Abstract
The advent of immune-checkpoint inhibitors (ICI) in modern oncology has significantly improved survival in several cancer settings. A subgroup of women with breast cancer (BC) has immunogenic infiltration of lymphocytes with expression of programmed death-ligand 1 (PD-L1). These patients may potentially benefit from ICI targeting the programmed death 1 (PD-1)/PD-L1 signaling axis. The use of tumor-infiltrating lymphocytes (TILs) as predictive and prognostic biomarkers has been under intense examination. Emerging data suggest that TILs are associated with response to both cytotoxic treatments and immunotherapy, particularly for patients with triple-negative BC. In this review from The International Immuno-Oncology Biomarker Working Group, we discuss (a) the biological understanding of TILs, (b) their analytical and clinical validity and efforts toward the clinical utility in BC, and (c) the current status of PD-L1 and TIL testing across different continents, including experiences from low-to-middle-income countries, incorporating also the view of a patient advocate. This information will help set the stage for future approaches to optimize the understanding and clinical utilization of TIL analysis in patients with BC.
Similar content being viewed by others
Introduction
The use of immune-checkpoint blockade (ICI) in clinical oncology has revolutionized patient care and improved survival outcomes in many patients with malignancies1. This therapeutic strategy has significantly expanded in the setting of advanced and early-stage breast cancer (BC), but much more work is needed to optimize patient selection based on tumor-based biomarkers. The presence of tumor-infiltrating lymphocytes (TILs) is believed to be predictive of response to immunotherapy, chemotherapy, and other targeted therapies2,3 in addition to their role as a prognostic biomarker4,5. Moreover, TILs in the tumor and the surrounding microenvironment are thought to reflect ongoing anti-tumor host immune response. Three main categories of the tumor microenvironment (TME) have been defined across different tumor types: immune-desert (“cold” tumors largely devoid of lymphocytes), immune-excluded (lymphocytes are present in the peritumoral stroma only), and immune-infiltrated/inflamed (“hot” tumors)6,7. Conceptually, each of these TME categories reflects a specific interaction between the tumor genotype/phenotype and the host immune system, which can impact the response to both conventional anticancer therapies and ICI8. However, there is considerable heterogeneity within each TME category, adding uncertainty to the reproducibility of the current classification of “cold” vs. “hot” vs. “intermediate” immune-subtypes. Also, there are no validated criteria to define these subtypes, either using morphology, immunostaining, transcriptomics, or their combination, limiting the impact of these descriptors in clinical trials and daily practice.
In BC, the molecular subtype of the tumor has a major influence on its interaction with the immune system. Triple-negative BC (TNBC) and HER2-positive BC are more frequently infiltrated by higher numbers of TILs than hormone receptor (HR)-positive tumors9,10. However, all BC subtypes include cases with TIL-infiltration. The degree of TIL infiltration has been hypothesized to reflect the tumor mutational burden (TMB), which is lower in HR-positive BC11,12. Higher TMB is linked to the expression of more neoantigens and has been shown to predict survival after ICI therapy in several cancer types, and recent evidence indicates this may also be the case for TNBC13,14,15. However, the correlation between mutational burden and immune composition is complex, with the degree and nature of clonality of the mutations playing a key role in determining whether they favor or hinder immune-mediated tumor control16. In TNBC, higher TMB and greater genomic heterogeneity have been associated with lower TILs17. Conceptually, this can be explained by immunoediting, which is a result of a selection of cancer cell clones with decreased immunogenicity despite the presence of many mutations18. This escape from immune surveillance is associated with a reduced TIL component and increased tumor clonal heterogeneity, explaining the negative association between TMB and TILs19,20,21.
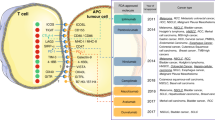
In TNBC, scoring TILs in the stromal compartment (sTILs) is demonstrably reproducible and generally well-correlated with intra-epithelial TILs, with higher stromal TILs (sTILs) predicting longer survival9,22,23,24. Generally, intra-epithelial TIL density tends to be lower than stromal TIL density23, raising the question of whether a tumor nest-stromal barrier precludes more robust T cell infiltration25, and/or whether the intra-epithelial TILs have always been present (in an inactive state), as tissue-resident T-cells. CD8+ tissue-resident memory T (TRM) cells were shown to mediate BC immunosurveillance26. Notably, high BC infiltration by TILs contained CD8+ T lymphocytes with TRM patterns which highly express immune-checkpoint molecules26.
Tertiary lymphoid structures (TLS) are lymph node-like structures that arise in tissues at sites of chronic inflammation27. TLS has been detected in the stroma of up to 60% of BC, with the highest frequencies in HER2+ and TNBC7,28,29. These findings support a critical role for the stroma in shaping the TME of BC. For example, fibroblastic reticular cells, concentrated in the T cell zone of TLS, promote, maintain or suppress T cell activities via their cytokine and chemokine secretion30. TLS architecture is distinguished by a T cell zone adjacent to a B cell follicle, similar to secondary lymphoid organs27. Immune responses generated in tumor-associated TLS would thus produce immunological memory to multiple BC neoantigens and could potentially control the growth of disseminated tumor cells31,32,33,34. However, there are significant concerns that TLS cannot be assessed in a reproducible manner by analysis of HE-stained slides, and that B- and T-cell immunostains are needed34. In addition, TLS is frequently found at the tumor perimeter, often contain high endothelial venules, and when present in the tumor area are generally considered as an aggregate when quantifying TILs in BC. Their role in BC still remains to be addressed.
The importance of expanding our current understanding of the complex TME in breast and other cancers has driven the development of diverse techniques, including molecular multi-omics profiling35 coupled with computational deconvolution of immune cell populations36,37, global and single-cell transcriptomics38,39, and multiplex imaging40, to quantify TIL distribution, functional orientation and relative frequencies in individual tumor types. However, these cutting-edge investigational tools are often limited by reproducibility across studies, particularly when attempting to resolve specific immune cell subtypes. In addition, tumors with low TILs seem to pose a significant challenge to any of these techniques and platforms41. Machine learning techniques are in development to evaluate TIL distribution patterns and integrate the spatial information with sTILs and with molecular profiling data42,43. A study examining clonal heterogeneity, TMB, copy number variations, somatic mutations, and germline polymorphisms, as well as the neoantigen load for their association with immune metagene expression in the BC subtypes, did not find any distinct recurrent single gene or pathway level mutations associated with immune infiltration21. However, lower clonal heterogeneity was observed in TNBC and HER2+ BC associated with higher immune gene expression, which is consistent with immunoediting21. There is also evidence for immune escape during the in situ to invasive BC transition, with a decrease in immune activation measurable using a combination of global profiling and single-cell transcriptomics44. It may be hypothesized that similar immune evasion mechanisms also occur during the transition from stage I TNBC to more advanced stages of the disease. This paradigm was also recently reported in lung cancer in which immune-evasion seems to be triggered by neoantigen editing during tumor evolution45. In the remainder of this review, the analytical and clinical impact of sTILs in BC management will be discussed based on recent updates from human studies, particularly clinical trials.
Updates on the analytical and clinical validity of TILs
Challenges in establishing the analytical validity of TILs
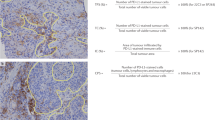
Analytical validity is defined as how accurately a test predicts the presence or absence of a biological variable. In other words, can the “test” correctly distinguish between TILs and other immune cells?. Previous TIL inter-pathologists-reproducibility studies (RING-studies) have shown that pathologists (using an H&E slide) can reliably assess TILs with very high concordance between many pathologists on a powered number of cases and at different cut-offs24. There is overwhelming evidence for the clinical validity of this evaluation46,47. However, analytical validity cannot be formally demonstrated due to the lack of a “gold standard” against which to assess the proposed method. This may not be that important for the clinical implementation of sTILs. However, for machine learning approaches this question is crucial. Comparing two methods, for example, a pathologist vs. an automated image-analysis system provides evidence on concordance, but not accuracy43. All the previous TIL-RING studies have been performed with the assumption of histological accuracy46,47.
In order to define accuracy in the context of assessing TILs using an H&E-stained slide, it is necessary to define a “gold standard” against which to assess the proposed method, i.e., an orthogonal-type method which is used as the “gold standard”. For example, a pan-lymphocyte marker can establish the accuracy of lymphocyte identification, answering “how often is what a pathologist calls a TIL actually a TIL?”. This is crucial when applying machine learning methods to identify TILs42, since the unequivocal differentiation of a myofibroblast, an invasive lobular carcinoma (ILC) cell, and lymphocyte is not always possible on an H&E slide. Moreover, the concordance between TIL assessment by machine learning-based methods and pathologist-TIL scores is unlikely to be 100%48. Currently, a TIL is defined as being a lymphocyte or a plasma cell, and both are scored and defined according to classic morphological definitions. Then, we need to define a “stromal TIL” (sTIL), i.e., what is the maximal distance between a tumor cell and a TIL to define it as an sTIL. Furthermore, uncertainty amongst pathologists exists about the inclusion of stroma abutting the tumor, or all of the stroma within the total tumoral area to include intervening stroma with low/very low sTILs. Indeed, accumulating evidence suggests that sTILs touching the tumor cells may have a different molecular phenotype than sTILs distant to the main tumor bulk25. Kos et al.24 have recently reported further factors that may impact the assessment of TILs including pre- and post-analytical histology factors, particularly in the setting of retrospective analysis of trial material, and the recognition of common artifacts. In an effort to improve concordance, the International Immuno-Oncology Biomarker Working Group, also called the TILs Working Group (https://www.tilsinbreastcancer.org/pitfalls/) recently provided reference images and digital slides as well as accessible guidance regarding the analysis of heterogeneous immune cell infiltrates.
