 60 days of bed rest using high‐resolution pQCT: WISE 2005. J. Bone Miner. Res. 26, 2399–2410 (2011).
Rittweger, J. et al. Prevention of bone loss during 56 days of strict bed rest by side-alternating resistive vibration exercise. Bone 46, 137–147 (2010).
Hughes-Fulford, M. Function of the cytoskeleton in gravisensing during spaceflight. Adv. Space Res. Ser. 32, 1585–1593 (2003).
Guignandon, A. et al. Effects of intermittent or continuous gravitational stresses on cell–matrix adhesion: quantitative analysis of focal contacts in osteoblastic ROS 17/2.8 cells. Exp. Cell Res. 236, 66–75 (1997).
Hughes-Fulford, M. & Lewis, M. L. Effects of microgravity on osteoblast growth activation. Exp. Cell Res. 224, 103–109 (1996).
Nabavi, N., Khandani, A., Camirand, A. & Harrison, R. E. Effects of microgravity on osteoclast bone resorption and osteoblast cytoskeletal organization and adhesion. Bone 49, 965–974 (2011).
Guignandon, A. et al. Rac1 GTPase silencing counteracts microgravity‐induced effects on osteoblastic cells. FASEB J. 28, 4077–4087 (2014).
Hughes-Fulford, M., Tjandrawinata, R., Fitzgerald, J., Gasuad, K. & Gilbertson, V. Effects of microgravity on osteoblast growth. Gravit. Space Biol. Bull. 11, 51–60 (1998).
Bradamante, S. et al. SCD – stem cell differentiation toward osteoblast onboard the international space station. Microgravity Sci. Technol. 30, 713–729 (2018).
Landis, W. J., Hodgens, K. J., Block, D., Toma, C. D. & Gerstenfeld, L. C. Spaceflight effects on cultured embryonic chick bone cells. J. Bone Miner. Res. 15, 1099–1112 (2000).
Blaber, E. A. et al. Mechanical unloading of bone in microgravity reduces mesenchymal and hematopoietic stem cell-mediated tissue regeneration. Stem Cell Res. 13, 181–201 (2014).
Blaber, E. A. et al. Microgravity reduces the differentiation and regenerative potential of embryonic stem cells. Stem Cells Dev. 24, 2605–2621 (2015).
Dai, Z. Q., Wang, R., Ling, S. K., Wan, Y. M. & Li, Y. H. Simulated microgravity inhibits the proliferation and osteogenesis of rat bone marrow mesenchymal stem cells. Cell Prolif. 40, 671–684 (2007).
Li, L. et al. Effects of simulated microgravity on the expression profiles of RNA during osteogenic differentiation of human bone marrow mesenchymal stem cells. Cell Prolif. 52, e12539 (2019).
Chen, Z., Luo, Q., Lin, C. & Song, G. Simulated microgravity inhibits osteogenic differentiation of mesenchymal stem cells through down regulating the transcriptional co-activator TAZ. Biochem. Biophys. Res. Commun. 468, 21–26 (2015).
Shi, W. et al. Primary cilia act as microgravity sensors by depolymerizing microtubules to inhibit osteoblastic differentiation and mineralization. Bone 136, 115346 (2020).
Gioia, M. et al. Simulated microgravity induces a cellular regression of the mature phenotype in human primary osteoblasts. Cell Death Discov. 4, 59 (2018).
Zayzafoon, M., Gathings, W. E. & McDonald, J. M. Modeled microgravity inhibits osteogenic differentiation of human mesenchymal stem cells and increases adipogenesis. Endocrinology 145, 2421–2432 (2004).
Hu, L. F., Qian, A. R., Wang, Y., Di, S. M. & Shang, P. Inhibitory effect of simulated microgravity on differentiating preosteoblasts. Adv. Space Res. Ser. 51, 107–114 (2013).
Ontiveros, C. & McCabe, L. R. Simulated microgravity suppresses osteoblast phenotype, Runx2 levels and AP‐1 transactivation. J. Cell. Biochem. 88, 427–437 (2003).
Qin, W. et al. Mir-494 inhibits osteoblast differentiation by regulating BMP signaling in simulated microgravity. Endocrine 65, 426–439 (2019).
Pardo, S. J. et al. Simulated microgravity using the Random Positioning Machine inhibits differentiation and alters gene expression profiles of 2T3 preosteoblasts. Am. J. Physiol. Cell Physiol. 288, C1211–C1221 (2005).
