Abstract
During cell migration, front-rear polarity is spatiotemporally regulated; however, the underlying design of regulatory interactions varies. In rod-shaped Myxococcus xanthus cells, a spatial toggle switch dynamically regulates front-rear polarity. The polarity module establishes front-rear polarity by guaranteeing front pole-localization of the small GTPase MglA. Conversely, the Frz chemosensory system, by acting on the polarity module, causes polarity inversions. MglA localization depends on the RomR/RomX GEF and MglB/RomY GAP complexes that localize asymmetrically to the poles by unknown mechanisms. Here, we show that RomR and the MglB and MglC roadblock domain proteins generate a positive feedback by forming a RomR/MglC/MglB complex, thereby establishing the rear pole with high GAP activity that is non-permissive to MglA. MglA at the front engages in negative feedback that breaks the RomR/MglC/MglB positive feedback allosterically, thus ensuring low GAP activity at this pole. These findings unravel the design principles of a system for switchable front-rear polarity.
Similar content being viewed by others
Introduction
Cell polarity with the asymmetric localization of proteins within cellular space is ubiquitous and foundational for many cellular functions, including growth and motility1,2,3. Nevertheless, how polarity emerges at cellular scales from local protein-protein interactions and how it is dynamically controlled is poorly understood. Polarity regulators are often connected to generate networks that include positive feedback, negative feedback and/or mutual inhibition2,4,5,6,7. In transcriptional regulation, it is well-established that different designs of regulatory circuits can result in functionally equivalent outcomes, e.g. double-negative is functionally equivalent to double-positive regulation8. Similarly, polarity-regulating networks with functionally equivalent outcomes can have different designs, raising the question of why a particular network design has been selected.
A recurring theme in polarity-regulating systems is the localization of the active GTP-bound form of a small GTPase at a single intracellular location6,7,9,10,11,12. The GTPase, in turn, interacts with downstream effectors to implement a specific response. These GTPases are molecular switches that alternate between an inactive, GDP-bound and an active, GTP-bound conformation13. The activation/deactivation cycle is regulated by a cognate guanine-nucleotide exchange factor (GEF), which facilitates the exchange of GDP for GTP, and a GTPase activating protein (GAP), which stimulates the low intrinsic GTPase activity14. Two experimentally and theoretically well-studied systems illustrate how polarity-regulating networks with different designs can result in equivalent outcomes. In Saccharomyces cerevisiae lacking the small GTPase Rsr1, the location of the single bud site depends on where the GTPase Cdc42 spontaneously forms a single cluster on the membrane. The responsible regulatory network centers on at least one positive feedback directly involving Cdc424,9. Briefly, Cdc42-GTP spontaneously forms a cluster on the membrane and then recruits a complex that includes the GEF Cdc249. Because Cdc24 activates additional Cdc42, Cdc24 recruitment stimulates the accumulation of additional Cdc42-GTP, closing the positive feedback9. Cdc42 GAPs inhibit Cdc42 cluster growth and may be part of a negative feedback9,15,16. In the alternative system, unidirectional migration of the rod-shaped cells of the bacterium Myxococcus xanthus depends on the localization of the GTPase MglA at the leading front pole. In this case, the positive feedback does not involve MglA but rather the GAP MglB and the RomR scaffold17. Ultimately, these two proteins establish a rear, lagging pole with high GAP activity leaving only the opposite pole free to recruit MglA-GTP17. Thus, both systems generate a single Cdc42/MglA cluster. Here, we focus on the mechanistic basis of polarity establishment in M. xanthus and the functional properties conferred by the underlying network compared to the circuit that brings about Cdc42 cluster formation.
M. xanthus migrates unidirectionally on surfaces using two motility machines that assemble at the leading pole11,18,19. In response to signalling by the Frz chemosensory system, cells reverse the direction of movement20. During reversals, cells invert polarity and the pole at which the motility machines assemble switches21,22. Motility and its regulation by the Frz system are essential for multicellular morphogenesis with the formation of predatory colonies and spore-filled fruiting bodies11,18,19. Active MglA-GTP stimulates the assembly of the motility machineries at the leading cell pole23,24,25. Front-rear polarity is regulated dynamically by two interconnected protein modules, i.e. the polarity module and the Frz chemosensory system, that in combination generate a spatial toggle switch19. The polarity module sets up the leading/lagging polarity axis and, in addition to MglA, comprises four proteins that also localize asymmetrically to the cell poles (Fig. 1a). The homodimeric roadblock domain protein MglB alone has GAP activity and together with its low-affinity co-GAP RomY, forms the MglB/RomY complex with even higher GAP activity26,27,28. RomX alone has GEF activity and forms the RomR/RomX complex with even higher GEF activity and also serves as a polar recruitment factor for MglA-GTP29.
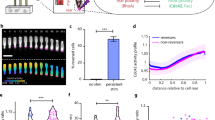
a Schematic of the localization of the polarity proteins. T4P indicate the leading pole. The circle size indicates relative amount of a protein at a pole. Colour code as in Fig. 1b. b Schematic of interactions between polarity proteins. Dashed box indicates the RomR/MglB positive feedback. c MglA, MglB and RomR polar localization depends on MglC. All fusion proteins were synthesized from their native locus. In the diagrams, the poles with the highest and lowest polar fraction of fluorescence are defined as pole 1 and pole 2, respectively. Filled circles, mean fraction of fluorescence at each pole. Dispersion of the single-cell measurements is represented by error bars and ellipses (colored dashed lines). Black dashed lines, symmetry lines; grey dashed lines, guidelines indicating fraction of total polar fluorescence. Number of cells analyzed (n), indicated in top right corners. Similar results were obtained in three independent experiments; data from one experiment is shown. mglA, mglB, mglC and romR genotypes are indicated with A, B, C and R, respectively. Schematics on the right, effects observed. d MglC-mVenus localizes asymmetrically and dynamically to the cell poles. The fusion was synthesized from the native locus. Cells were imaged at 30 sec intervals. 200 cells were imaged in two independent experiments; one representative cell is shown. Scale bar, 1μm. e MglC polar localization depends partially on MglB and strongly on RomR. Experiments were done and data presented as in Fig. 1c. f Quantification of the polar localization of MglC-mVenus, MglB-mCherry and RomR-mCherry in the absence of MglA. Experiments were done and data presented as in Fig. 1c. g MglC is a component of the RomR/MglC/MglB positive feedback (dashed box). h. MglC is essential for establishing correct RomR polarity. Cells were imaged as in Fig. 1d, and the fractions of cells with the brightest cluster at the leading or lagging pole determined. Left panel, summary of fractions of cells with indicated localization pattern. Total number of cells in three independent experiments is indicated on the top. Right panel, quantification of RomR-mCherry localization in moving cells.