The importance to assess the clinical validity of TILs in the context of clinical utility
Clinical validity refers to the presence of sufficient evidence, usually level 1 evidence of the effect of a test (biomarker) to demonstrate its validity in a clinical setting. That is, there is robust statistically validated evidence that the test (biomarker) relates to a clinical outcome (prognostic or predictive) or a specific phenotype (TILs), etc. However clinical validity alone, whilst required for changes in clinical practice, does not drive a change in practice. For this to occur, the test must have clinical utility. Clinical utility essentially requires that the test in question addresses a direct clinical need (prediction of response to therapy, prognosis, or diagnostic classification of subtypes) and will when implemented, impact patient management. Simply put, a test only has clinical utility when it impacts physician and patient choice on treatment or management options. The level 1 requirement for clinical validity clearly differs according to the setting49. However, it is clearly not optimal to assess the clinical validity of TILs without first framing the question of clinical utility correctly. Once the correct clinical question is framed, appropriate studies must be designed to generate evidence to support the clinical validity of TILs in the setting of clinical utility. In this respect, we agree with Simon et al. that prospective trials not originally designed to address tissue biomarker studies can be used to “accommodate biomarker utility” using archived samples49. Since only simple tools, like a microscope, are needed for the assessment of TILs, this provides tremendous opportunities to test the “clinical utility” of TILs in various settings.
The clinical validity and utility of TILs
TILs in TNBC
Level 1 evidence for a biomarker49 can either be reached by incorporating a biomarker into a properly powered prospective clinical trial (level 1A) or by achieving reproducible results in archived tissues from independent randomized trials, designed, conducted, and analyzed as per REMARK criteria (level 1B)50. Using these widely accepted criteria, level 1B evidence for clinical validity of TILs as a prognostic biomarker in early-stage TNBC is well established9,22. Two pooled analyses of TILs, in the adjuvant setting for TNBC22, and in the neoadjuvant setting across BC-subtypes9, included studies that have evaluated TILs on archived tissue samples based on our published guidelines23.
In a pooled analysis (n = 2148), the clinical value of TILs in predicting prognosis of early-stage TNBC, including adjuvant trials of anthracycline-based chemotherapy alone or in combination with taxanes was investigated22. The average age of enrolled patients was 50 years, and 33% of them were lymph node-negative. The quantification of TILs showed that their average was 23%, and 77% of patients had at least 1% sTILs. Notably, sTILs were found to be significantly reduced with advanced age, larger tumor size, more positive lymph nodes, and lower histological grade. In the multivariable Cox regression model, sTILs were an independent prognostic predictor for all endpoints; each 10% increment in sTILs corresponding to an invasive disease-free survival (DFS) hazard ratio (HR) of 0.87 (95% CI: 0.83–0.91), a distant DFS HR of 0.83 (95% CI: 0.79–0.88) and an overall survival (OS) HR of 0.84 (95% CI: 0.79–0.89) (p < 10−6)22. Histological grade was not a prognostic factor in this study. The second pooled analysis included women with primary BC treated with neoadjuvant chemotherapy (NACT) in six randomized trials conducted by the German Breast Cancer Group9. It assessed the predictive value of sTILs for chemotherapy response and prognostic estimation in patients with TNBC, HER2-positive, and luminal A/B–HER2-negative BC. sTILs quantified in diagnostic core biopsies from 3771 patients were associated with pathologic complete response (pCR) after NACT across all BC subgroups. For instance, based on three predefined groups of low (0–10% immune cells in peritumoral stromal tissue), intermediate (11–59%), and high TILs (≥60%), pCR was achieved in 31% (80/260) of TNBC patients with low TILs, 31% (117/373) of TNBC patients with intermediate TILs, and 50% (136/273) of TNBC patients with high TILs (p < 0.0001). OS was analyzed in 2560 patients across all BC subtypes from five of the six neoadjuvant clinical trial cohorts. However, increased sTILs were associated with longer OS only in TNBC (HR: 0.92; CI: 0.86–0.99, p = 0.032).
Recently, Park et al.51 investigated the prognostic impact of sTILs in early-stage TNBC based on four multicenter cohorts (476 patients) who were not treated with (neo)adjuvant chemotherapy. The presence of sTILs at baseline was correlated with several clinical endpoints including OS. Multivariate analyses demonstrated that sTILs are an independent prognostic biomarker of OS (p = 0.015), invasive DFS and distant DFS for TNBC (p < 0.001 for both)51. A 10% increase in sTILs also positively correlated with OS (HR: 0.88; 95% CI: 0.79–0.98), invasive DFS (HR: 0.90; 95% CI: 0.82–0.97) and distant DFS (HR: 0.86; 95% CI: 0.77–0.95). In a subgroup of TNBC patients with stage I tumors and sTILs ≥ 30%, excellent 5-year survival outcomes were reached including 98% 5-year OS51. Indeed, the expert opinion at the 16th St. Gallen International Breast Cancer Conference has endorsed the routine reporting of sTILs in TNBC as a prognostic factor52 although guidelines have not yet recommended de-escalation of standard systemic therapy according to TILs. The WHO Classification of Tumors: Breast Tumors, 5th Edition has also endorsed histopathological TIL quantification in TNBC and HER2-positive BCs, expressed as a mean percentage of lymphoplasmacytic infiltration of the tumor stroma53.
The 5th Edition of the WHO classification also re-classifies medullary carcinoma, in which prominent TILs have long been recognized, into invasive breast carcinoma of no special type with medullary pattern53. These carcinomas have been characterized as showing high immune-related gene54 expression and it is likely that the high TILs component of these tumors contributes significantly to their favorable clinical outcomes55,56. The role of TILs in the histological spectrum of TNBC is yet to be fully elucidated although early data on metaplastic carcinoma suggest that TILs may have prognostic relevance57,58. The role of TILs in TNBC subtypes recognized as low grade and with good prognosis histological features, such as adenoid cystic carcinoma of the breast59, is at present unknown. The importance of histological grade in TNBC is likely to be of utility in identifying the so-called low-grade TNBC, including the rarer subtypes (for example adenoid cystic carcinoma), that have low TILs but an excellent outcome compared to the lack of clinical utility and prognostic significance of histological grading alone in TNBC NOS.
TILs and PD-L1 in triple-negative BC
The phase 2 double-blind placebo-controlled GeparNuevo study (NCT02685059), randomized 174 early stage TNBC patients to receive durvalumab (a PD-L1 inhibitor) combined with standard NACT and used sTILs as a biomarker for patient stratification during randomization60. This proof-of-concept study showed that patients with higher sTILs in both arms of the trial had significantly improved pCR rates (p < 0.01), although the sTILs were not specifically related to durvalumab-response. A recent translational analysis of this trial demonstrated that both continuous TMB and TILs were independent predictors of pCR61. Patients with high TMB had excellent pCR rates of 82% (95% CI: 60–95%) highlighting the potential of this emerging biomarker in tailoring therapy in this setting61. The German phase II trial (NCT03289819) compares neoadjuvant pembrolizumab combined with nab-paclitaxel vs. pembrolizumab with epirubicin and cyclophosphamide in patients with early TNBC will also investigate sTILs at baseline, in addition to other potential biomarkers such as mutational load and microbiota. In this setting, the recently released findings of IMpassion03162, KEYNOTE-52263, and I-SPY264 studies suggest that response to ICIs is independent of PD-L1 status. Thus, other biomarkers of response are needed to predict outcomes in TNBC patients treated with NACT and immune-checkpoint blockade.
In metastatic or locoregionally advanced TNBC, several immunotherapy trials have demonstrated the value of immune cell infiltrate in estimating survival outcomes including phase I (NCT01375842)65, phase II KEYNOTE-086 (NCT02447003)66,67, and phase III IMpassion130 (NCT02425891)68 clinical trials. TILs were used to assess the activity of atezolizumab as monotherapy in a phase Ia expansion cohort in metastatic TNBC69. Patients’ stratification based on TILs at baseline indicated that median OS was improved in those with >10% of sTILs cut-off (12.6 vs. 6.6 months, p = 0.0028)69. In the phase III study, Schmid et al. showed that the addition of atezolizumab to nab-paclitaxel improved progression-free survival (PFS) in TNBC as compared with the addition of placebo to nab-paclitaxel, particularly in those with PD-L1 positive tumors. Median OS in this subgroup was consistently improved in both interim analyses68,70, but no formal statistical testing of OS in the PD-L1 positive subgroup could be performed due to the absence of OS benefit for the entire study population70. Hence, the FDA approval for this particular drug and assay was based on PFS, not on OS. The available tumor samples from this study were also re-assessed for the analytical concordance of various assays for PD-L1 immunohistochemistry (IHC) and the clinical utility of these assays71. This post-hoc analysis demonstrated that all subgroup results based on different PD-L1-based assays were suggestive of some clinical benefit (with SP142 immunopositivity apparently indicating most clinical benefit), with different HR and low concordance between the immunohistochemical assays used (SP142 vs. 22C3 vs. SP263)71,72. This analysis also raises confusion as to whether HR or underpowered subgroup-analyses combining different antibodies, should be used to make claims on the performance of assays or not; taking into consideration that only a biomarker-treatment interaction analysis, in the biomarker positive vs biomarker negative population and only in powered subgroup-analysis, can inform the performance of assays. It was also shown that when >20% of sTILs were present, nearly all patients had a positive PD-L1 essay, irrespective of the assay used. This indicates that sTILs are important drivers of response and that sTILs could help mitigate the well-known assay- and reproducibility issues associated with PD-L1 assessment71,72. In fact, numerically higher counts of sTILs were noted in TNBC patients with a positive FDA-approved SP142 assay71. Furthermore, in the recent biomarker-analysis of Impassion13073, it was shown that TILs predict benefit to atezolizumab for any PD-L1-expression.
KEYNOTE-086 enrolled 170 patients with advanced TNBC who were treated with at least one prior line of therapy and showed a 5% response rate (RR) and 20% stable disease of the subgroup with PD-L1 positive tumors (based on the 22C3 pharmDx assay) when treated with pembrolizumab monotherapy66. Similarly, the cohort of the trial treated with first-line pembrolizumab displayed a durable response in patients with positive-PD-L1 (21.4%; 95% CI: 13.9–31.4)67. These findings confirm the previously noted improved outcomes in this setting with higher TIL levels74. In fact, higher sTILs were associated with significantly increased ORR (OR: 1.26, 95% CI: 1.03–1.55, p = 0.01) and disease control rates (OR: 1.22, 95% CI: 1.02–1.46; p = 0.01). PD-L1 expression was also significantly correlated with the levels of sTILs (p < 0.001) in metastatic TNBC treated with pembrolizumab74.