Dong, J. et al. The combined effects of simulated microgravity and X-ray radiation on MC3T3-E1 cells and rat femurs. Npj Microgravity 7, 3 (2021).
Makihira, S., Kawahara, Y., Yuge, L., Mine, Y. & Nikawa, H. Impact of the microgravity environment in a 3‐dimensional clinostat on osteoblast‐ and osteoclast‐like cells. Cell Biol. Int. 32, 1176–1181 (2008).
Sun, Z. et al. Simulated microgravity reduces intracellular‐free calcium concentration by inhibiting calcium channels in primary mouse osteoblasts. J. Cell. Biochem. 120, 4009–4020 (2019).
He, B. et al. Blockade of IL-6 alleviates bone loss induced by modeled microgravity in mice. Can. J. Physiol. Pharmacol. 98, 678–683 (2020).
Bucaro, M. A. et al. The effect of simulated microgravity on osteoblasts is independent of the induction of apoptosis. J. Cell. Biochem. 102, 483–495 (2007).
Pan, Z. et al. Effects of hindlimb unloading on ex vivo growth and osteogenic/adipogenic potentials of bone marrow-derived mesenchymal stem cells in rats. Stem Cells Dev. 17, 795–804 (2008).
Yuge, L. et al. Cell differentiation and p38MAPK cascade are inhibited in human osteoblasts cultured in a three-dimensional clinostat. In Vitro Cell Dev. Biol. Anim. 39, 89–97 (2003).
Lin, C. et al. Sclerostin mediates bone response to mechanical unloading through antagonizing Wnt/β‐catenin signaling. J. Bone Miner. Res. 24, 1651–1661 (2009).
Mann, V. et al. Changes in human foetal osteoblasts exposed to the random positioning machine and bone construct tissue engineering. Int. J. Mol. Sci. 20, 1357 (2019).
Crawford-Young, S. J. Effects of microgravity on cell cytoskeleton and embryogenesis. Int. J. Dev. Biol. 50, 183–191 (2003).
Vorselen, D., Roos, W. H., MacKintosh, F. C., Wuite, G. J. L. & Loon, J. J. W. A. The role of the cytoskeleton in sensing changes in gravity by nonspecialized cells. FASEB J. 28, 536–547 (2014).
Khan, A. U., Qu, R., Fan, T., Ouyang, J. & Dai, J. A glance on the role of actin in osteogenic and adipogenic differentiation of mesenchymal stem cells. Stem Cell Res. Ther. 11, 283 (2020).
Heng, Y.-W. & Koh, C.-G. Actin cytoskeleton dynamics and the cell division cycle. Int. J. Biochem. Cell Biol. 42, 1622–1633 (2010).
BUCARO, M. A. et al. Bone cell survival in microgravity: evidence that modeled microgravity increases osteoblast sensitivity to apoptogens. Ann. N. Y. Acad. Sci. 1027, 64–73 (2004).
Yuge, L. et al. Microgravity potentiates stem cell proliferation while sustaining the capability of differentiation. Stem Cells Dev. 15, 921–929 (2006).
Cazzaniga, A., Maier, J. A. M. & Castiglioni, S. Impact of simulated microgravity on human bone stem cells: New hints for space medicine. Biochem. Biophys. Res Commun. 473, 181–186 (2016).
Tamma, R. et al. Microgravity during spaceflight directly affects in vitro osteoclastogenesis and bone resorption. FASEB J. 23, 2549–2554 (2009).
Ikegame, M. et al. Melatonin is a potential drug for the prevention of bone loss during space flight. J. Pineal Res. 67, e12594 (2019).
Yamamoto, T. et al. Expression of sclerostin in the regenerating scales of goldfish and its increase under microgravity during space flight. Biomed. Res. 41, 279–288 (2020).
Apone, S., Lee, M. Y. & Eyre, D. R. Osteoclasts generate cross-linked collagen N-telopeptides (NTx) but not free pyridinolines when cultured on human bone. Bone 21, 129–136 (1997).
Colucci, S. et al. Irisin prevents microgravity‐induced impairment of osteoblast differentiation in vitro during the space flight CRS‐14 mission. FASEB J. 34, 10096–10106 (2020).
Rucci, N., Rufo, A., Alamanou, M. & Teti, A. Modeled microgravity stimulates osteoclastogenesis and bone resorption by increasing osteoblast RANKL/OPG ratio. J. Cell. Biochem. 100, 464–473 (2007).