Experiments and mathematical modelling have uncovered an intricate set of regulatory interactions between the proteins of the polarity module17,26,27,28,29,30,31,32 (Fig. 1b). The RomR scaffold is at the base of all other polarity proteins’ polar localization and also reinforces its own polar localization, thereby establishing a positive feedback17. RomR also engages in a positive feedback with MglB by an unknown mechanism17. Additionally, RomR directly recruits RomX to form the RomR/RomX GEF complex29. High concentrations of polar MglB stimulate polar recruitment of its low-affinity interaction partner RomY28. At the RomR node of the RomR/MglB positive feedback, RomR/RomX promotes MglA-GTP polar recruitment (Fig. 1b – connector from RomR/RomX to MglA)29, and at the MglB node, MglB/RomY inhibits MglA-GTP polar recruitment (Fig. 1b – connector from MglB/RomY to MglA)26,27,28. Finally, MglA-GTP disrupts the RomR/MglB positive feedback by an unknown mechanism (Fig. 1b – connector from MglA to dashed box)17. Together these interactions have been suggested to result in the system’s emergent properties (Fig. 1a, b)17,28. Briefly, at the pole with the highest RomR concentration, the RomR/MglB positive feedback establishes a pole with high concentrations of RomR/RomX and MglB/RomY. Due to the presence of the MglB/RomY complex, GAP activity dominates over GEF activity at this pole, thus inhibiting MglA-GTP recruitment, and this pole becomes the lagging pole. At the opposite pole, RomR/RomX GEF activity dominates over GAP activity because the low concentration of MglB is insufficient to recruit RomY28. Consequently, MglA-GTP is recruited to this pole and engages in the negative feedback to inhibit the RomR/MglB positive feedback, thereby ensuring the low concentration of the other polarity regulators. The Frz system is the second module of the spatial toggle switch, and the polarity module is the downstream target of this system. Frz signaling causes the inversion of polarity of the proteins of the polarity module by an unknown mechanism, thus laying the foundation for the assembly of the motility machineries at the new leading pole30,31,33,34.
Among the interactions of the proteins of the polarity module, the positive feedback of RomR on itself, the RomR/MglB positive feedback, and the inhibitory effect of MglA-GTP on this positive feedback are poorly understood. MglC is also a homodimeric roadblock domain protein35,36,37 and is involved in cell polarity regulation by an unknown mechanism36. Because MglC interacts with RomR and MglB35,36, MglC was a candidate for acting in the RomR/MglB positive feedback.
Here, we show that MglC forms a complex with RomR and MglB, thereby establishing a RomR/MglC/MglB positive feedback and that MglA-GTP inhibits this positive feedback by breaking the interaction between the MglC and MglB roadblock domain proteins. Moreover, we demonstrate that the RomR/MglC/MglB positive feedback lays the foundation for switchable polarity.
Results
MglC is important for Frz-induced cellular reversals
To investigate the function of MglC in polarity, we recharacterized the motility defects of a mutant with an in-frame deletion of mglC (ΔmglC). In agreement with previous findings36, the ΔmglC mutant has defects in both gliding and T4P-dependent motility in population-based motility assays, and ectopic expression of mglC complemented these defects (Supplementary Fig. 1a, b). In single cell-based motility assays (Supplementary Fig. 1c), and consistent with previous observations36, ΔmglC cells moved with the same speed as wild-type (WT) for both motility systems; however, similarly to the ΔfrzE negative control that lacks the FrzE kinase, ΔmglC cells had a significantly lower reversal frequency than WT.
To discriminate whether the ΔmglC mutant is unresponsive to or has reduced sensitivity to Frz signaling, we treated WT and ΔmglC cells with the short-chain alcohol isoamyl alcohol (IAA) that highly stimulates reversals in a FrzE-dependent manner38. WT and the ΔmglC mutant responded similarly to 0.3% IAA with the formation of colonies that had smooth edges and no visible flares on 0.5% agar, which is optimal for T4P-dependent motility, and few single cells at the edge on 1.5% agar, which is optimal for gliding motility (Supplementary Fig. 1a). Such smooth colony edges indicate a high reversal frequency20,39. We conclude that the ΔmglC mutant does not have a defect in motility per se but reduced sensitivity to Frz signalling resulting in a reduced reversal frequency.
MglC is important for the polar localization of MglA, MglB and RomR
Because the polarity module is the downstream target of the Frz system, we quantified the polar localization of active, fluorescently labelled fusions of the polarity proteins in the absence of MglC. Because RomX localization follows that of RomR29 and RomY localization follows the highest concentration of MglB28, we used RomR and MglB localization as readouts for the localization of the RomR/RomX complex and MglB/RomY complex, respectively.
In snapshots of ΔmglC cells (Fig. 1c), polar localization of MglA-mVenus and MglB-mCherry was strongly reduced, while RomR-mCherry polar localization was only partially lost. MglA, MglB and RomR accumulated independently of MglC (Supplementary Fig. 1d).
MglC polar localization depends partially on MglB and strongly on RomR
To study MglC localization, we first observed that a fully active MglC-mVenus fusion expressed from the native site (Supplementary Fig. 1a, b) localized in a bipolar asymmetric pattern with a large cluster at the lagging pole in WT cells and switched polarity during reversals (Fig. 1d). The bipolar asymmetric pattern was also evident in snapshots (Fig. 1e). In the absence of MglA, MglC-mVenus was more polar (Fig. 1e). However, in the absence of MglB, MglC-mVenus polar localization was partially lost; and, in the absence of RomR, it was almost completely lost (Fig. 1e). MglC-mVenus accumulated independently of MglA, MglB and RomR (Supplementary Fig. 1e). Thus, MglA inhibits MglC polar localization while MglC depends partially on MglB and strongly on RomR. Notably, in the absence of RomR, MglB fails to support significant MglC polar localization.
MglC establishes the RomR/MglC/MglB positive feedback
Because the interpretation of the results for polar localization of MglC, MglB and RomR can be challenging due to the inhibitory effect of MglA-GTP on the RomR/MglB positive feedback, we quantified their polar fluorescence in strains lacking MglA.