A recent advance resulted from the KEYNOTE-119 phase III trial (NCT02555657) in which 622 previously treated metastatic TNBC patients were randomized to receive pembrolizumab as monotherapy or cytotoxic chemotherapy13. This study did not demonstrate significantly prolonged OS in the overall cohort nor in the PD-L1-positive subgroup (combined positive score -CPS- ≥1 or ≥10)13. Exploratory analysis revealed that a marked increase in the efficacy of pembrolizumab was seen when the cut-off for a combined positive score was increased to ≥20. In this study, TILs were then evaluated according to our established guidelines23. In the pembrolizumab arm, high distribution of TILs was observed in responders with better survival outcomes, an effect not observed in the chemotherapy arm75. Since sTILs were not prespecified in the trial protocol, sTILs were not considered “regulatory-proof”, despite the OS benefit demonstrated in this phase 3 setting. TILs and combined positive scores were moderately correlated and were independent predictors of outcome in patients treated with pembrolizumab. More recently, KEYNOTE-355 (NCT02819518) randomized 847 women with metastatic TNBC to receive pembrolizumab in combination with chemotherapy vs. chemotherapy plus placebo76. This demonstrated that patients with CPS ≥10 treated in the immunotherapy arm had improved median PFS as compared to the placebo group (9.7 vs. 5.6 months, HR: 0.65, 95% CI: 0.49–0.86; p = 0.0012) but not in those with CPS of 1 or more (7.6 vs. 5.6 months (HR: 0.74, 95% 0.61–0.90; one-sided p value not significant)76.
Nevertheless, biomarker analyses of TILs in such studies are all exploratory. These findings should, therefore, ideally be validated in independent prospective studies that use suitably powered phase III randomized and controlled trial design to accelerate the clinical validation of TIL-guided therapy and to provide Level 1A evidence. We strongly propose pre-specifying the use of TILs as an integral biomarker in future trial protocols. However, the FDA has recently approved pembrolizumab for adults and children with TMB-H solid tumors. Treatment efficacy was studied in a prospectively planned retrospective analysis of 10 cohorts of patients with solid malignancies with unresectable or metastatic TMB-H tumors. These patients were enrolled in a multicenter, non-randomized, open-label clinical trial (KEYNOTE-158; NCT02628067). This approval raises interesting questions, for example: is the old paradigm for obtaining level 1A evidence for biomarkers becoming obsolete or is it simply underused?. Are prospective-retrospective analyses for biomarkers that are predictive for immune therapy and indicate OS, with the level of evidence IB, and with proven analytical and clinical validity (like TILs) sufficient for regulatory approval as a predictive marker for selection of patients for immune checkpoint inhibition? Will level 1B evidence drive clinical change? Regulatory agencies approve assays and drugs, but the scientific community still must apply that knowledge in a clinical context based on a synthesis of all evidence. Is it the role of regulatory agencies to approve clinical practice changes or should this be the role of the scientific community, in a partnership with industry and the regulatory agencies?
TILs in ER-positive BC
Although a high percentage of sTILs in primary TNBC and HER2-positive BCs suggests a favorable prognosis, the significance in estrogen receptor (ER)-positive, HER2-negative tumors remains uncertain. This is partly because there has been less focus on ER-positive carcinomas, which are traditionally considered to demonstrate lower immunogenicity9,77,78. For example, the mean TILs count is significantly lower than in TNBC and HER2-positive BC9,79,80. The average TMB, which frequently correlates with neoantigen load, is also lower in these tumors81. Small studies of single-agent immune checkpoint blockade have yielded low responses in patients with pretreated metastatic ER-positive cancer82,83. Nevertheless, there is marked heterogeneity for TILs infiltration and mutational burden seen in ER-positive BCs, with a considerable proportion above the mean observed in triple-negative tumors77. The importance of TILs in ER+/HER2-ve BC can be most easily demonstrated by combining two aspects of BC; firstly, recent population-based surveys of over 350,000 BCs in the UK and USA suggest that 73% of newly diagnosed BCs are ER+/HER2−84,85. Given that TILs have been detected in the stroma of up to 60% of BC7,28,29 the majority (over half) of all BCs with TILs must therefore be in the ER+/HER2- subgroup. Put simply, the population of ER+/HER2− BCs with TILs exceeds the combined population of HER2+/TNBCs with this feature. This demonstrates the large unmet potential for exploiting immune targeted agents in ER+/HER2− patients.
Several studies have examined the impact of TIL levels on patient prognosis in primary ER-positive BC, and five of the largest studies are summarized in Table 19,78,86,87,88. These studies retrospectively examined histological slides either from randomized controlled trials or annotated clinical cohorts. However, the material varied considerably (tissue microarray vs. core biopsy vs full face section) as did the TILs analysis methodology (H&E staining vs. IHC) and the TILs quantification statistical methods (continuous vs categorical variable). With these caveats in mind, the most notable finding from these analyses is that no study demonstrated a favorable impact of TILs on disease-free, BC-specific, or OS in ER-positive BC. In some analyses, TILs were associated with an unfavorable prognosis. For example, in a recent meta-analysis of six NACT trials, higher TILs levels were associated with a significant reduction in OS9. Furthermore, two studies analyzing mixed trial and/or clinical cohorts (of almost 6000 and 1400 ER-positive tumors, respectively) reported that higher levels of intratumoral CD8+ TILs were associated with a significant reduction in BC-specific survival80,86.
These observations must be interpreted with caution given that the molecular subtype of ER+ BC is a major confounder in all these analyses. With the introduction of PAM-50 assays in the last decade, ER-positive cancers have been divided into luminal A and luminal B tumors—the latter characterized by higher proliferation and reduced dependence on endocrine signaling89. Luminal A tumors have both lower TIL infiltration and TMB than luminal B tumors79 and luminal B cancers are associated with worse clinical outcomes90. In keeping with this, the significant associations between CD8+ TIL infiltration and worse prognosis in the studies described above were lost after adjusting for tumor grade. Similarly, the significant reduction in OS observed in the ER+/HER2− cancers in the BIG 02-98 analysis was not upheld in a multivariate analysis adjusting for other prognostic features78.
ILC is the second most common histological subtype of BC and is frequently ER+/HER2−. Compared to IDC, fewer data are currently available on the immune microenvironment in ILC91. Nevertheless, ILC has been shown to harbor lower TIL levels when compared to IDC92,93, although gene expression profiling has indicated that the transcriptomic immune response signatures may in fact be upregulated in ILC94. High TIL ILC has been associated with poor prognostic factors and, to date, emergent data suggest that high TILs are suggestive of less favorable clinical outcomes in ILC95,96. Indeed, it has been suggested that high TILs may drive aromatase inhibitor resistance which may in part account for some of the ILC-TIL signal identified97,98. However, the role of TILs in ILC is still unsolved, and more and larger, preferably pooled studies are needed to define the importance of TILs in this specific subtype.
The data on TIL levels and benefits from anti-estrogen endocrine therapy is even sparser. Sobral-Leite and colleagues evaluated CD8 expression in over 500 ER-positive BC patients who were randomized between no adjuvant endocrine treatments or one or three years of tamoxifen88. Patients with high CD8 infiltration in the tumor had a significant unfavorable prognosis; however, this effect was seen regardless of tamoxifen treatment. In addition, a study of 79 patients demonstrated a smaller reduction in tumor cell proliferation after two weeks of neoadjuvant aromatase inhibitor treatment in tumors with a significant lymphocytic infiltrate91. Another analysis from a prospective study of neoadjuvant letrozole ± lapatinib (n = 73) showed that significant Ki-67 reduction was observed in both patients with high and low baseline TILs, with high TILs tumors achieving more frequently a relative Ki-67 suppression >50% (55% vs. 35% of patients with low TILs)99. The long-term clinical significance of these observations remains unclear. There is also very limited data regarding the impact of targeted systemic therapy on TILs infiltration in ER-positive BC. Recently, CDK4/6 inhibitors have been reported to enhance antitumor immune responses associated with the upregulation of interferon-driven gene expression signatures100. However, this has not been associated with an increased TILs level in tumors101.
At present, there is no role for routine assessment of TILs in primary ER-positive BC, and their presence cannot be used to guide prognosis nor as a predictive biomarker. We propose that new studies should take a uniform approach to the assessment of TILs, such as those outlined in recent guidelines23,102 and should report data for luminal A and B tumors separately, In addition, a more refined understanding of the ER-positive BC immune environment, including the significance of immunosuppressive regulatory T cells, the spatial distribution of lymphocytes, and the role of estrogenic signaling will be required to define meaningful immune biomarkers in ER-positive disease103,104,105. Notably, a recent retrospective analysis (n = 563) of the multicenter IKA trial that randomized stage 1–3 ER-positive BC patients to receive tamoxifen or no adjuvant therapy showed that CD8-positive sTILs were significantly higher in patients with PIK3CA-mutated tumors (OR: 1.65; 95% CI: 1.03–2.68)88. In addition, this population of BC patients had an increased risk of recurrence (HR: 1.98; 95% CI: 1.14–3.41) on multivariate analysis as compared to those with CD4 and FOXP3 sTILs that were not statistically associated with prognostic outcomes88. This enrichment of TILs in tumors with mutated PIK3CAand their association with outcomes provided additional evidence on the crosstalk between mutational status and TME.
TILs in HER2-positive BC
Patients with HER2-positive early BC have a higher infiltration of TILs and improved pCR with NACT and trastuzumab106,107. Moreover, improved event-free survival in primary disease treated with trastuzumab and lapatinib was also noticed107. The predictive and prognostic value of TILs as a continuous variable in HER2-positive BC in the neoadjuvant setting was demonstrated in another analysis (n = 498) that evaluated TILs from two trials (GeparQuattro and GeparQuinto)108. In the group of patients with lymphocyte-predominant (≥60% sTILs) phenotype, a marked increase of pCR rates was noted108. Moreover, an increment of TILs by 10% was found to be an independent predictor of pCR in multivariate analysis (p = 0.014)108. Notably, this effect was more relevant in patients with ER+, PR+, and HER2+ tumors108. In the N9831 phase III trial (NCT00005970), tumor samples from patients with early HER2-positive BC were collected at baseline for TILs quantification in deciles with a prespecified categorical cutoff of ≥60%109. TILs were associated with recurrence-free survival in the cohort treated in the control arm with chemotherapy alone. Unexpectedly, higher TILs predicted a lack of response to trastuzumab in HER2 positive BC patients109. However, an immune gene-expression analysis on the same dataset did show a strong association with benefit110. A recent meta-analysis of 5 randomized and controlled trials that pooled data from 1256 patients provided additional evidence of the prognostic impact of TILs in the neoadjuvant setting111. Higher TILs at baseline were predictive of pCR irrespective of the neoadjuvant treatments used in HER2 positive BC111.
