Abstract
Sex chromosome evolution is thought to be tightly associated with the acquisition and maintenance of sexual dimorphisms. Plant sex chromosomes have evolved independently in many lineages1,2 and can provide a powerful comparative framework to study this. We assembled and annotated genome sequences of three kiwifruit species (genus Actinidia) and uncovered recurrent sex chromosome turnovers in multiple lineages. Specifically, we observed structural evolution of the neo-Y chromosomes, which was driven via rapid bursts of transposable element insertions. Surprisingly, sexual dimorphisms were conserved in the different species studied, despite the fact that the partially sex-linked genes differ between them. Using gene editing in kiwifruit, we demonstrated that one of the two Y-chromosome-encoded sex-determining genes, Shy Girl, shows pleiotropic effects that can explain the conserved sexual dimorphisms. These plant sex chromosomes therefore maintain sexual dimorphisms through the conservation of a single gene, without a process involving interactions between separate sex-determining genes and genes for sexually dimorphic traits.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
All sequencing data from Illumina and three Actinidia genomes have been deposited in the DDBJ database: Sequence Read Archives database (BioProject ID PRJDB13958, Run ID DRR396285–DRR396297 for the Illumina sequencing reads; BRYE01000001–BRYE01000030 for the A. arguta genome; BRYF01000001–BRYF01000030 for the A. polygama genome; BRYG01000001–BRYG01000029 for the A. rufa genome).
References
Ming, R., Bendahmane, A. & Renner, S. S. Sex chromosomes in land plants. Annu. Rev. Plant Biol. 62, 485–514 (2011).
Henry, I. M., Akagi, T.,Tao, R. & Comai, L. One hundred ways to invent the sexes: theoretical and observed paths to dioecy in plants. Annu. Rev. Plant Biol. 69, 553–575 (2018).
Bachtrog, D. et al. Sex determination: why so many ways of doing it? PLoS Biol. https://doi.org/10.1371/journal.pbio.1001899 (2014).
Akagi, T. et al. A Y-encoded suppressor of feminization arose via lineage-specific duplication of a cytokinin response regulator in kiwifruit. Plant Cell 30, 780–795 (2018).
Akagi, T. et al. Two Y-chromosome-encoded genes determine sex in kiwifruit. Nat. Plants 5, 801–809 (2019).
Harkess, A. et al. The asparagus genome sheds light on the origin and evolution of a young Y chromosome. Nat. Commun. 8, 1279 (2017).
Harkess, A. et al. Sex determination by two Y-linked genes in garden asparagus. Plant Cell 32, 1790–1796 (2020).
Massonnet, M. et al. The genetic basis of sex determination in grapes. Nat. Commun. 11, 2902 (2020).
Akagi, T., Henry, I. M., Tao, R. & Comai, L. A Y-chromosome–encoded small RNA acts as a sex determinant in persimmons. Science 346, 646–650 (2014).
Yang, H. W., Akagi, T., Kawakatsu, T. & Tao, R. Gene networks orchestrated by MeGI: a single‐factor mechanism underlying sex determination in persimmon. Plant J. 98, 97–111 (2019).
Vicoso, B. Molecular and evolutionary dynamics of animal sex-chromosome turnover. Nat. Ecol. Evol. 3, 1632–1641 (2019).
Pan, Q. et al. Evolution of master sex determiners: TGF-β signalling pathways at regulatory crossroads. Phil. Trans. R. Soc. B 376, 20200091 (2021).
Müller, N. A. et al. A single gene underlies the dynamic evolution of poplar sex determination. Nat. Plants 6, 630–637 (2020).
Hallingbäck, H. R., Pucholt, P., Ingvarsson, P. K., Rönnberg-Wästljung, A. C. & Berlin, S. Genome-wide association mapping uncovers sex-associated copy number variation markers and female hemizygous regions on the W chromosome in Salix viminalis. BMC Genom. 22, 710 (2021).
Tennessen, J. A. et al. Repeated translocation of a gene cassette drives sex-chromosome turnover in strawberries. PLoS Biol. 16, e2006062 (2018).
Charlesworth, B. & Charlesworth, D. A model for the evolution of dioecy and gynodioecy. Am. Nat. 112, 975–997 (1978).
Charlesworth, D., Charlesworth, B. & Marais, G. Steps in the evolution of heteromorphic sex chromosomes. Heredity 95, 118–128 (2005).