MglC-mVenus polar localization in the ΔmglAΔmglB mutant was partially lost compared to the ΔmglA mutant, almost completely abolished in the ΔmglAΔromR mutant, and completely abolished in the triple ΔmglAΔmglBΔromR mutant (Fig. 1f). These observations confirm that MglC-mVenus polar localization depends partially on MglB and strongly on RomR. They also confirm that in the absence of RomR, MglB fails to support MglC polar localization significantly.
MglB-mCherry polar localization in the ΔmglAΔmglC, the ΔmglAΔromR and the ΔmglAΔmglCΔromR mutants was almost completely lost (Fig. 1f). These results confirm that MglB-mCherry polar localization depends strongly on MglC and, as previously shown17, on RomR. Moreover, neither MglC nor RomR alone can establish efficient polar MglB-mCherry localization.
RomR-mCherry polar localization was partially abolished in the ΔmglAΔmglB, ΔmglAΔmglC and ΔmglAΔmglBΔmglC mutants (Fig. 1f). Thus, both MglB and MglC are important but not essential for RomR polar localization. Moreover, only when MglB and MglC are both present can they further stimulate RomR polar localization.
These observations demonstrate that RomR alone localizes polarly, and they support that RomR recruits MglC, which then recruits MglB. The observations that (1) MglB stimulates MglC polar localization in the presence of RomR, and (2) MglB together with MglC stimulates RomR polar localization support that the three proteins establish a positive feedback that reinforces their polar localization (Fig. 1g). These observations also suggest that the previously established RomR/MglB positive feedback depends on MglC, i.e. MglC helps to generate a RomR/MglC/MglB positive feedback by acting between RomR and MglB (Fig. 1g). Because MglA inhibits the RomR/MglB positive feedback17, this model also explains the observation that MglA inhibits MglC polar localization (Fig. 1e). Moreover, the reduced MglA polar localization in the absence of MglC (Fig. 1c) is a direct outcome of the reduced RomR polar localization in the absence of MglC (Fig. 1c, f).
To further test the idea of the RomR/MglC/MglB positive feedback, we leveraged an established approach to monitor the cooperative polar recruitment of RomR-mCherry17. In this approach, a vanillate-inducible promoter drives romR-mCherry expression; upon induction, RomR-mCherry polar localization is followed by time-lapse fluorescence microscopy. To monitor RomR-mCherry synthesis over time, we estimate the RomR-mCherry concentration in individual cells, referred to as the fluorescence concentration, by measuring total cellular fluorescence and then normalizing by cell area, which we use as a proxy for cell volume.
Upon induction of romR-mCherry expression in the ΔmglAΔmglBΔromRΔmglC quadruple mutant (Supplementary Fig. 2a) and the ΔmglAΔromRΔmglC triple mutant (Supplementary Fig. 2b), RomR-mCherry localized asymmetrically to the poles at all fluorescence concentrations and quantitatively followed the pattern previously observed in the ΔmglAΔmglBΔromR triple mutant (Supplementary Fig. 2c). As described17, the observations that the fractions of RomR-mCherry at both poles increase with fluorescence concentration at low induction levels provide evidence for positive cooperativity in RomR-mCherry polar localization (Supplementary Fig. 2a-d). Because RomR-mCherry polar localization is quantitatively similar in these three strains, we conclude that MglC, similar to MglB, is not essential for the positive feedback of RomR on itself. By contrast, in the ΔmglAΔromR double mutant, RomR-mCherry polar localization was increased and more asymmetric, with the brighter pole accounting for a larger fraction of RomR-mCherry fluorescence (Supplementary Fig. 2d). This observation confirms that MglC is essential for establishing the RomR/MglB positive feedback and that the RomR/MglB positive feedback is, in fact, a RomR/MglC/MglB positive feedback (Fig. 1g).
MglC is essential for establishing correct RomR polarity
The model for polarity establishment (Fig. 1g) predicts that in the absence of MglC and, therefore, the RomR/MglC/MglB positive feedback, the residual polar RomR, together with RomX, will recruit MglA-GTP. As a result, MglA-GTP and RomR/RomX will have their highest polar fluorescence at the leading pole. To test this prediction, we performed time-lapse fluorescence microscopy of moving cells. In WT, MglA-mVenus localized with a large cluster at the leading pole and RomR-mCherry with a large cluster at the lagging pole in most cells (Fig. 1h). Importantly, and as predicted, in the ΔmglC mutant, MglA-mVenus and RomR-mCherry had their highest polar fluorescence at the leading pole in most cells (Fig. 1h). We conclude that MglC is important not only for the polar localization of MglA, MglB and RomR but also for establishing the correct polarity of RomR-mCherry.
RomR, MglC and MglB interact to form a complex
To investigate the mechanism underlying the RomR/MglC/MglB positive feedback, we tested for direct interactions between RomR, MglC, MglB and MglA using pull-down experiments in vitro with purified proteins. In agreement with previous observations in in vitro pull-down experiments35 and Bacterial Adenylate Cyclase-Based Two-Hybrid (BACTH) assays36, Strep-MglC pulled-down His6-MglB and MalE-RomR in pairwise combinations, but not MglA-His6 preloaded with GTP (Fig. 2a; Supplementary Fig. 3a). In pairwise combinations using MalE-RomR as a bait, MalE-RomR pulled-down Strep-MglC but not His6-MglB; notably, in the presence of all three proteins, RomR-MalE pulled down Strep-MglC as well as His6-MglB (Fig. 2b; Supplementary Fig. 3b). Finally, in pairwise combinations, His6-MglB pulled-down Strep-MglC but not MalE-RomR; however, in the presence of all three proteins, His6-MglB pulled-down Strep-MglC as well as MalE-RomR (Fig. 2c; Supplementary Fig. 3c).
a–c Proteins were mixed at final concentrations of 10 µM and applied to the indicated matrices. Matrices were washed and bound proteins eluted. The bait protein is indicated by the black circle. In experiments with MglA-His6, the protein was preloaded with GTP and all buffers contained 40 µM GTP. Equivalent volumes of the load (L), last wash (W) and eluate (E) were separated on the same SDS-PAGE gel and stained with Coomassie Brilliant Blue. Gap between lanes indicates lanes deleted for presentation purposes. Calculated molecular masses of the indicated proteins are indicated on the right and molecular weight markers (M) on the left. In the schematics on the right, a direct interaction with the bait is indicated by a black and an indirect interaction by a dashed arrow. For samples with MalE-RomR, the faster migrating proteins are degradation products. Similar results were obtained in three independent experiments.