Recently, supporting evidence of the association of sTILs with distant DFS in this setting has emerged from the Short-HER phase III randomized study that compared different adjuvant regimens combined with trastuzumab (9 weeks vs. 12 months) (NCT00629278). On multivariate analysis, TILs predicted improved distant DFS for each 10% increment (HR: 0.73, 95% CI: 0.59–0.89, p = 0.006)112. At five years of follow-up, distant DFS rates were significantly higher in patients with TILs ≥ 20% (p = 0.025)112. Importantly, the 10% TIL increments were significantly associated with distant DFS only in patients randomized to receive nine weeks trastuzumab-based regimen (HR: 0.60, 95% CI: 0.41–0.88; p = 0.009)112.
In the National Surgical Adjuvant Breast and Bowel Project B-31 stromal TILs were also assessed47. Not only was the concordance between 6 different pathologists 90.8% when evaluated on a set of 100 representative cases from this trial, but sTILs were associated also with improved DFS. Higher sTILs were found associated with a higher response to trastuzumab-based on the 8-gene prediction model (p < 0.001)47. The association between TILs and prognosis in the advanced HER2-positive setting is less well established. A recent analysis of the pre-treatment samples from the CLEOPATRA study population revealed that higher quantities of TILs predicted an improved survival benefit in first-line treatment with taxane-based chemotherapy, trastuzumab, and pertuzumab113. Conversely, analysis of the samples from MA.31, a randomized clinical trial of lapatinib or trastuzumab in combination with taxane-based chemotherapy, reported that 35% of the population were categorized as high TIL but that this did not show significant prognostic or predictive effects114. It is worthwhile noting that the cutoff used for high TILs was 5%, much lower than the 20% cutoff used in the binary component of the CLEOPATRA analysis. MA.31 assessed TILs also on the primary tumor samples and found a mean of 9.2%; median, 5%; IQR, 1–10%. Provision of tumor tissue from a metastatic site pre and post-study treatment was an optional subsidy with low uptake—hence metastatic samples were not included in the TIL analysis. Conversely, the CLEOPATRA TILs analysis used samples of either the primary (93%) or metastatic (7%) sites and found the mean stromal TILs value was 21.07% and the median stromal TILs value was 10% (IQR 5–30). Although only a small number of metastatic samples were analyzed, the TILs values of samples from lung and lymph nodes were significantly higher than those from primary tumor, liver, bone, or skin. Furthermore, in the PANACEA-trial115, combined TILs and PD-L1 predicted benefit for pembrolizumab combined with trastuzumab in trastuzumab-resistant advanced HER2 positive BC. Whilst the early TNBC studies used a similar cutoff for high TIL groups, more recent analyses using TIL as a continuous variable has shown a linear association with survival outcomes. The ideal threshold for high TILs remains unclear in advanced HER2-positive disease, but variation in cut-offs may account for the conflicting results seen thus far114.
The incorporation of TILs into routine clinical practice and clinical trial design
It should be emphasized that formally speaking, the clinical utility of sTILs is not proven since no prospectively designed phase 3 TILs-driven trials have yet been performed, and evidence is lacking that treatment decisions based on sTILs favorably affect patient outcomes. Therefore, the TILs Working Group does not currently recommend the use of sTILs in isolation to guide treatment decisions in clinical practice. However, sTILs could be used to help identify patients with an excellent outcome to study de-escalation approaches and thereby reduce toxicity. In addition, based on the data of Luen et al., escalation of treatment regimens may be clinically beneficial for TNBC patients with a high post-neoadjuvant residual disease burden and low sTILs due to the poor prognosis116. Along those lines, the retrospective evaluation of sTILs in trials that have evaluated adjuvant treatments for those patients who did not reach a pCR, such as the CREATE-X trial—is planned117. In practice, some clinicians are using TILs in conjunction with other clinical variables to decide on the type and duration of cytotoxic chemotherapy. It may be emphasized that for most of the prognostic factors currently used for decades in BC daily practices, there is no level of 1A-evidence. To achieve clinical utility in TNBC, TILs must be included as pre-specified biomarkers in clinical trials and/or prospectively/retrospectively evaluated in randomized trials.
To successfully integrate TILs as a stratification factor in clinical trials, it is necessary to develop digital pathology tools to ensure accurate and rapid sTILs scoring by independent pathologists. Web-based platforms that allow easy scanning, viewing, and scoring of H&E slides should be optimized. A risk-based framework, aiming to reduce variability in TIL assessment within a clinical trial was used in the first stage of an adaptive non-comparative phase II trial (TONIC-trial)118. A total of 67 patients with metastatic TNBC were randomized to nivolumab (anti-PD-1) without induction or to one of four induction treatments, consisting of irradiation of one metastatic lesion (3 × 8 Gy) or a 2-week low-dose regimen of cyclophosphamide, cisplatin, or doxorubicin, all followed by nivolumab (NCT02499367)118. Concordance values between four pathologists were >90% in this trial, showing the applicability of this concept in real-world trial-settings119. The added value of web based-tools is that the biomarker scores of local pathologists can be integrated into the flow of a clinical trial, thereby enhancing local pathologists’ compliance and experience within a trial, bridging trial- with daily pathology practices. This is in contrast to the current situation, where the tissue may be sent to a central laboratory with limited to no involvement of local pathologists. Is the current trial-paradigm of “single-reference laboratory-approach” therefore in need of revision?
PD-L1 testing and TILs in worldwide settings for BC
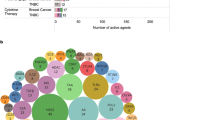
Access to novel therapeutic agents, including ICIs in low- and middle-income countries needs global support and importantly, standardization of testing. The “interchangeability of PD-L1 antibodies”, with a recent ESMO presentation showing all PD-L1 antibodies predict some benefit in TNBC for immunotherapy71 is still debated, illustrating the complexity and confusion that exists on these assays in the scientific community. In addition, the TILs Working Group, in a partnership with the College of American Pathologists, the European Society of Pathology, the Latin American Society of Pathology, and the European Working Group of Breast Screening Pathology argued how the current assay-approval policies, exemplified by the current PD-L1-assay situation, are leading to (unintended) imprecision medicine, which is not in the best interest of our patients. Hence, this pathology partnership proposed concrete solutions to improve the current assay approval pathway120. A framework for better evaluation of risks associated with suboptimal patient selection and stratification for ICI-based treatments in future clinical trials and real-world settings based on TILs/PD-L1 is proposed by the TILs Working Group72. The use of TILs as a predictive biomarker of response to ICI’s may considerably support cancer care in countries that have difficulty with PD-L1 implementation, mainly related to costs. Many ongoing clinical trials will report data that are expected to support TILs as a valid biomarker for response (see Table 2).
In Europe
In the United Kingdom (UK), the National Institute for Health and Care Excellence (NICE) reported that atezolizumab combined with nab-paclitaxel (Abraxane®) does not have plausible potential to be cost-effective in advanced TNBC. Nevertheless, PD-L1 testing and atezolizumab can be offered to patients with advanced TNBC. In Sweden, TILs have been incorporated in the Swedish Breast Cancer Guidelines as of June 2020121. Based on the results of Impassion 131, the New Therapies Council (NT-Rådet) does not recommend the use of atezolizumab in combination with paclitaxel or nab-paclitaxel for the treatment of advanced TNBC. The implementation of PD-L1 testing (SP142 assay) in Denmark has been hampered by the lack of analytical validity and reproducibility of immunostaining. Consequently, PD-L1 IHC is not in widespread use. In addition, the process concerning approval of atezolizumab for metastatic TNBC is ongoing in the Danish Medicines Council. TILs are included in the national Danish Breast Cancer Cooperative Group (DBCG) pathology guidelines. In Romania, PD-L1 testing is not routinely used. The testing of patients is made primarily by private pathology laboratories on request. National Health Insurance does not reimburse the bill for testing, so patients can enroll in a clinical trial or get free testing from the Pharmaceutical Industry accessing special testing programs. Atezolizumab is currently not approved for BC in Romania. Atezolizumab is approved and reimbursed in Italy for the treatment of patients with locally advanced or metastatic TNBC, and the SP142 assay has been identified as a companion diagnostic.
In Africa
In Morocco, many breast pathologists practicing at university hospitals have benefited from the Roche® training for the implementation of SP142 PD-L1 IHC. However, this assay has a high cost, and most of the national efforts to support cancer patients including health insurance do not cover this testing. Alternatively, several private pathology laboratories offer this assay on-demand with some oncological institutions providing access to ICI. Data from Kenya show HER-2 type BC prevalence of 17.6% and TNBC prevalence of 20.2%. Most patients with BC are still unable to meet the costs of expensive immunohistochemical PD-L1-testing and subsequent immunotherapy. In South Africa, PD-L1 testing is available on Dako and Ventana platforms but the cost is very high and not routinely performed in the public health sectors, which covers 75% of the population. In the private sector, PD-L1 testing is performed upon clinician request. The cost of ICIs drugs remains prohibitively high for use in the public sector. ICIs in BC are available only in the clinical trial setting, as these drugs are not licensed for use in TNBC. Assessment of TILs in BC care is currently not routinely done in either sector.
In North and South America
In the United States, the PD-L1 SP142 assay has been approved as a companion assay for the selection of patients with TNBC to offer atezolizumab and taxane chemotherapy (http://www.svfp.se/foreningar/uploads/L15178/kvast/brostpatologi/Brostjuni2020.pdf). In Brazil, some central pathology laboratories perform PD-L1 testing based on principal investigator-sponsored agreements. PD-L1 IHC is tested mostly in private institutions with some programs offering pro bono testing to assess eligibility for approved ICI. The Brazilian Pathology Society follows WHO recommendations; hence the inclusion of TILs in daily practice can be envisaged in the near future. In Chile, PD-L1 testing is available on several platforms but their cost is high and this is not covered by public health insurance which covers 75% of the population. Anti-PD-L1 drugs remain at a high cost and are usually only funded by additional insurance or in clinical trial settings. In Argentina, PD-L1 testing is not covered by public funds. A few central private pathology laboratories perform PD-L1 testing on-demand funded by industry. Some pathologists have started to incorporate TILs into pathology reports for TNBC; however, there are no current national guidelines. In Perú, TILs evaluation has been included in TNBC protocols by pathologists in public and private centers. SP142 IHC is available in a few private labs while Dako 22C3 is being processed for non-breast malignancies in private and public centers. There are a few BC patients who have received atezolizumab funded via private insurance or clinical trials. It is not expected that the public system will support anti-PD-1 drugs in the near future.