Bergero, R. & Charlesworth, D. The evolution of restricted recombination in sex chromosomes. Trends Ecol. Evol. 24, 94–102 (2009).
Van Doorn, G. & Kirkpatrick, M. Turnover of sex chromosomes induced by sexual conflict. Nature 449, 909–912 (2007).
Varkonyi-Gasic, E. et al. Shy Girl, a kiwifruit suppressor of feminization, restricts gynoecium development via regulation of cytokinin metabolism and signalling. N. Phytol. 230, 1461–1475 (2021).
Testolin, R., Cipriani, G. & Costa, G. Sex segregation ratio and gender expression in the genus Actinidia. Sex. Plant Reprod. 8, 129–132 (1995).
Tahir, J. et al. First chromosome-scale assembly and deep floral-bud transcriptome of a male kiwifruit. Front. Genet. https://doi.org/10.3389/fgene.2022.852161 (2022).
Charlesworth, B., Sniegowski, P. & Wright, S. The evolutionary dynamics of repetitive DNA in eukaryotes. Nature 371, 215–220 (1994).
Kapun, M. & Flatt, T. The adaptive significance of chromosomal inversion polymorphisms in Drosophila melanogaster. Mol. Ecol. 28, 1263–1282 (2019).
Charlesworth, D. Young sex chromosomes in plants and animals. N. Phytol. 224, 1095–1107 (2019).
Akagi, T. & Charlesworth, D. Pleiotropic effects of sex-determining genes in the evolution of dioecy in two plant species. Proc. R. Soc. B https://doi.org/10.1098/rspb.2019.1805 (2019).
Komatsuda, T. et al. Six-rowed barley originated from a mutation in a homeodomain-leucine zipper I-class homeobox gene. Proc. Natl Acad. Sci. USA 104, 1424–1429 (2007).
González-Grandío et al. Abscisic acid signaling is controlled by a BRANCHED1/HD-ZIP I cascade in Arabidopsis axillary buds. Proc. Natl Acad. Sci. USA 114, E245–E254 (2017).
Darwin, C. R. The Different Forms of Flowers on Plants of the Same Species (John Murray, 1877).
Barrett, S. C. & Hough, J. Sexual dimorphism in flowering plants. J. Exp. Bot. 64, 67–82 (2013).
Cheng, H., Concepcion, G. T., Feng, X., Zhang, H. & Li, H. Haplotype-resolved de novo assembly using phased assembly graphs with hifiasm. Nat. Methods 18, 170–175 (2021).
Pilkington, S. M. et al. A manually annotated Actinidia chinensis var. chinensis (kiwifruit) genome highlights the challenges associated with draft genomes and gene prediction in plants. BMC Genom. 19, 257 (2018).
Alonge, M. et al. RaGOO: fast and accurate reference-guided scaffolding of draft genomes. Genome Biol. 20, 224 (2019).
Cantarel, B. L. et al. MAKER: an easy-to-use annotation pipeline designed for emerging model organism genomes. Genome Res. 18, 188–196 (2008).
Wu, H. et al. A high-quality Actinidia chinensis (kiwifruit) genome. Hortic. Res. 6, 117 (2019).
Tang, W. et al. Chromosome-scale genome assembly of kiwifruit Actinidia eriantha with single-molecule sequencing and chromatin interaction mapping. GigaScience 8, giz027 (2019).
Smit, A. F. A., Hubley, R. & Green, P. RepeatMasker Open v.4.0 http://www.repeatmasker.org (2013–2015).
Jurka, J. et al. Repbase Update, a database of eukaryotic repetitive elements. Cytogenet. Genome Res. 110, 462–467 (2005).
Smit, A. F. A. & Hubley, R. RepeatModeler Open v.1.0 http://www.repeatmasker.org (2008–2015).
R Core Team. R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, 2021); https://www.R-project.org/
Gu, Z., Gu, L., Eils, R., Schlesner, M. & Brors, B. circlize implements and enhances circular visualization in R. Bioinformatics 30, 2811–2812 (2014).
Wang, Y. et al. MCScanX: a toolkit for detection and evolutionary analysis of gene synteny and collinearity. Nucleic Acids Res. 40, e49 (2012).