Next, we determined whether MglC and/or RomR/MglC have MglA GAP activity or interfere with MglB and/or MglB/RomY GAP activity. To this end, we determined MglA-His6 GTPase activity in the presence of RomR, MglC, MglB and/or RomY. Neither Strep-MglC nor MalE-RomR/Strep-MglC affected MglA GTPase activity in the presence or absence of MglB-His6 and/or Strep-RomY (Supplementary Fig. 4).
We conclude that RomR, MglC and MglB interact to form a complex in which MglC is sandwiched between RomR and MglB.
The MglB KRK surface region represents the interface for interaction with MglC
To elucidate the structural basis for the RomR→MglC→MglB interactions, we took advantage of structural information for MglA, MglB and MglC35,40,41,42. Each MglB protomer in the homodimer consists of a five-stranded β-sheet sandwiched between the α2-helix and the α1/α3-helices40. In the dimer, the α2-helices generate the so-called two-helix side and the pairs of α1/α3-helices the so-called four-helix side (Fig. 3a). In the crystallographic structure of the MglA-GTPɣS:MglB2 complex, the MglA monomer interacts asymmetrically with the two-helix side of the MglB dimer40,41,42.
a Crystallographic structure of MglB dimer (pdb ID: 6hjm40) viewed from the two-helix and four-helix sides. Lower panels, surface representation of MglB dimer based on electrostatic surface potential contoured from +5 to −5 kT e−1. The K14, R115 and K120 residues are indicated in red on the four-helix side and the corresponding positively charged surface regions by black circles in the electrostatic surface potential diagrams. b The MglBKRK variant does not interact with MglC. Pull-down experiment was performed with His6-MglBKRK as bait on the indicated resin, and the data presented as in Fig. 2. Similar results were obtained in three independent experiments. c. MglBKRK-mCherry has reduced polar localization. For comparison, MglBWT-mCherry is included (red dot). MglBKRK-mCherry was synthesized from the native locus. d MglBKRK causes reduced polar localization of MglA, MglC and RomR. For comparison, the localization of the three fusion proteins are included in the presence of MglBWT (yellow, brown and green dots). MglBKRK was synthesized from the native locus. In c–d, experiments were done and data presented as in Fig. 1c.
Galicia et al. reported that the K14, R115, K120 residues in MglB are highly conserved in MglB homologs, surface-exposed in the solved structure of the MglB dimer generating two positively charged surface regions on the four-helix side of the MglB dimer40 (Fig. 3a). Moreover, they reported that the MglBK14A R115A K120A variant (henceforth, MglBKRK) with substitutions of these three positively charged residues to Ala still has GAP activity in vitro but localizes diffusely in vivo by an unknown mechanism40. Because MglB localizes diffusely in the absence of MglC, we, therefore, hypothesized that the positively charged surface regions in the MglB dimer defined by the K14, R115, K120 residues could be involved in the interaction between MglB and MglC.
In in vitro pull-down experiments, His6-MglBKRK did not detectably bind Strep-MglC (Fig. 3b). Consistently, polar localization of MglBKRK-mCherry in otherwise WT cells was strongly reduced independently of the presence or absence of MglC and MglA (Fig. 3c; Supplementary Fig. 5a). In the inverse experiment, MglBKRK caused a strong reduction in MglA-mVenus localization, while MglC-mVenus and RomR-mCherry polar localization was partially abolished (Fig. 3d; Supplementary Fig. 5a). We conclude that MglBKRK is deficient in interacting with MglC and suggest that the positively charged KRK surface regions in the MglB dimer represent the interface to MglC, in agreement with the suggestion by Kapoor et al.35. Moreover, we suggest that the effect of the MglBKRK variant on RomR and MglA localization is caused by the interruption of the RomR/MglC/MglB positive feedback, resulting in reduced polar RomR/RomX localization and, consequently, reduced MglA polar localization.
The MglC FDI surface region represents the interface for interaction with MglB
The MglC homodimer’s structure is similar to that of MglB with two-helix and four-helix sides (Fig. 4a)35. McLoon et al. reported that the F25, D26, I28 residues in MglC are highly conserved in MglC homologs36 (Supplementary Fig. 6). In the solved structure of the MglC dimer, these three residues are surface-exposed and generate two separated negatively charged surface regions on the two-helix side (Fig. 4a). McLoon et al. also reported that the MglCF25A D26A I28A variant (henceforth, MglCFDI) with substitutions of these three residues to Ala was abolished in its interaction with MglB but not with RomR based on BACTH assays36. We, therefore, hypothesized that the two negatively charged surface regions defined by the F25, D26, I28 residues could represent the interaction interface of MglC to MglB.
a Crystallographic structure of MglC dimer (pdb ID: 7CY135) viewed from two-helix and four-helix sides. Lower panels, surface representation of MglC dimer based on electrostatic surface potential contoured from +5 to −5 kT e−1. The F25, D26, I28 residues and the K104, R106, R110 residues are indicated in red on the two-helix and the four-helix sides, respectively, and the corresponding negatively and positively charged surface regions indicated by black circles in the electrostatic surface potential diagrams. b MglCFDI-mVenus has reduced polar localization. For comparison, MglCWT-mVenus is included (brown dot). MglCFDI-mVenus was synthesized from the native locus. c MglCFDI causes reduced polar localization of MglA, MglB and RomR. For comparison, the localization of the three fusion proteins is included in the presence of MglCWT (yellow, red and green dots). MglCFDI was synthesized ectopically. d MglCKRR-mVenus has strongly reduced polar localization. For comparison, MglCWT-mVenus is included (brown dot). MglCKRR-mVenus was synthesized from the native locus. e MglCKRR causes reduced polar localization of MglA, MglB and RomR. For comparison, the localization of the three fusion proteins is included in the presence of MglCWT (yellow, red and green dots). MglCKRR was synthesized ectopically. In b-e, experiments were done and data presented as in Fig. 1c.