In Asia
In India, PD-L1 and TIL assessments are not being performed routinely with significant discrepancies of results due to the different clones available to date. PD-L1 reporting is assessed on the platform as recommended by the standard reporting guidelines. TILs reporting is performed simultaneously with PD-L1 testing. Insurance companies do not offer reimbursement for PD-L1 testing. In April 2020, atezolizumab in combination with nab-paclitaxel received Drug Controller General of India (DCGI) approval as a first-line treatment for metastatic TNBC patients. This may pave the way for its use in selected patients if affordable (https://www.expresspharma.in/amp/latest-updates/roches-atezolizumab-receives-dcgi-approval-for-treatment-of-metastatic-triple-negative-breast-cancer-in-india/). OptiView PD-L1 (SP142) on the Ventana platform was approved in Japan as the companion diagnostic for metastatic TNBC treatment with atezolizumab. Other antibodies including 22C3, 28-8, and SP236 or OptiView PD-L1 (SP-142) on other platforms are un-funded. Pathology laboratories can use any PD-L1 assay for clinical research but only PD-L1-testing using SP142 on the Ventana platform companion diagnostic is permitted for funded treatment with atezolizumab in clinical practice. Many Japanese pathologists have limited clinical experience interpreting PD-L1 staining with no current national guidelines. The Japanese Society of Pathology does not provide oversight for quality control, which leaves this mostly to the pharmaceutical industry and individual laboratories. In Taiwan, PD-L1 assays are obligatory when considering immune checkpoint inhibition for BC. The fee for a PD-L1 assay is very high and the assay is not covered by the national health insurance and has to be paid at the patients’ own expense. The PD-L1 essay is only available in medical centers and some large hospitals.
In Malaysia, some ministry/government-based pathologists do not deem the PD-L1 interpretation as practical given the fact that immunotherapy is not generally provided to patients due to high costs and the lack of subsidized initiatives from the government. On the other hand, similar to that seen in Morocco, assays are being actively performed in the private setting with private health insurance covering the costs of the tests and the ICI. PD-L1 testing is not standardized in Iran, and this assay is not routinely requested in the setting of BC by oncologists. Additionally, sTILs are not routinely reported by pathologists in Iran, and those who do, use the method developed by the International TILS working group.
Cost issues are an extremely relevant topic for low-to-middle-income countries (LMIC) pertaining to PD-L1 testing. There is a considerable difference between the costing of the standard PD-L1-antibodies which are for most patients unaffordable. The high cost of PD-L1 assays in LMIC can be mitigated by selecting patients using the sTILs on H&E-slides to include cases for further analysis. Furthermore, as argued in detail before72, TILs and PD-L1 assays for immune cells are complementary as both are part of the same immune spectrum in cancer. However, the landscape of PD-L1 testing is clearly complex for both oncologists and pathologists. The guidelines for interpretation of immunohistochemical results as well as the selection of the appropriate companion diagnostic antibody vary with both tumor type and specific ICI under consideration. The Canadian evidence-based recommendations are a good example of the efforts provided to guide pathologists in establishing fit-for-purpose PD-L1 biomarker assays to select patients to benefit from ICIs in any tumor type122.
The International Immuno-Oncology Biomarker Working Group supports efforts to advance knowledge and best practices for immune-based therapies in all solid tumors. In this regard, the TIL Working Group has created an online tool (www.tilsinbreastcancer.org, “NEW PD-L1 Help Desk) to help oncologists and pathologists select and interpret appropriate anti-PD-1/PD-L1 therapies and associated companion diagnostics. This will be updated regularly to reflect the most current standards for eligibility for ICI for all solid tumors.
A personal patient advocate perspective on TILs
“As a 5-year TNBC survivor who supports other BC patients to navigate and cope with their treatment, I know there’s no standard set of information that a patient uses to make their informed choice on how to treat their tumor. Some use Ki-67, some use PET scans; some are shown online risk tools like Predict, most use receptor status. Patients want as much information as possible to make the informed decisions forced upon them. We understand that nothing is “absolute”, that one hospital’s benchmark of a “clear margin” is different to another’s. We know that the thresholds defining hormone receptor subtypes differ between hospitals and that Ki-67 scoring can vary as much as three times between different pathologists. We understand the variability of response to drugs in different patients. We accept that pathology is an art but struggle to accept that cancer treatment plans arise from a random clutch of data-points and estimates of risk. Patients would benefit from owning their pathology reports. We live longer, we move between hospitals, and even when IT systems are compatible (which is rare) much of our data exist in unstructured reports that future clinicians do not access, either because they do not know it exists or they can’t find it amidst the hundreds of files that we generate. If patients had a checklist of markers that were important to their disease, we could help ensure evidence-based treatment plans. Patients suffer unnecessarily; it is stressful enough to face your own mortality but to trawl social media comparing your treatment to that of strangers creates more stress. Patient-ownership of pathology reports would help remove fears of age-bias and variation in care, and furthermore could help generate complete datasets for audits and real-world evidence studies.
We know that cancer is an ecosystem of cancer cells and the stromal cells between them. We know the well-defined markers for the cancer cells that direct chemotherapy and hormone therapy. What markers do we have for the intra-tumor stroma? PD-L1 SP142 is an expensive assay and notoriously difficult to achieve consensus on, but nevertheless if you ever once had PD-L1 recorded for any one of your TNBC primary tumors or metastases, you are eligible for one of the latest drugs, atezolizumab. Would it be wise therefore for all TNBC patients to request PD-L1 testing of their primaries for the event that their metastases can’t be biopsied? Shouldn’t we be seeking an easier and cheaper marker (e.g., TILs) to understand the stroma and direct treatment? I still feel very lucky that my TILs were scored in my pathology report. They have given me a more positive outlook and this alone is a reason for scoring them. I’m also very aware (through atezolizumab’s licensing) that details on my pathology report today might be my gateway to a life-prolonging drug tomorrow.”
Conclusion
TILs are an inexpensive, robust prognostic biomarker that represents a surrogate for anti-tumor T cell-mediated immunity. Incorporating TILs into standard clinical practice should be strongly considered in both early and advanced TNBC and HER2-positive BC. TILs assessment at the time of diagnosis may enable a clinician to assess prognosis and in future inform therapeutic decision-making more accurately, is informative for predictive purposes, can help to interpret PD-L1 assays, and, certainly in LMIC, may be considered as a screening tool before embarking on expensive immune-assays, being PD-L1 or others. Further information on the clinical utility of TILs in HR positive BC is needed to identify their role in this subtype of the disease.
Education and standardization of testing across the pathology community by providing centralized training and educational tools are required to up-skill clinicians to utilize this new biomarker. The TIL-WG host’s pathologists from academic hospitals, community hospitals, and industry, supported by expert clinicians and statisticians, incorporating the view of patients also. We encourage transparent and efficient communication with the regulatory authorities. This collaboration of experts and patients is imperative to guide the development of this new biomarker and optimize its role across academia, industry, and the clinical setting. Looking to the future, a collaboration between the oncology and pathology communities across countries and continents is integral to define how best TILs can be integrated into a multivariate prognostic model with standard variables such as age, tumor size, and nodal status to optimize outcomes of patients with BC. In addition, trials being developed using baseline, on-treatment, and post-treatment TILs may improve our understanding of the complex interaction between host immunity and the TME and will improve our approach to fit as best as possible our patient’s needs.
References
Kruger, S. et al. Advances in cancer immunotherapy 2019—latest trends. J. Exp. Clin. Cancer Res. 38, 268 (2019).
Gómez-Aleza, C. et al. Inhibition of RANK signaling in breast cancer induces an anti-tumor immune response orchestrated by CD8+ T cells. Nat. Commun. 11, 6335 (2020).
Dushyanthen, S. et al. Agonist immunotherapy restores T cell function following MEK inhibition improving efficacy in breast cancer. Nat. Commun. 8, 606 (2017).
de Melo Gagliato, D. et al. Tumour-infiltrating lymphocytes in breast cancer and implications for clinical practice. Biochim. Biophys. Acta Rev. Cancer 1868, 527–537 (2017).
Baxevanis C. N., Fortis S. P. & Perez S. A. The balance between breast cancer and the immune system: challenges for prognosis and clinical benefit from immunotherapies. Semin. Cancer Biol. https://doi.org/10.1016/j.semcancer.2019.12.018 (2019).
Chen, D. S. & Mellman, I. Elements of cancer immunity and the cancer-immune set point. Nature 541, 321–330 (2017).
Gu-Trantien, C. et al. CD4+ follicular helper T cell infiltration predicts breast cancer survival. J. Clin. Invest. 123, 2873–2892 (2013).
Binnewies, M. et al. Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat. Med. 24, 541–550 (2018).
Denkert, C. et al. Tumour-infiltrating lymphocytes and prognosis in different subtypes of breast cancer: a pooled analysis of 3771 patients treated with neoadjuvant therapy. Lancet Oncol. 19, 40–50 (2018).
Stanton, S. E., Adams, S. & Disis, M. L. Variation in the incidence and magnitude of tumour-infiltrating lymphocytes in breast cancer subtypes: a systematic review. JAMA Oncol. 2, 1354–1360 (2016).
Hammerl, D. et al. Breast cancer genomics and immuno-oncological markers to guide immune therapies. Semin. Cancer Biol. 52(Pt 2), 178–188 (2018).
Thomas, A. et al. Tumour mutational burden is a determinant of immune-mediated survival in breast cancer. Oncoimmunology 7, e1490854 (2018).
Cortés, J. et al. LBA21 KEYNOTE-119: phaseIII study of pembrolizumab (pembro) versus single-agent chemotherapy (chemo) for metastatic triple negative breast cancer (mTNBC). Ann. Oncol. 94, 010 (2019).