Cabanettes, F. & Klopp, C. D-GENIES: dot plot large genomes in an interactive, efficient and simple way. PeerJ https://doi.org/10.7717/peerj.4958 (2018).
Bayer, M. et al. Comparative visualization of genetic and physical maps with Strudel. Bioinformatics 27, 1307–1308 (2011).
Li, H. & Durbin, R. Fast and accurate short read alignment with Burrows–Wheeler transform. Bioinformatics 25, 1754–1760 (2009).
Robinson, J. T. et al. Integrative genomics viewer. Nat. Biotechnol. 29, 24–26 (2011).
Ellinghaus, D., Kurtz, S. & Willhoeft, U. LTRharvest, an efficient and flexible software for de novo detection of LTR retrotransposons. BMC Bioinform. 9, 18 (2008).
Ou, S. & Jiang, N. LTR_FINDER_parallel: parallelization of LTR_FINDER enabling rapid identification of long terminal repeat retrotransposons. Mob. DNA 10, 48 (2019).
Ou, S. & Jiang, N. LTR_retriever: a highly accurate and sensitive program for identification of long terminal repeat retrotransposons. Plant Physiol. 176, 1410–1422 (2018).
Shi, J. & Liang, C. Generic repeat finder: a high-sensitivity tool for genome-wide de novo repeat detection. Plant Physiol. 180, 1803–1815 (2019).
Su, W., Gu, X. & Peterson, T. TIR-Learner, a new ensemble method for TIR transposable element annotation, provides evidence for abundant new transposable elements in the maize genome. Mol. Plant 12, 447–460 (2019).
Han, Y. & Wessler, S. R. MITE-Hunter: a program for discovering miniature inverted-repeat transposable elements from genomic sequences. Nucleic Acids Res. 38, e199 (2010).
Xiong, W., He, L., Lai, J., Dooner, H. K. & Du, C. HelitronScanner uncovers a large overlooked cache of Helitron transposons in many plant genomes. Proc. Natl Acad. Sci. USA 111, 10263–10268 (2014).
Li, W. & Godzik, A. Cd-hit: a fast program for clustering and comparing large sets of protein or nucleotide sequences. Bioinformatics 22, 1658–1659 (2006).
Wang, T., Atkinson, R. & Janssen, B. The choice of Agrobacterium strain for transformation of kiwifruit. ISHS Acta Hortic. 753, 227–232 (2007).
Masuda, K. et al. Reinvention of hermaphroditism via activation of a RADIALIS-like gene in hexaploid persimmon. Nat. Plants 8, 217–224 (2022).
Love, M. I., Huber, W. & Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550 (2014).
Gu, Z. Complex heatmaps reveal patterns and correlations in multidimensional genomic data. Bioinform 32, 2847–2849 (2016).
Tamura, K. et al. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 28, 2731–2739 (2011).
Kumar, S., Stecher, G., Li, M., Knyaz, C. & Tamura, K. MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 35, 1547–1549 (2018).
Acknowledgements
We thank L. Comai (Department of Plant Biology and Genome Center, University of California, Davis, USA) for discussion and comments on this study and K. Miyata (Board of Education of Miyoshi city, Tokushima, Japan) for sampling of native Actinidia germplasm collections. This work was supported by PRESTO from Japan Science and Technology Agency (grant no. JPMJPR20Q1) and Grant-in-Aid for Transformative Research Areas (A) from JSPS (grant nos 22H05172 and 22H05173) to T.A.
Author information
Authors and Affiliations
Contributions
T.A., D.C. and I.K. conceived the study. T.A., E.V.-G., A.C., I.M.H. and D.C. designed the experiments. T.A., E.V.-G., A.C., K.S., K.M., N.F. and E.K. conducted the experiments. T.A., E.V.-G., A.C., K.S., P.D., D.M., K.M. and N.F. analysed the data. T.A., E.V.-G., K.S., I.M.H., P.D., K.U., K.B., A.C.A. and I.K. contributed to the plant resources and facilities. T.A., E.V.-G., A.C., I.M.H., D.C. and I.K. drafted the manuscript. All authors approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Plants thanks Richard Moore and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Conserved sexual dimorphisms in a wide variety of Actinidia species.
a, Representative female and male flowers in Actinidia species (from a segregated F1 population of A. chinensis x A. rufa, named KE-population in Akagi et al. 2018). Male flowers frequently form big clusters. B Floral shoots of four Actinidia species: A. chinensis, A. rufa, A. polygama, and A. arguta var. hypoleuca. Males produced more flower buds (fb) than female. This tendency is thought to be linked to two factors, increased number of flowers per inflorescence in male than in female (c), and increased number of floral nodes per shoot in male than in female (d). *p < 0.05, **p < 0.01, ***p < 0.001 Student’s t-test (biological replicates N = 10 for c, N = 5 for d).