We initially sought to verify the effect of the MglCFDI variant on the MglC/MglB interaction in vitro; however, the Strep-tagged variant formed inclusion bodies in Escherichia coli under all conditions tested, precluding its purification. Importantly, in M. xanthus, MglCFDI-mVenus was soluble (Supplementary Fig. 5b); however, it accumulated at a reduced level compared to MglC-mVenus (Supplementary Fig. 5c). Polar localization of MglCFDI-mVenus in otherwise WT cells was partially lost in comparison to MglC-mVenus (Fig. 4b). Moreover, MglCFDI–mVenus polar localization did not change much upon removal of MglA or MglB but was abolished by removal of RomR (Fig. 4b). In the inverse experiment, MglCFDI accumulated like MglC and, similar to the ΔmglC mutation, caused strong reductions in MglA-mVenus and MglB-mCherry polar localization while RomR-mCherry polar localization was only partially abolished (Fig. 4c; Supplementary Fig. 5c). We conclude that MglCFDI is deficient in interacting with MglB but not with RomR and suggest that the negatively charged FDI surface regions in the MglC dimer represent the interface to MglB. Moreover, we suggest that the effect of the MglCFDI variant on RomR and MglA localization is caused by the interruption of the RomR/MglC/MglB positive feedback, resulting in reduced polar RomR/RomX localization and, consequently, reduced MglA polar localization, as observed in the ΔmglC mutant.
The MglC KRR surface region represents the interface for interaction with RomR
In addition to the FDI residues, the K104, R106, R110 residues (MglC numbering) are highly conserved in MglC homologs (Supplementary Fig. 6). In the solved structure of the MglC dimer, these three residues are surface exposed on the four-helix side and define a continuous, positively charged, surface-exposed region in the dimer (Fig. 4a). Because this region is apart from the FDI regions and MglC interacts with MglB and RomR in parallel, we hypothesized that the positively charged surface region defined by the K104, R106, R110 residues could represent the interaction interface of MglC to RomR.
To this end, we generated MglCK104A R106A R110A variants (henceforth, MglCKRR). However, the Strep-tagged variant formed inclusion bodies in E. coli under all conditions tested, precluding its purification. Importantly, in M. xanthus, MglCKRR-mVenus was soluble (Supplementary Fig. 5b) and accumulated at the same level as MglC-mVenus (Supplementary Fig. 5d). Polar localization of MglCKRR-mVenus in otherwise WT cells was strongly reduced compared to MglC-mVenus (Fig. 4d) and remained strongly reduced upon removal of MglA, MglB or RomR (Fig. 4d). In the inverse experiment, MglCKRR accumulated like MglC and, similar to the ΔmglC mutation and MglCFDI, caused strong reductions in MglA-mVenus and MglB-mCherry polar localization while RomR-mCherry polar localization was only partially abolished (Fig. 4e; Supplementary Fig. 5d). Based on these observations, we conclude that MglCKRR is deficient in interacting with RomR and suggest that the two positively charged KRR surface regions in the MglC dimer represent the interface to RomR. Moreover, we suggest that the effect of the MglCKRR variant on MglA localization is caused by the interruption of the RomR/MglC/MglB positive feedback, as observed in the ΔmglC and mglCFDI mutants.
The α-helical RomRC has three functions and represents the interface to MglC
RomR homologs comprise an N-terminal receiver domain of response regulators, an intrinsically disordered region (IDR), and an α-helical, negatively charged Glu-rich region at the C-terminus (henceforth, RomRC) (Fig. 5a)30. In BACTH assays, RomRC interacts with MglC36. To examine whether RomRC is the only interface to MglC, we generated a RomR1–368 variant that lacks RomRC and a variant only containing RomRC.
a Domain architecture and charge score of RomR. Charge score was calculated using a sliding window of 20 residues. b MP analysis of MalE-RomR, MalE-RomR1-368 and MalE-RomRC. Molecular masses corresponding to the respective Gaussian fits are shown in kDa above the fitted curves. Calculated molecular masses of the monomeric, dimeric and trimeric MalE-RomR variants are indicated in brackets together with symbols of oligomeric states. Similar results were obtained in three independent experiments. c RomRC interacts with MglC while RomR1–368 does not. Pull-down experiment was performed with Strep-MglC as bait on the indicated resin and presented as in Fig. 2. The multiple bands below MalE-RomRC and MalE-RomR1–368 are degradation products. Similar results were obtained in three independent experiments. d RomR1–368-mCherry has strongly reduced polar localization. For comparison, RomRWT-mCherry is included (green dot). RomR1–368-mCherry was synthesized from the native locus. e RomR1–368 causes strongly reduces polar localization of MglA, MglB and MglC. For comparison, the localization of the three fusion proteins is included in the presence of RomRWT (yellow, red and brown dots). RomR1–368 was synthesized from the native locus. f RomRC-mCherry localizes polarly. For comparison, RomRWT-mCherry is included (green dot). RomRC-mCherry was synthesized ectopically. In d-f, experiments were done and data presented as in Fig. 1c.
First, using mass photometry (MP), we investigated the oligomeric structure of RomR. We detected MalE-RomR with masses matching well with monomers, dimers and trimers (Fig. 5b). MalE-RomRC was also detected with masses matching well monomers, dimers and trimers, while MalE-RomR1–368 was only detected at a mass matching monomers (Fig. 5b). Trimeric MalE-RomR was more prevalent at 50 nM compared to 25 nM, while trimeric MalE-RomRC was equally present at 25 nM and 50 nM (Fig. 5b). We conclude that RomRC is required and sufficient for RomR oligomerization and that the receiver domains and the IDRs do not interact. Moreover, these results support that RomR forms up to trimers and full-length RomR begins to dissociate to dimers below 50 nM. Based on quantitative immunoblot analysis, an M. xanthus cell contains ~6000 ± 2000 RomR molecules (Supplementary Fig. 5e), resulting in a cellular RomR concentration of ~2.5 ± 0.8 µM. We, therefore, suggest that RomR is predominantly present as a trimer in vivo.
In pull-down experiments, MalE-RomR1–368 did not detectably interact with Strep-MglC while MalE-RomRC did (Fig. 5c). In M. xanthus, RomR1–368-mCherry accumulated at the same level as RomR-mCherry (Supplementary Fig. 5f), however polar localization in otherwise WT cells was abolished (Fig. 5d). In the inverse experiment, RomR1–368 accumulated like RomR and, similar to the ΔromR mutation, caused strong reductions in the polar localization of MglA-mVenus, MglB-mCherry and MglC-mVenus (Fig. 5e17,30,31; Supplementary Fig. 5f). RomRC-mCherry, even when expressed from the strong pilA promoter, accumulated at a strongly reduced level (Supplementary Fig. 5f); importantly, most cells had a weak polar signal (Fig. 5f).
We conclude that the negatively charged RomRC has three functions: It is required and sufficient for RomR oligomerization, represents the RomR interface to MglC, and is required and at least partly responsible for the polar localization of RomR.