Samstein, R. M. et al. Tumour mutational load predicts survival after immunotherapy across multiple cancer types. Nat. Genet. 51, 202–206 (2019).
Nanda, R. et al. Pembrolizumab plus standard neoadjuvant therapy for high-risk breast cancer (BC): results from I-SPY 2. J. Clin. Oncol. 35(15 suppl), 506 (2017).
McGranahan, N. et al. Clonal neoantigens elicit T cell immunoreactivity and sensitivity to immune checkpoint blockade. Science 351, 1463–1469 (2016).
Karn, T. et al. Association between genomic metrics and immune infiltration in triple-negative breast cancer. JAMA Oncol. 3, 1707–1711 (2017).
Schreiber, R. D., Old, L. J. & Smyth, M. J. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science 331, 1565–1570 (2011).
Karn, T. et al. Homogeneous datasets of triple negative breast cancers enable the identification of novel prognostic and predictive signatures. PLoS ONE 6, e28403 (2011).
Karn, T. et al. Association between genomic metrics and immune infiltration in triple-negative breast cancer. JAMA Oncol. 3, 1707–1711 (2017).
Safonov, A. et al. Immune gene expression is associated with genomic aberrations in breast cancer. Cancer Res. 77, 3317–3324 (2017).
Loi, S. et al. Tumour-infiltrating lymphocytes and prognosis: a pooled individual patient analysis of early-stage triple-negative breast cancers. J. Clin. Oncol. 37, 559–569 (2019).
Salgado, R. et al. The evaluation of tumour-infiltrating lymphocytes (TILs) in breast cancer: recommendations by an International TILs Working Group 2014. Ann. Oncol. 26, 259–271 (2015).
Kos, Z. et al. Pitfalls in assessing stromal tumor infiltrating lymphocytes (sTILs) in breast cancer. NPJ Breast Cancer 6, 17 (2020).
Keren, L. et al. A structured tumour-immune microenvironment in triple negative breast cancer revealed by multiplexed ion beam imaging. Cell 174, 1373–1387.e19 (2018).
Savas, P. et al. Single-cell profiling of breast cancer T cells reveals a tissue-resident memory subset associated with improved prognosis [published correction appears in Nat Med. 2018 Dec;24(12):1941]. Nat. Med. 24, 986–993 (2018).
Colbeck, E. J., Ager, A., Gallimore, A. & Jones, G. W. Tertiary lymphoid structures in cancer: drivers of antitumour immunity, immunosuppression, or bystander sentinels in disease? Front. Immunol. 8, 1830 (2017).
Buisseret, L. et al. Tumour-infiltrating lymphocyte composition, organization and PD-1/ PD-L1 expression are linked in breast cancer. Oncoimmunology 6, e1257452 (2016).
Solinas, C. et al. Immune checkpoint molecules on tumour-infiltrating lymphocytes and their association with tertiary lymphoid structures in human breast cancer. Front. Immunol. 8, 1412 (2017).
Buechler, M. B. & Turley, S. J. A short field guide to fibroblast function in immunity. Semin. Immunol. 35, 48–58 (2018).
Cabrita, R. et al. Tertiary lymphoid structures improve immunotherapy and survival in melanoma [published correction appears in Nature. 2020 Apr;580(7801):E1]. Nature 577, 561–565 (2020).
Lee, M. et al. Presence of tertiary lymphoid structures determines the level of tumour-infiltrating lymphocytes in primary breast cancer and metastasis. Mod. Pathol. 32, 70–80 (2019).
Sautès-Fridman, C., Petitprez, F., Calderaro, J. & Fridman, W. H. Tertiary lymphoid structures in the era of cancer immunotherapy. Nat. Rev. Cancer 19, 307–325 (2019).
Buisseret, L. et al. Reliability of tumour-infiltrating lymphocyte and tertiary lymphoid structure assessment in human breast cancer. Mod. Pathol. 30, 1204–1212 (2017).
Finotello, F. & Trajanoski, Z. Quantifying tumour-infiltrating immune cells from transcriptomics data. Cancer Immunol. Immunother. 67, 1031–1040 (2018).
Dannenfelser, R. et al. Data-driven analysis of immune infiltrate in a large cohort of breast cancer and its association with disease progression, ER activity, and genomic complexity. Oncotarget 8, 57121–57133 (2017).
Li, B. et al. Comprehensive analyses of tumour immunity: implications for cancer immunotherapy. Genome Biol. 17, 174 (2016).
Chung, W. et al. Single-cell RNA-seq enables comprehensive tumour and immune cell profiling in primary breast cancer. Nat. Commun. 8, 15081 (2017).
Singer, M. & Anderson, A. C. Revolutionizing cancer immunology: the power of next-generation sequencing technologies. Cancer Immunol. Res. 7, 168–173 (2019).
Parra, E. R., Francisco-Cruz, A. & Wistuba, I. I. State-of-the-art of profiling immune contexture in the era of multiplexed staining and digital analysis to study paraffin tumor tissues. Cancers (Basel). 11, 247 (2019).
Nederlof, I. et al. Comprehensive evaluation of methods to assess overall and cell-specific immune infiltrates in breast cancer. Breast Cancer Res. 21, 151 (2019).
Klauschen, F. et al. Scoring of tumour-infiltrating lymphocytes: from visual estimation to machine learning. Semin. Cancer Biol. 52(Pt 2), 151–157 (2018).
Amgad, M. et al. Report on computational assessment of tumor infiltrating lymphocytes from the International Immuno-Oncology Biomarker Working Group. NPJ Breast Cancer 6, 16 (2020).
Gil Del Alcazar, C. R. et al. Immune escape in breast cancer during in situ to invasive carcinoma transition. Cancer Discov. 7, 1098–1115 (2017).
Rosenthal, R. et al. Neoantigen-directed immune escape in lung cancer evolution. Nature 567, 479–485 (2019).
Denkert, C. et al. Standardized evaluation of tumour-infiltrating lymphocytes in breast cancer: results of the ring studies of the international immuno-oncology biomarker working group. Mod. Pathol. 29, 1155–1164 (2016).
Kim, R. S. et al. Stromal tumour-infiltrating lymphocytes in NRG oncology/NSABP B-31 adjuvant trial for early-stage HER2-positive breast cancer. J. Natl Cancer Inst. 111, 867–871 (2019).
Amgad, M. et al. Structured crowd sourcing enables convolutional segmentation of histology images. Bioinformatics 35, 3461–3467 (2019).
Simon, R. M., Paik, S. & Hayes, D. F. Use of archived specimens in evaluation of prognostic and predictive biomarkers. J. Natl Cancer Inst. 101, 1446–1452 (2009).
McShane, L. M. et al. Reporting recommendations for tumour marker prognostic studies. J. Clin. Oncol. 23, 9067–9072 (2005).
Park, J. H. et al. Prognostic value of tumour-infiltrating lymphocytes in patients with early-stage triple-negative breast cancers (TNBC) who did not receive adjuvant chemotherapy. Ann. Oncol. 30, 1941–1949 (2019).
Burstein, H. J. et al. Estimating the benefits of therapy for early-stage breast cancer: the St. Gallen International Consensus Guidelines for the primary therapy of early breast cancer 2019. Ann. Oncol. 30, 1541–1557 (2019).
WHO Classification of Tumours Editorial Board. Breast Tumours: WHO Classification of Tumours, 5th Edition, 2.
Lehmann, B. D. et al. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J. Clin. Invest. 121, 2750–2767 (2011).
Geyer, F. C. et al. The spectrum of triple-negative breast disease: high- and low-grade lesions. Am. J. Pathol. 187, 2139–2151 (2017).
Rakha, E. A. et al. The prognostic significance of inflammation and medullary histological type in invasive carcinoma of the breast. Eur. J. Cancer 45, 1780–1787 (2009).
Kalaw, E. et al. Metaplastic breast cancers frequently express immune checkpoint markers FOXP3 and PD-L1. Br. J. Cancer 123, 1665–1672 (2020).
Lien, H. C. et al. Tumor-infiltrating lymphocyte abundance and programmed death-ligand 1 expression in metaplastic breast carcinoma: implications for distinct immune microenvironments in different metaplastic components. Virchows Arch. 478, 669–678 (2021).
Marchiò, C., Weigelt, B. & Reis-Filho, J. S. Adenoid cystic carcinomas of the breast and salivary glands (or ‘The strange case of Dr Jekyll and Mr Hyde’ of exocrine gland carcinomas). J. Clin. Pathol. 63, 220–228 (2010).
Loibl, S. et al. A randomised phase II study investigating durvalumab in addition to an anthracycline taxane-based neoadjuvant therapy in early triple-negative breast cancer: clinical results and biomarker analysis of GeparNuevo study. Ann. Oncol. 30, 1279–1288 (2019).
Karn, T. et al. Tumor mutational burden and immune infiltration as independent predictors of response to neoadjuvant immune checkpoint inhibition in early TNBC in GeparNuevo. Ann. Oncol. https://doi.org/10.1016/j.annonc.2020.05.015 (2020). S0923-7534(20)39836-7.
Mittendorf, E. A. et al. Neoadjuvant atezolizumab in combination with sequential nab-paclitaxel and anthracycline-based chemotherapy versus placebo and chemotherapy in patients with early-stage triple-negative breast cancer (IMpassion031): a randomised, double-blind, phase 3 trial. Lancet 396, 1090–1100 (2020).
Schmid, P. et al. KEYNOTE-522 investigators. pembrolizumab for early triple-negative breast cancer. N. Engl. J. Med. 382, 810–821 (2020).
Nanda, R. et al. Effect of pembrolizumab plus neoadjuvant chemotherapy on pathologic complete response in women with early-stage breast cancer: an analysis of the ongoing phase 2 adaptively randomized I-SPY2 trial. JAMA Oncol. 6, 676–684 (2020).
Emens, L. A. et al. Long-term clinical outcomes and biomarker analyses of atezolizumab therapy for patients with metastatic triple-negative breast cancer: a phase 1 study. JAMA Oncol. 5, 74–82 (2019).
Adams, S. et al. Pembrolizumab monotherapy for previously untreated, PD-L1-positive, metastatic triple-negative breast cancer: cohort B of the phase II KEYNOTE-086 study. Ann. Oncol. 30, 405–411 (2019b).