Sambandam, Y. et al. Microarray profile of gene expression during osteoclast differentiation in modelled microgravity. J. Cell. Biochem. 111, 1179–1187 (2010).
Sambandam, Y. et al. Microgravity control of autophagy modulates osteoclastogenesis. Bone 61, 125–131 (2014).
Ethiraj, P. et al. Microgravity modulation of syncytin‐A expression enhance osteoclast formation. J. Cell. Biochem. 119, 5696–5703 (2018).
Li, Y. et al. Knockdown of CD44 inhibits the alteration of osteoclast function induced by simulated microgravity. Acta Astronaut. 166, 607–612 (2020).
Saxena, R., Pan, G., Dohm, E. D. & McDonald, J. M. Modeled microgravity and hindlimb unloading sensitize osteoclast precursors to RANKL-mediated osteoclastogenesis. J. Bone Miner. Metab. 29, 111–122 (2011).
Aguirre, J. et al. Osteocyte apoptosis is induced by weightlessness in mice and precedes osteoclast recruitment and bone loss. J. Bone Miner. Res. 21, 605–615 (2006).
Simonet, W. S. et al. Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell 89, 309–319 (1997).
Lacey, D. L. et al. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell 93, 165–176 (1998).
Metzger, C. E., Narayanan, S. A., Phan, P. H. & Bloomfield, S. A. Hindlimb unloading causes regional loading-dependent changes in osteocyte inflammatory cytokines that are modulated by exogenous irisin treatment. Npj Microgravity 6, 28 (2020).
Rodionova, N. V., Oganov, V. S. & Zolotova, N. V. Ultrastructural changes in osteocytes in microgravity conditions. Adv. Space Res. Ser. 30, 765–770 (2002).
Basso, N. & Heersche, J. N. M. Effects of hind limb unloading and reloading on nitric oxide synthase expression and apoptosis of osteocytes and chondrocytes. Bone 39, 807–814 (2006).
Andreev, D. et al. Osteocyte necrosis triggers osteoclast-mediated bone loss through macrophage-inducible C-type lectin. J. Clin. Invest. 130, 4811–4830 (2020).
Ru, J. & Wang, Y. Osteocyte apoptosis: the roles and key molecular mechanisms in resorption-related bone diseases. Cell Death Dis. 11, 846 (2020).
Spatz, J. M. et al. The Wnt inhibitor sclerostin is up-regulated by mechanical unloading in osteocytes in vitro. J. Biol. Chem. 290, 16744–16758 (2015).
Metzger, C. E. et al. Differential responses of mechanosensitive osteocyte proteins in fore- and hindlimbs of hindlimb-unloaded rats. Bone 105, 26–34 (2017).
Li, X. et al. Sclerostin binds to LRP5/6 and antagonizes canonical Wnt signaling*. J. Biol. Chem. 280, 19883–19887 (2005).
van Bezooijen, R. L. et al. Sclerostin is an osteocyte-expressed negative regulator of bone formation, but not a classical BMP antagonist. J. Exp. Med. 199, 805–814 (2004).
Winkler, D. G. et al. Osteocyte control of bone formation via sclerostin, a novel BMP antagonist. EMBO J. 22, 6267–6276 (2003).
Yang, X., Sun, L.-W., Liang, M., Wang, X.-N. & Fan, Y.-B. The response of wnt/ß-catenin signaling pathway in osteocytes under simulated microgravity. Microgravity Sci. Technol. 27, 473–483 (2015).
Xiong, J. et al. Matrix-embedded cells control osteoclast formation. Nat. Med. 17, 1235–1241 (2011).
Tatsumi, S. et al. Targeted ablation of osteocytes induces osteoporosis with defective mechanotransduction. Cell Metab. 5, 464–475 (2007).
Gambacurta, A. et al. Human osteogenic differentiation in Space: proteomic and epigenetic clues to better understand osteoporosis. Sci. Rep. 9, 8343 (2019).
Colaianni, G. et al. The myokine irisin increases cortical bone mass. Proc. Natl Acad. Sci. USA 112, 12157–12162 (2015).
Qiao, X. et al. Irisin promotes osteoblast proliferation and differentiation via activating the MAP kinase signaling pathways. Sci. Rep. 6, 18732 (2016).
Zhang, J. et al. Exercise-induced irisin in bone and systemic irisin administration reveal new regulatory mechanisms of bone metabolism. Bone Res. 5, 16056 (2017).
Colaianni, G. et al. Irisin prevents and restores bone loss and muscle atrophy in hind-limb suspended mice. Sci. Rep. 7, 2811 (2017).