A structural model of the RomR/MglC/MglB complex
To gain structural insights into the RomR/MglC/MglB complex, we used structural information35,40,41, our functional data and AlphaFold-Multimer43,44,45 structural predictions to model this complex. The AlphaFold-Multimer models of the MglB and MglC dimers were predicted with high confidence and agreed well with the solved, crystallographic structures of the MglB and MglC dimers (Supplementary Fig. 7a-c), documenting the validity of the structural predictions.
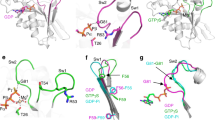
A low-resolution structure of the MglC/MglB complex supports that one MglC dimer binds two MglB dimers35. In AlphaFold-Multimer models with the same stoichiometry, two MglB dimers are predicted with high accuracy to interact using their four-helix sides with the “lateral” edges of the two-helix side of the MglC dimer giving rise to an MglC2:(MglB2)2 complex (Fig. 6a; Supplementary Fig. 7d). In this complex, Pymol-based analyses support that the R115 residues in the two KRK regions of an MglB dimer are in close proximity to and involved in establishing contact with D26 and I28 of an MglC FDI region (Fig. 6a, inset). Thus, this structural model agrees with a 2:4 stoichiometry of the MglC/MglB complex and together with our experimental findings support that the oppositely charged MglB KRK and MglC FDI surface regions interface. We note that the MglB dimers bound to MglC are structurally different compared to the MglB dimer in isolation, i.e. the four-helix side is in a more closed state when complexed with MglC2 (Supplementary Fig. 7e). On the other hand, the MglC dimer bound to MglB only has minor structural differences compared to the dimer in isolation (Supplementary Fig. 7f).
a AlphaFold-Multimer structural model of MglC2:(MglB2)2 complex. Model rank 1 is shown. Inset shows R115 in each of the MglB protomers together with the D26 and I28 residues in a MglC protomer. b MP analysis of MalE-MglC (top) and mixtures of MglC with MalE-RomR or MalE-RomRC. Molecular masses corresponding to the respective Gaussian fits are shown in kDa above the fitted curves. The Gaussian fits are colored according the respective protein MalE-MglC (brown), MalE-RomR (green), MalE-RomRC (green), MalE-RomR:MalE-MglC (purple) and MalE-RomRC:MalE-MglC (purple). Blue Gaussian fit (bottom panel) indicates a mixture of MalE-MglC and MalE-RomRC. Calculated molecular masses of monomeric and dimeric MalE-MglC, monomeric, dimeric and trimeric MalE-RomR/MalE-RomRC, and MalE-RomR/MalE-RomRC:MalE-MglC complexes with stoichiometries of 2:2 and 3:2 are indicated in brackets together with symbols of the oligomeric states. Similar results were obtained in three independent experiments. c Schematic of the RomR/MglC/MglB complex with a 3:2:4 stoichiometry. d Measurement of in vivo recovery kinetics of polar RomR-mCherry clusters in FRAP experiments. Upper panel, white circle indicates the bleached region of interest (ROI) at a lagging pole and the stippled line the bleaching event. Lower panel, normalized fluorescence intensity of the ROI before bleaching was set to 1.0. Colored dots indicate the mean and error bars STDEV. Dark lines show the recovery fitted to a single exponential. n, number of bleaching events at a lagging pole. Scale bar, 2μm. e Summary of T1/2 and Fmob. Cells were treated as in d with bleaching of clusters at the lagging (Lag) or leading (Lead) pole. Total number of bleaching events in three biological replicates are listed above. Error bars, mean ± STDEV. *P < 0.05, ns, no significant difference two-sided Student’s t-test. The exact P values are listed in the Source Data File.
To determine the stoichiometry of the RomR/MglC complex, we used MP. We detected a MalE-MglC fusion protein with masses matching a monomer and dimer (Fig. 6b). In the presence of both MalE-MglC and MalE-RomR, we detected, in addition to the masses of the individual proteins, complexes with masses consistent with a RomR:MglC stoichiometry of 2:2 and 3:2 (Fig. 6b; see also Fig. 5b). Similarly, in the presence of both MalE-MglC and MalE-RomRC, we detected additional peaks with masses consistent with a RomRC:MglC stoichiometry of 2:2 and 3:2 (Fig. 6b; see also Fig. 5b).
To obtain structural insights into the RomR/MglC complexes, we attempted to generate AlphaFold-Multimer structural models of dimeric and trimeric RomR, dimeric and trimeric RomRC, RomR2:MglC2 and RomR3:MglC2, as well as RomRC2:MglC2 and RomRC3:MglC2 complexes. However, none of these complexes was predicted with high confidence. Altogether, our experimental data support that the MglC dimer can interact with dimeric and trimeric RomR and that the interface between MglC and RomR are represented by the oppositely charged KRR regions in the MglC dimer and the negatively charged RomRC in the RomR dimer and trimer.
In total, these data support that a single MglC dimer is sandwiched between two MglB dimers and a RomR dimer or trimer, giving rise to a RomR:MglC:MglB complex with a 2:2:4 or a 3:2:4 stoichiometry. Because quantitative immunoblot analysis (Supplementary Fig. 5e) support that RomR is predominantly present as a trimer in vivo, we suggest that the dominant form of the RomR:MglC:MglB complex in vivo has a 3:2:4 stoichiometry (Fig. 6c).
MglC and MglB decrease RomR-mCherry polar turnover
The structural model of the RomR/MglC/MglB complex support a model for how RomR, MglC and MglB interact and how polar RomR recruits MglC, which recruits MglB. However, from this model, it is not clear how the positive RomR/MglC/MglB feedback is closed. We speculated that this loop could be closed if RomR would bind more stably to the poles in the RomR/MglC/MglB complex compared to RomR alone. To obtain a metric for the stability of RomR in polar clusters, we used Fluorescence Recovery after Photobleaching (FRAP) experiments in which polar RomR-mCherry clusters were bleached and half-maximal recovery time (T1/2) and the mobile fraction (Fmob) used to assess RomR-mCherry turnover. In WT, RomR-mCherry at the lagging/leading pole dynamically exchanged with the cytoplasm with T1/2 of 25.7 ± 15.2/17.3 ± 8.6 s, similar to previous results34, and Fmob of 0.7 ± 0.1/0.9 ± 0.1 (Fig. 6d, e). Thus, RomR-mCherry turnover is significantly slower and less at the lagging than at the leading pole. These observations agree with MglA-GTP at the leading pole engaging in a negative feedback to inhibit the RomR/MglC/MglB positive feedback. Consistently, in the non-motile ΔmglA mutant in which leading and lagging poles cannot be distinguished, T1/2 was increased and Fmob decreased compared to the leading pole in WT (Fig. 6e). Importantly, in ΔmglB and ΔmglC cells, T1/2 and Fmob of RomR-mCherry at the two poles were similar, and the T1/2 values significantly lower and the Fmob values significantly higher than at the lagging pole in WT (Fig. 6e).