Adams, S. et al. Pembrolizumab monotherapy for previously treated metastatic triple-negative breast cancer: cohort A of the phase II KEYNOTE-086 study. Ann. Oncol. 30, 397–404 (2019a).
Schmid, P., Adams, S., Rugo, H. S. & Schneeweiss, A. Atezolizumab and nab‐paclitaxel in advanced triple‐negative breast cancer. N. Engl. J. Med. 379, 2108–2121 (2018).
Schmid P. et al. Atezolizumab in metastatic triple-negative breast cancer: long-term clinical outcomes and biomarker analyses [abstract]. in: Proc. American Association for Cancer Research Annual Meeting 2017; Apr; Washington, DC. (AACR, Philadelphia (PA), 2017) Abstract nr 2986.
Schmid, P. et al. Atezolizumab plus nab-paclitaxel as first-line treatment for unresectable, locally advanced or metastatic triple-negative breast cancer (IMpassion130): updated efficacy results from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 21, 44–59 (2020).
Rugo, H. S. et al. Performance of PD-L1 immunohistochemistry assays in unresectable locally advanced or metastatic triple-negative breast cancer: post hoc analysis of IMpassion130. Ann. Oncol. 30(Suppl. 5), v851–v934 (2019).
Gonzalez-Ericsson P. I. et al. The path to a better biomarker: application of a risk management framework for the implementation of PD-L1 and TILs as immuno-oncology biomarkers into breast cancer clinical trials and daily practice. J. Pathol. https://doi.org/10.1002/path.5406 (2020).
Emens L. A. et al. Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer: biomarker evaluation of the IMpassion130 study. J. Natl Cancer Inst. https://doi.org/10.1093/jnci/djab004 (2021).
Loi S. et al. Relationship between tumor infiltrating lymphocyte (TIL) levels and response topembrolizumab (pembro) in metastatictriple-negative breast cancer (mTNBC):results from KEYNOTE-086. Ann. Oncol. 2017;28:Suppl:LBA13. abstract.
Loi et al. Relationship between tumour-infiltrating lymphocytes (TILs) and outcomes in the KEYNOTE-119 study of pembrolizumab vs chemotherapy for previously treated metastatic triple-negative breast cancer (mTNBC), PD5-03, SABC 2019.
Cortes, J. et al. Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for previously untreated locally recurrent inoperable or metastatic triple-negative breast cancer (KEYNOTE-355): a randomised, placebo-controlled, double-blind, phase 3 clinical trial. Lancet 396, 1817–1828 (2020).
Luen, S., Virassamy, B., Savas, P., Salgado, R. & Loi, S. The genomic landscape of breast cancer and its interaction with host immunity. Breast 29, 241–250 (2016).
Loi, S. et al. Prognostic and predictive value of tumour-infiltrating lymphocytes in a phase III randomized adjuvant breast cancer trial in node-positive breast cancer comparing the addition of docetaxel to doxorubicin with doxorubicin-based chemotherapy: BIG 02-98. J. Clin. Oncol. 31, 860–867 (2013).
Tsang, J. Y. S. et al. Lymphocytic infiltrate is associated with favorable biomarkers profile in HER2-overexpressing breast cancers and adverse biomarker profile in ER-positive breast cancers. Breast Cancer Res. Treat. 143, 1–9 (2013).
Baker, K. et al. Prognostic significance of CD8+ T lymphocytes in breast cancer depends upon both oestrogen receptor status and histological grade. Histopathology 58, 1107–1116 (2011).
Haricharan, S., Bainbridge, M. N., Scheet, P. & Brown, P. H. Somatic mutation load of estrogen receptor-positive breast tumours predicts overall survival: an analysis of genome sequence data. Breast Cancer Res. Treat. 146, 211–220 (2014).
Rugo, H. S. et al. Safety and antitumour activity of pembrolizumab in patients with estrogen receptor–positive/human epidermal growth factor receptor 2—negative advanced breast cancer. Clin. Cancer Res. 24, 2804–2811 (2018).
Dirix, L. Y. Avelumab, an anti-PD-L1 antibody, in patients with locally advanced or metastatic breast cancer: a phase 1b tumour study. Breast Cancer Res. Treat. 167, 671–686 (2018).
Dodson, A. et al. Breast cancer biomarkers in clinical testing: analysis of a UK national external quality assessment scheme for immunocytochemistry and in situ hybridisation database containing results from 199 300 patients [published correction appears in J Pathol Clin Res. 2020 Jul;6(3):227]. J. Pathol. Clin. Res. 4, 262–273 (2018).
Howlader, N., Cronin, K. A., Kurian, A. W. & Andridge, R. Differences in breast cancer survival by molecular subtypes in the United States. Cancer Epidemiol. Biomark. Prev. 27, 619–626 (2018).
Ali H. R. Association between CD8+ T-cell infiltration and breast cancer survival in 12,439 patients. Ann. Oncol. 25,1536–1543 (2014).
Dieci, M. V. et al. Prognostic and predictive value of tumour-infiltrating lymphocytes in two phase III randomized adjuvant breast cancer trials. Ann. Oncol. 26, 1698–1704 (2015).
Sobral-Leite, M. et al. Cancer-immune interactions in ER-positive breast cancers: PI3K pathway alterations and tumor-infiltrating lymphocytes. Breast Cancer Res. 21, 90 (2019).
Ades, F. et al. Luminal B breast cancer: molecular characterization, clinical management, and future perspectives. J. Clin. Oncol. 32, 2794–2803 (2014).
Metzger-Filho, O. et al. Patterns of recurrence and outcome according to breast cancer subtypes in lymph node–negative disease: results from International Breast Cancer Study Group Trials VIII and IX. J. Clin. Oncol. 31, 3083–3090 (2013).
Thompson, E. D. et al. PD-L1 expression and the immune microenvironment in primary invasive lobular carcinomas of the breast. Mod. Pathol. 30, 1551–1560 (2017).
Droeser, R. et al. Differential pattern and prognostic significance of CD4+, FOXP3+ and IL-17+ tumor infiltrating lymphocytes in ductal and lobular breast cancers. BMC Cancer 12, 134 (2012).
Desmedt, C. et al. Immune infiltration in invasive lobular breast cancer. J. Natl Cancer Inst. 110, 768–776 (2018).
Du, T. et al. Invasive lobular and ductal breast carcinoma differ in immune response, protein translation efficiency and metabolism. Sci. Rep. 8, 7205 (2018).
Desmedt C. et al. Lymphocytic infiltration in invasive lobular breast cancer. [abstract]. in Proc. Thirty-Eighth Annual CTRC-AACR San Antonio Breast Cancer Symposium: 2015 Dec; San Antonio, TX. (AACR, Philadelphia (PA), 2016) Abstract nr S1-02.
Tille, J. C. et al. Tumor-infiltrating lymphocytes are associated with poor prognosis in invasive lobular breast carcinoma. Mod. Pathol. 33, 2198–2207 (2020).
Dunbier, A. K. et al. Molecular profiling of aromatase inhibitor-treated postmenopausal breast tumors identifies immune-related correlates of resistance. Clin. Cancer Res. 19, 2775–2786 (2013).
Heindl A. et al Relevance of spatial heterogeneity of immune infiltration for predicting risk of recurrence after endocrine therapy of ER+ breast cancer. J. Natl Cancer Inst. https://doi.org/10.1093/jnci/djx137 (2018).
Dieci, M. V. et al. Tumour-infiltrating lymphocytes and molecular response after neoadjuvant therapy for HR+/HER2- breast cancer: results from two prospective trials [published correction appears in Breast Cancer Res Treat. 2017 Jun;163(3):637]. Breast Cancer Res. Treat. 163, 295–302 (2017).
Goel, S. et al. CDK4/6 inhibition triggers anti-tumour immunity. Nature 548, 471–475 (2017).
Hurvitz S, et al. Abstract PD2-10: Treatment with abemaciclib modulates the immune response in gene expression analysis of the neoMONARCH neoadjuvant study of abemaciclib in postmenopausal women with HR+, HER2 negative breast cancer. Poster Discuss. Abstr. https://doi.org/10.1158/1538-7445.sabcs18-pd2-10 (2019).
Hendry, S. et al. Assessing tumour-infiltrating lymphocytes in solid tumours: a practical review for pathologists and proposal for a standardized method from the International Immunooncology Biomarkers Working Group: Part 1: assessing the host immune response, TILs in invasive breast carcinoma and ductal carcinoma in situ, metastatic tumour deposits and areas for further research. Adv. Anat. Pathol. 24, 235–251 (2017).
Chung, Y. R., Kim, H. J., Jang, M. H. & Park, S. Y. Prognostic value of tumour infiltrating lymphocyte subsets in breast cancer depends on hormone receptor status. Breast Cancer Res. Treat. 161, 409–420 (2016).
Bates, G. J. et al. Quantification of regulatory T cells enables the identification of high-risk breast cancer patients and those at risk of late relapse. J. Clin. Oncol. 24, 5373–5380 (2006).
Heindl, A. et al. Relevance of spatial heterogeneity of immune infiltration for predicting risk of recurrence after endocrine therapy of ER+ breast cancer. J. Natl Cancer Inst. 110, 166–175 (2017).
Denkert, C. et al. Tumour-infiltrating lymphocytes and response to neoadjuvant chemotherapy with or without carboplatin in human epidermal growth factor receptor 2-positive and triple-negative primary breast cancers. J. Clin. Oncol. 33, 983–991 (2015).
Salgado, R. et al. Tumour-infiltrating lymphocytes and associations with pathological complete response and event-free survival in HER2-positive early-stage breast cancer treated with lapatinib and trastuzumab: a secondary analysis of the NeoALTTO trial. JAMA Oncol. 1, 448–454 (2015).
IngoldHeppner, B. et al. Tumor-Infiltrating LymphocyTes: A Predictive and Prognostic Biomarker in Neoadjuvant-treated HER2-positive breast cancer. Clin. Cancer Res. 22, 5747–5754 (2016).
Perez, E. A. et al. Association of stromal tumour-infiltrating lymphocytes with recurrence-free survival in the N9831 adjuvant trial in patients with early-stage HER2-positive breast cancer. JAMA Oncol. 2, 56–64 (2016).