Carpenter, R. D., LeBlanc, A. D. Evans, H., Sibonga, J. D. & Lang, T. F. Long-term changes in the density and structure of the human hip and spine after long-duration spaceflight. Acta Astronaut. 67, 71–81 (2010).
Lang, T., LeBlanc, A., Evans, H., Lu, Y., Genant, H. & Yu, A. Cortical and Trabecular Bone Mineral Loss From the Spine and Hip in Long‐Duration Spaceflight. J. Bone Miner. Res. 19, 1006–1012 (2004).
Rittweger, J. et al. Muscle atrophy and bone loss after 90 days’ bed rest and the effects of flywheel resistive exercise and pamidronate: results from the LTBR study. Bone. 36, 1019–1029 (2005).
Zwart, S. R. et al. Lower body negative pressure treadmill exercise as a countermeasure for bed rest-induced bone loss in female identical twins. Bone. 40, 529–537 (2007).
Cavolina, J. M., Evans, G. L., Harris, S. A., Zhang, M., Westerlind, K. C. & Turner, R. T. The effects of orbital spaceflight on bone histomorphometry and messenger ribonucleic acid levels for bone matrix proteins and skeletal signaling peptides in ovariectomized growing rats. Endocrinology 138, 1567–1576 (1997).
Chatani, M. et al. Acute transcriptional up-regulation specific to osteoblasts/osteoclasts in medaka fish immediately after exposure to microgravity. Sci. Rep. 6, 39545 (2016).
Camirand, A., Goltzman, D., Gupta, A., Kaouass, M., Panda, D. & Karaplis, A. The Role of Parathyroid Hormone-Related Protein (PTHrP) in Osteoblast Response to Microgravity: Mechanistic Implications for Osteoporosis Development. PLoS ONE 11, e0160034 (2016).
Yang, M. et al. Treatment with hydrogen sulfide donor attenuates bone loss induced by modeled microgravity. Can. J. Physiol. Pharmacol. 97, 655–660 (2019).
Yang, J. et al. Blocking glucocorticoid signaling in osteoblasts and osteocytes prevents mechanical unloading-induced cortical bone loss. Bone 130, 115108 (2020).
Ding, Y. et al. Anti-RANKL monoclonal antibody and bortezomib prevent mechanical unloading-induced bone loss. J. Bone Miner. Metab. 39, 1–10 (2021).
Dai, Z. et al. Actin microfilament mediates osteoblast Cbfa1 responsiveness to BMP2 under simulated microgravity. PLoS ONE 8, e63661 (2013).
Saxena, R., Pan, G. & McDonald, J. M. Osteoblast and osteoclast differentiation in modeled microgravity. Ann. N. Y. Acad. Sci. 1116, 494–498 (2007).
Hu, L. F., Li, J. B., Qian, A. R., Wang, F. & Shang, P. Mineralization initiation of MC3T3-E1 preosteoblast is suppressed under simulated microgravity condition. Cell Biol. Int. 39, 364–372 (2015).
Author information
Authors and Affiliations
Contributions
J.M.: conception and design of the project or output, analysis or interpretation of research data, and drafting or critically revising significant parts of the manuscript; T.G.: drafting or critically revising significant parts of the manuscript; G.S.-D.: drafting parts of the manuscript; A.L.: conception and design of the project or output, drafting or critically revising significant parts of the manuscript, and acquirement of funding.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Man, J., Graham, T., Squires-Donelly, G. et al. The effects of microgravity on bone structure and function. npj Microgravity 8, 9 (2022). https://doi.org/10.1038/s41526-022-00194-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41526-022-00194-8
This article is cited by
-
Acute and short-term fluctuations in gravity are associated with changes in circulatory plasma protein levels
npj Microgravity (2024)
-
Rescuing SERCA2 pump deficiency improves bone mechano-responsiveness in type 2 diabetes by shaping osteocyte calcium dynamics
Nature Communications (2024)
-
Cordymin alleviates osteoporosis induced by hindlimb unloading via regulating the gut - microelements -bone axis --for non-clinical studies
BMC Musculoskeletal Disorders (2023)
-
Microgravity triggers ferroptosis and accelerates senescence in the MG-63 cell model of osteoblastic cells
npj Microgravity (2023)
-
Mechanical stimulation controls osteoclast function through the regulation of Ca2+-activated Cl− channel Anoctamin 1
Communications Biology (2023)