These observations support that MglB and MglC jointly reduce polar RomR-mCherry turnover, thus supporting that more stable polar binding of RomR in the presence of both MglC and MglB closes the RomR/MglC/MglB positive feedback.
MglA-GTP breaks the MglC/MglB interaction
To dissect how MglA-GTP inhibits the positive RomR/MglC/MglB feedback, we hypothesized that MglA-GTP breaks the interaction between RomR/MglC, MglC/MglB or both. To this end, we performed pull-down experiments with Strep-MglC as bait. Strep-MglC pulled-down His6-MglB and MalE-RomR but not MglA-His6 in the presence of either the non-hydrolyzable GTP analogue GppNHp or GDP (Fig. 7a, b; Supplementary Fig. 8a). Intriguingly, in the presence of MglA-His6 loaded with GppNHp, Strep-MglC no longer pulled-down His6-MglB but still pulled-down His6-MglB in the presence of MglA-His6 loaded with GDP (Fig. 7a). By contrast, Strep-MglC pulled-down MalE-RomR in the presence of MglA-His6 loaded with either GppNHp or GDP (Fig. 7b). We conclude that MglA-GTP specifically breaks the MglC/MglB interaction but not the MglC/RomR interaction. This observation is also in agreement with neither MglC nor RomR/MglC affecting MglB and MglB/RomY GAP activity (Supplementary Fig. 4). Thus, addition of MglA-GTP to the RomR/MglC/MglB complex results in sequestration of MglB by MglA-GTP, thus allowing MglB GAP activity to proceed.
a, b MglA-GTP breaks the interaction between MglC and MglB. Pull-down experiments were performed with Strep-MglC as bait on the indicated resin as described in Fig. 2. MglA-His6 was preincubated with GppNHp or GDP (final concentration 40 µM). All buffers contained 40 µM GppNHp or GDP. In a, the SDS-PAGE gels were probed by immunoblotting with α-MglB antibodies. In b, the multiple bands below MalE-RomR are degradation products. Similar results were obtained in three independent experiments. c. Crystallographic structure of MglA-GTPγS:MglB2 (grey/yellow) (pdb ID: 6izw41) superimposed on the AlphaFold-Multimer model of a MglC2:MglB2 complex (brown/red). Inset, R115 in each of the MglB protomers in the MglA-GTPγS:MglB2 complex is shown in yellow and in red in the MglC2:MglB2 model; D26 and I28 are shown in brown for one of the MglC protomers. Arrows indicate the repositioning of R115 in the two complexes. For simplicity, the MglC dimer is shown to interact with only one MglB dimer. d Schematic of the breaking of the MglB/MglC interaction by MglA-GTP. Bent arrows indicate the conformational change in the MglB dimers upon binding of MglA-GTP. e Regulatory interactions that establish and maintain front-rear polarity in M. xanthus. f Different interactions between the polarity proteins dominate at the leading and lagging poles in WT and the ΔmglC mutant. Full arrows show locally strong interactions, dashed arrows show interactions that are locally suppressed. The arrow from RomR on itself indicates the positive feedback that reinforces its polar localization but it is not known how RomR becomes polarly localized. Color code as in e.
To understand the basis for MglA-GTP inhibition of the MglC/MglB interaction, we compared the solved structure of MglA-GTPɣS:MglB2 to the MglC2:(MglB2)2 AlphaFold-Multimer model (Fig. 6a). We identified significant conformational differences in the MglB dimers in the two complexes. Specifically, the MglB2 four-helix side is in a more open state when complexed with MglA-GTPɣS than with MglC2 (Fig. 7c). As a result, the two R115 residues in the MglB KRK regions are likely positioned in such a way in the complex with MglA-GTPɣS that they cannot interact with D26 and I28 in the MglC FDI region (Fig. 7c, d; see also Fig. 6a). We note that in the solved structure of the MglB dimer with MglA-GTPɣS, the four-helix side is more open than in the solved structure of the MglB dimer in isolation (Supplementary Fig. 8b). Thus, these solved structures together with the AlphaFold-Multimer model of the MglC2:(MglB2)2 complex support that the MglB dimer can exist in three different conformational states, where the degree of “openness” on the four-helix side varies (Supplementary Fig. 8c).
Discussion
Here, we identify MglC as a critical component of the polarity module for switchable front-rear polarity in M. xanthus. We demonstrate that the previously proposed RomR/MglB positive feedback incorporates and depends on MglC. These three proteins form a heteromeric RomR/MglC/MglB complex in which MglC is sandwiched between RomR and MglB. In vivo, they establish the RomR/MglC/MglB positive feedback that results in the colocalization of the RomR/RomX GEF and MglB/RomY GAP at high concentrations at the lagging pole (Fig. 7e and f, upper panel). Moreover, we demonstrate that the previously reported inhibitory effect of MglA-GTP on the RomR/MglB positive feedback is the result of MglA-GTP breaking the MglC/MglB interaction without interfering with the RomR/MglC interaction in the RomR/MglC/MglB positive feedback (Fig. 7e and f, upper panel). By way of this inhibitory effect, MglA-GTP at the leading pole limits the accumulation of the other polarity regulators at this pole. By engaging in these interactions, MglC stimulates polar localization of the remaining polarity proteins and is also key to enabling dynamic inversion of polarity in response to Frz signaling.
In vitro observations together with an AlphaFold-Multimer structural model of the MglC/MglB complex and in vivo experiments, support that the RomR:MglC:MglB complex has a 3:2:4 stoichiometry in vivo. Specifically, our data support that the negatively charged α-helical RomRC interacts with the two juxtaposed positively charged KRR surface regions in the MglC dimer, and that each of the two negatively charged FDI surface regions in the MglC dimer interface with the positively charged KRK surface regions in a MglB dimer. These interactions between oppositely charged surface regions allow polar RomR to recruit MglC, which recruits MglB. FRAP experiments in vivo demonstrated that MglC and MglB enable more stable polar RomR occupancy. Based on these findings, we infer that the RomR/MglC/MglB positive feedback for polar localization involves direct recruitment via the RomR→MglC→MglB interactions. These interactions stabilize polar RomR binding, thereby closing the positive feedback. Because neither RomR, MglC, nor RomR/MglC has measurable GAP activity or measurably affects GAP activity by MglB/RomY and MglB, we infer that one function of MglC is to connect MglB and RomR to establish the positive feedback.