Perez, E. A. et al. Genomic analysis reveals that immune function genes are strongly linked to clinical outcome in the North Central Cancer Treatment Group n9831 Adjuvant Trastuzumab Trial. J. Clin. Oncol. 33, 701–708 (2015).
Solinas, C. et al. Tumour-infiltrating lymphocytes in patients with HER2-positive breast cancer treated with neoadjuvant chemotherapy plus trastuzumab, lapatinib or their combination: a meta-analysis of randomized controlled trials. Cancer Treat. Rev. 57, 8–15 (2017).
Dieci, M. V. et al. Association of tumour-infiltrating lymphocytes with distant disease-free survival in the ShortHER randomized adjuvant trial for patients with early HER2+ breast cancer. Ann. Oncol. 30, 418–423 (2019).
Luen, S. J. et al. Tumour-infiltrating lymphocytes in advanced HER2-positive breast cancer treated with pertuzumab or placebo in addition to trastuzumab and docetaxel: a retrospective analysis of the CLEOPATRA study [published correction appears in Lancet Oncol. 2018 Dec;19(12):e667]. Lancet Oncol. 18, 52–62 (2017).
Liu, S. et al. Role of cytotoxic tumour-infiltrating lymphocytes in predicting outcomes in metastatic HER2-positive breast cancer: a secondary analysis of a randomized clinical trial. JAMA Oncol. 3, e172085 (2017).
Loi, S. et al. Pembrolizumab plus trastuzumab in trastuzumab-resistant, advanced, HER2-positive breast cancer (PANACEA): a single-arm, multicentre, phase 1b-2 trial. Lancet Oncol. 20, 371–382 (2019).
Luen, S. J. et al. Prognostic implications of residual disease tumour-infiltrating lymphocytes and residual cancer burden in triple-negative breast cancer patients after neoadjuvant chemotherapy. Ann. Oncol. 30, 236–242 (2019).
Masuda, N. et al. Adjuvant capecitabine for breast cancer after preoperative chemotherapy. N. Engl. J. Med. 376, 2147–2159 (2017).
Voorwerk, L. et al. Immune induction strategies in metastatic triple-negative breast cancer to enhance the sensitivity to PD-1 blockade: the TONIC trial. Nat. Med. 25, 920–928, https://doi.org/10.1038/s41591-019-0432-4 (2019).
Hudeček, J. et al. Application of a risk-management framework for integration of stromal tumor-infiltrating lymphocytes in clinical trials. NPJ Breast Cancer 6, 15 (2020).
Salgado R. et al. How current assay approval policies are leading to unintended imprecision medicine. Lancet Oncol. https://doi.org/10.1016/S1470-2045(20)30592-1 (2020).
https://www.fda.gov/medical-devices/recently-approved-devices/ventana-pd-l1-sp142-assay-p160002s009. Accessed 15 June 2020.
Cheung, C. C. et al. Diagnostic accuracy in fit-for-purpose PD-L1 testing. Appl. Immunohistochem. Mol. Morphol. 27, 251–257 (2019).
Acknowledgements
Shom Goel’ work is supported by the National Health and Medical Research Council of Australia (Investigator Grant GNT1177357 to S.G.); Susan G. Komen for the Cure (CCR18547966 to S.G.); the Royal Australasian College of Physicians (Research Establishment Fellowship to S.G.); and the NIH/NCI (P50 CA168504 to S.G.). Sherene Loi is supported by the National Breast Cancer Foundation of Australia Endowed Chair and the Breast Cancer Research Foundation, New York. Khalid El Bairi would like to report that the content of this review reflects the authors’ perspectives and not of his institution and/or affiliation. Roberto Salgado is supported by the Breast Cancer Research Foundation (BCRF, grant N° 17-194).
Author information
Authors and Affiliations
Consortia
Contributions
All authors made a substantial contribution to the conception or design of the work. All authors participated in either drafting the work or revising it critically for important intellectual content. All authors have approved the final completed version of this paper and assume accountability for all aspects of the work.
Corresponding author
Ethics declarations
Competing interests
S. Loi has received research funding to her institution from Novartis, Bristol Meyers Squibb, Merck, Roche-Genentech, Puma Biotechnology, Pfizer, Eli Lilly, Nektar Therapeutics AstraZeneca, and Seattle Genetics. S. Loi has acted as a consultant (not compensated) to Seattle Genetics, Pfizer, Novartis, BMS, Merck, AstraZeneca, and Roche-Genentech. S. Loi has acted as a consultant (paid to her institution) to Aduro Biotech, Novartis, GlaxoSmithKline, Roche-Genentech, Puma Biotechnology, AstraZeneca, and G1 Therapeutics. S. Loi is a Scientific Advisory Board Member of Akamara Therapeutics. S.D. has received research funding from institutions from Lytix Biopharma and Nanobiotix is a scientific advisory board member of Lytix Biopharma and has acted as an ad-hoc consultant (compensated) to Ono Pharma USA Inc, EMD Serono, and Mersana Therapeutics. S.G. is the recipient of lab research funding from Eli Lilly and Co and G1 Therapeutics. Shom Goel has served as a paid advisory board member for Eli Lilly and Co, G1 Therapeutics, Pfizer, and Novartis. S.G. also conducts clinical research sponsored by Eli Lilly and Co. S. Loibl reports grants and others from Abbvie, grants and other from Amgen, grants and other from Celgene, grants and other from Novartis, grants and other from Roche, other from Seattle Genetics, other from PriME/Medscape, personal fees from Chugai, grants and other from Daiichi-Sankyo, other from Lilly, other from Samsung, other from BMS, other from Puma, other from MSD, grants from Immunomedics, grants and other from AstraZeneca, grants and other from Pfizer, other from Pierre Fabre and other from Merck outside the submitted work; In addition, Dr. Loibl has a patent EP14153692.0 pending. J.M.S.B. BSc, PhD, FRCPath has consultancies for Insight Genetics, Inc. BioNTech AG Biotheranostics, Inc. Pfizer RNA Diagnostics Inc. oncoXchange/MedcomXchange Communications Inc. Herbert Smith French Solicitors OncoCyte Corporation SCIENTIFIC ADVISORY BOARD MedcomXchange Communications Inc. HONORARIA NanoString Technologies, Inc. Oncology Education Biotheranostics, Inc. MedcomXchange Communications Inc. RESEARCH FUNDING Thermo Fisher Scientific GenoptixAgendiaNanoString Technologies, Inc. Stratifyer GmbH Biotheranostics, Inc. TRAVEL, ACCOMMODATIONS, EXPENSES Biotheranostics, Inc. NanoString Technologies, Inc. Breast Cancer Society of Canada PATENTS—APPLIED Jan 2017: Methods and Devices for Predicting Anthracycline Treatment Efficacy, US utility—15/325,472; EPO—15822898.1; Canada—not yet assigned Jan 2017: Systems, Devices and Methods for Constructing and Using a Biomarker, US utility—15/328,108; EPO—15824751.0; Canada—not yet assigned Oct 2016: Histone gene module predicts anthracycline benefit, PCT/CA2016/000247 Dec 2016: 95-Gene Signature of Residual Risk Following Endocrine Treatment, PCT/CA2016/000304 Dec 2016: Immune Gene Signature Predicts Anthracycline Benefit, PCT/CA2016/000305 June 2020: Use of Molecular Classifiers to Diagnose, Treat and Prognose Prostate Cancer, US Provisional 63/040.692 INVENTION DISCLOSURE Disclosure Name: A Molecular Classifier for Personalized Risk Stratification for Patients with Prostate Cancer, Date: 21/08/2019. P.A.F. has received Honoraria from AstraZeneca and Novartis and Traveled to give overseas lectures from Ipsen. B.L.R. reports non-financial support from HalioDx, in the form of a collaborative association on a non-remunerative basis, during the conduct of the study. D.R. reports grants and personal fees from Amgen and AstraZeneca, Cepheid, Konica Minolta InVicro, NextCure, Ultivue, and Eli Lilly; personal fees from Bristol Myers Squibb, Cell Signaling Technology, Daiichi Sankyo, Danaher, GSK, Merck, Nanostring Technologies, Odonate, Paige. AI, Roche, Sanofi, and Ventana; grants from Navigate Biopharma; and personal royalties from RareCyte related to a patent on circulating cancer cells outside of the submitted work. A.E. is a Roche advisory board and Lecturer paid by Roche, Amgen, and Novartis. M.V.D. has personal fees for advisory/consultancy roles from Eli Lilly, Celgene, Novartis, and Genomic Health. R.Y. has received support from Chugai pharmaceutical company. S.M. has conflicts of interest not related to this study including statistical advice for IDDI and Janssen Cilag and is an Independent Data Monitoring Committee member for Hexal, Steba, IQVIA, Roche, Sensorion, Biophytis, Servier, and Yuan. M.K. Institution receives funding from BMS, Roche, AZ/Medimmune. M.K. has an advisory role for BMS, Roche, MSD, and Daiichi. R.S. reports non-financial support from Merck and Bristol Myers Squibb; research support from Merck, Puma Biotechnology, and Roche; and personal fees from Roche for an advisory board related to a trial-research project. The remaining authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
El Bairi, K., Haynes, H.R., Blackley, E. et al. The tale of TILs in breast cancer: A report from The International Immuno-Oncology Biomarker Working Group. npj Breast Cancer 7, 150 (2021). https://doi.org/10.1038/s41523-021-00346-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41523-021-00346-1
This article is cited by
-
Circulating blood biomarkers correlated with the prognosis of advanced triple negative breast cancer
BMC Women's Health (2024)
-
HER2-low and tumor infiltrating lymphocytes in triple-negative breast cancer: Are they connected?
Breast Cancer Research (2024)
-
S100A8/A9 predicts response to PIM kinase and PD-1/PD-L1 inhibition in triple-negative breast cancer mouse models
Communications Medicine (2024)
-
High tumor infiltrating lymphocytes are significantly associated with pathological complete response in triple negative breast cancer treated with neoadjuvant KEYNOTE-522 chemoimmunotherapy
Breast Cancer Research and Treatment (2024)
-
Distinct profiles of proliferating CD8+/TCF1+ T cells and CD163+/PD-L1+ macrophages predict risk of relapse differently among treatment-naïve breast cancer subtypes
Cancer Immunology, Immunotherapy (2024)