Extended Data Fig. 2 Genome-wide syntenic relationships among A. chinensis, A. rufa, A. polygama, and A. arguta.
Syntenic blocks were defined based on conserved physical order of homologous gene pairs (<1e-50), using MCSanX (Wang et al. 2012) with default parameters. Basic collinearity was conserved amongst the four species for most chromosomes, although some chromosomes exhibited large scale inversions or translocations (for example, Chr19 and 23).
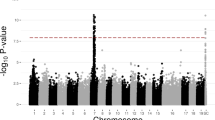
Extended Data Fig. 3 Genetic evidence for transition of the sex determining regions in the genus Actinidia.
Circos plots showing the genomic locations and significance (–log10 P values) of SNPs segregating with sex. These SNPs were identified after mapping reads of interspecific hybrid populations to different genome assemblies. a-d, Reads from populations CR (A. chinensis X A. rufa) (a), CM (A. chinensis X A. arguta var. melanandra) (b), CP (A. chinensis X A. arguta var. purpurea) (c) and CV (A. chinensis X A. valvata) (d) were aligned to the cv. Russell male A. chinensis genome assembly (Tahir et al., 2022). In this assembly, the sex determining region is located on the edge of chr. 25. e, Reads from population CR was aligned to the de novo assembled A. rufa genome. f-g, Reads from populations CM (f) and CP (g) were aligned to the de novo assembled A. arguta genome. h, Reads from population CV was aligned to the de novo assembled A. polygama genome. i-k, Linkage maps of populations CR (i), CM (j), and CP (k), are consistent with the results that the sex determining genes are located on Chr. 4 and Chr. 3 in A. rufa and A. arguta, respectively. l, Summary of the location of the sex determining regions (or sex chromosomes), based on these genomic and genetic analyses.
Extended Data Fig. 4 Sequence contexts of the sex determining regions in Chr25 of A. chinensis and A. polygama.
a-b, close-up of the Y-haplotype region of Chr25 in A. chinensis. a, An approximately 1.3 Mb region (putative MSY) exhibited mostly male-specific coverage of genomic reads. b, Synteny between Y- and X-haplotypes. Green bands indicate predicted genes. c, close-up view of the Chr25 Y-haplotype region in A. polygama. The MSY corresponds to a ~1.1 Mb region.
Extended Data Fig. 5 No synteny is conserved amongst the MSYs of four Actinidia species.
a, Syntenic dotplots between sex determining regions of A. chinensis and A. polygama, in Chr25. Although PAR regions exhibited significant collinearity, no long syntenic blocks were observed within the MSYs. b-c, Syntenic dotplot of the neo-sex determining regions, Chr3 in A. arguta (b) and Chr4 in A. rufa (c), against A. chinensis Chr25. d-e, Synteny analyses between the two neo-sex determining regions in A. arguta and A. rufa. No collinearity was detected, except for the presence of three conserved genes in the MSYs, Shy Girl, Friendly Boy, and YFT. Green bands indicate predicted genes (not including TE-like genes) for panel e.
Extended Data Fig. 6 Dynamic inversions of the three conserved genes in the MSYs of four Actinidia species.
Physical location of the three conserved genes, Shy Girl (SyGI, magenta), Friendly Boy (FrBy, light green), and Y-encoded FT-like (YFT, orange) genes in the MSYs of 4 Actinidia species. Their physical orders were different in all 4 species, implying frequent lineage-specific inversions.
Extended Data Fig. 7 TE enrichments in neo-MSY were not observed in the counterpart region in X chromosome.
a, Distribution of four TE classes (LTR-unknown, CACTA, hAT, and Helitron) in the A. rufa Y- and X-chromosomes (Chr 4), surrounding the neo-MSY. b, Distribution of Gypsy TE in the A. arguta Y- and X-chromosomes (Chr 3), surrounding the neo-MSY. Hyper enrichment of these TEs was specific to the neo-MSY in both species.
Extended Data Fig. 8 Shy Girl is solely explainable for most of the representative sexual dimorphisms in the genus Actinidia.
a, Disruptive mutations in the sygl lines. Red arrows indicated the gRNA sequences used for gene-editing. b-h, Sexual dimorphism traits are comparable between sygl gene-edited A. chinensis cv. Bruce and female A. chinensis cv. Hort16A grown in glasshouse conditions. (b, c) Solitary flowers with aborted bracts and lateral flower initials (arrows) in sygl and female lines. (d) Terminal and lateral flowers and multiple bracts (orange arrows) in male lines. (e) Comparison of floral node numbers in glasshouse-grown female, sygl and male A. chinensis. (f, g) Typical sygl and female shoots bearing fruit after pollination. (h) Schematic representation of the number of lateral shoots (horizontal axis) and position of vegetative (green) and floral nodes (yellow) on lateral shoots (vertical axis) in representative male, hermaphrodite sygl (gene-edited) and female lines.
Extended Data Fig. 9 Transcriptomic characterization of flower buds in comparison of the original male and the sygl (edited) lines.
a, Principal component analysis (PCA) of gene expression profiles in inflorescences of male and sygl hermaphrodite lines. Day 0 and day 4 represented embryonic inflorescence from dormant and breaking buds, respectively. Day 10 and day 14 represented inflorescences developing in leaf axils of expanding shoots. b, Representative images demonstrating early inflorescence development. Comparable development was observed in male and sygl lines with dome shaped meristems (dsm) and early inflorescence (inf) and bract (br) development at day 0, initiation of lateral flowers (lf) and sepals (s) at day 4, and presence of lateral and terminal flowers (tf) at day 10, but only terminal and aborted lateral flowers (alf) were present in sygl lines at day 14. c, Heatmap and hierarchical clustering of the DEGs between the male and the sygl-edited hermaphrodite inflorescences. Clust 2 (d) and Clust 5 (f) include genes upregulated in the sygl-edited hermaphrodite during the time period of day 4 to 10, and constantly downregulated in the sygl-edited hermaphrodite for the time period from day 0 to 14, respectively (Supplementary Data S5). GO analysis of Clust2 (e), and Clust5 (g).
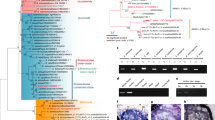
Extended Data Fig. 10 Evolutionary topology of HB21/40/53-MeGI-Vrs1-like genes and their functional conservation.
Maximum-likelihood (ML)-based phylogenetic analysis of HB21/40/53-MeGI-Vrs1 orthologs including Acc06091 from the kiwifruit genome, which is a DEG nested into Clust 5 (see Extended Data Fig. 9). Three clades of angiosperms (Asterid, Rosid, and Monocot) are shown in different colors. MeGI silencing by the Y-encoded OGI gene is responsible for development of trifurcated cyme-like male, instead of solitary female flowers in persimmon (Asterid, blue shade), absence of HB21, HB40 and HB53 is associated with a highly branched phenotype in Arabidopsis (Rosid, green shade), and absence of Vrs1 gives rise to six-rowed instead of wild-type two-rowed spike in barley (Monocot, yellow shade). Consistently, differential expression of Acc06091 is correlated with variation in branching in male vs sygl inflorescences.
Supplementary information
Supplementary Data
Supplementary Data 1: Genome assembly qualities. Supplementary Data 2: Genome-wide repeats statistics. Supplementary Data 3: MSY gene lists in four Actinidia species. Supplementary Data 4: TE counts in MSYs of four Actinidia species. Supplementary Data 5: List of the DEGs in Clusters 2 and 5.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Akagi, T., Varkonyi-Gasic, E., Shirasawa, K. et al. Recurrent neo-sex chromosome evolution in kiwifruit. Nat. Plants 9, 393–402 (2023). https://doi.org/10.1038/s41477-023-01361-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41477-023-01361-9
This article is cited by
-
Haplotype-resolved genome assembly provides insights into evolutionary history of the Actinidia arguta tetraploid
Molecular Horticulture (2024)
-
Subgenome dominance shapes novel gene evolution in the decaploid pitcher plant Nepenthes gracilis
Nature Plants (2023)
-
DNA Methylation is Involved in Sex Determination in Spinach
Biochemical Genetics (2023)