In vitro, MglA-GTP breaks the MglC/MglB interaction in the RomR/MglC/MglB complex without interfering with the RomR/MglC interaction. A comparison of the solved structure of the MglA-GTPγS:MglB2 complex with an AlphaFold-Multimer model of the MglC2:(MglB2)2 complex supports that MglA-GTP breaks the MglC/MglB interaction using an allosteric mechanism. In this model, MglA-GTP by binding to the two-helix side of a MglB dimer induces a conformational change that alters the four-helix side of the MglB dimer, thereby breaking the interaction between the MglC FDI and MglB KRK interfaces. Supporting these observations, RomR/MglC neither affects MglB nor MglB/RomY GAP activity (Supplementary Fig. 4). Thus, the second function of MglC is to enable the inhibitory effect of MglA-GTP on the RomR/MglC/MglB positive feedback in vivo. In our model, the high concentration of the MglB/RomY GAP complex at the lagging pole inhibits MglA-GTP recruitment to this pole by stimulating MglA GTPase activity. In this process, MglA-GTP breaks the RomR/MglC/MglB positive feedback resulting in the detachment of MglB. However, due to the high concentration of RomR and MglC at this pole, MglB will rapidly be recaptured to restore the RomR/MglC/MglB complex. Consistent with this notion, polar MglB exchanges rapidly with the cytoplasm with a T1/2 of ~6 sec in FRAP experiments34.
Our study raises several intriguing questions for future research regarding the proteins of the polarity module. First, RomRC has three functions: It interacts not only with MglC but also mediates oligomerization with dimer and trimer formation and is required and at least partly responsible for RomR polar localization. In vivo quantification of the RomR concentration (Supplementary Fig. 5e) suggests that trimeric RomR is the predominant form in vivo; however, which of the two forms represent the active form remains to be determined. Similarly, it is not known how RomRC brings about the polar localization of RomR, and how RomR stimulates its polar binding. Second, experimental evidence and AlphaFold-Multimer structural models support that MglB binds its co-GAP RomY with low affinity on the two-helix side28. We, therefore, suggest that the RomR/MglC/MglB complex at the lagging pole also contains RomY forming a RomR/MglC/MglB/RomY complex. Third, RomR interacts with RomX to generate the polarly localized Rom/RomX GEF complex. While this complex’s structural details are unknown, they raise the possibility that the RomR/MglC/MglB complex may also include RomX. The complexes formed will be addressed in future work.
The ΔmglC mutant resembles WT concerning unidirectional motility but is less sensitive to Frz signaling, supporting that the ultimate function of MglC is to establish sensitivity to Frz signaling, thereby enabling polarity inversions. The two output response regulators of the Frz system, FrzX and FrzZ, act on the polarity module by unknown mechanisms to enable polarity inversions30,31,33,34. The observation that the ΔmglC mutant still responds to high levels of Frz signaling argues that MglC is not the downstream molecular target of the Frz system but enables Frz responsiveness by a different mechanism. As predicted by the model for polarity establishment (Fig. 7e, f, upper panels), neither MglC nor the RomR/MglC/MglB positive feedback is important for MglA localization at the leading pole. Instead, in the absence of MglC, and therefore, the RomR/MglC/MglB positive feedback, the highest polar concentration of the RomR/RomX complex colocalizes with MglA at the leading pole (Fig. 7f, lower panel). In the ΔmglC mutant, RomR/RomX and MglA polar localization is driven by RomR stimulating its own polar binding in a positive feedback and then recruiting RomX and MglA (Fig. 7f, lower panel). Thus, in this configuration, the polarity module is less sensitive to the Frz system, while front-rear polarity is robustly maintained. Based on theoretical arguments, we previously argued that the configuration with a high concentration of the RomR/RomX GEF at the lagging pole would allow for the rapid accumulation of MglA-GTP at this pole in response to Frz signaling, allowing the inversion of polarity. We, therefore, suggest that the spatial configuration of the polarity proteins in the ΔmglC mutant makes it less sensitive to Frz signaling because there is too little RomR/RomX GEF at the lagging pole to recruit MglA-GTP during reversals. Thus, the RomR/MglC/MglB positive feedback resulting in the peculiar colocalization of the RomR/RomX GEF and MglB/RomY GAP at the lagging pole in WT serves two purposes: First, the GAP activity displaces MglA-GTP from this pole to enable unidirectional translocation; and, second, the GEF activity is necessary to provide the system with the ability to rapidly and efficiently invert polarity. In other words, an important function of MglC and the RomR/MglC/MglB positive feedback is to establish the configuration of the polarity proteins that confer the polarity module with responsiveness to the Frz system. Returning to the question raised in the introduction, i.e. why different network designs have been selected for in various polarity-regulating networks with functionally equivalent outcomes, in the S. cerevisiae polarity system that establishes the single Cdc42 cluster, the positive feedback is centered on Cdc42 and the Cdc24 GEF9. Therefore, once the Cdc42 cluster is established, this polarity is stably maintained, and the decay of a nascent bud site or the formation of competing bud sites is efficiently avoided. In the ΔmglC mutant, RomR, by stimulating its own polar binding in a positive feedback, brings about RomR/RomX and MglA polar localization at the same pole (Fig. 7f, lower panel). This design is conceptually similar to the yeast system driving Cdc42 cluster formation. Thus, while the network designs of the M. xanthus and the S. cerevisiae polarity systems enable the formation of a single MglA/Cdc42 cluster, the different wirings can be rationalized as the M. xanthus polarity module being part of a spatial toggle switch that is optimal for stable polarity as well for rapid polarity inversions. By contrast, the S. cerevisiae system is optimized to provide stable polarity.
In principle, it would seem that the RomR/MglC/MglB positive feedback could have been established by RomR interacting directly with MglB, raising the question of the advantage of incorporating MglC into the RomR/MglC/MglB positive feedback. The roadblock domain protein family is ancient, abundantly present in all domains of life, and often involved in regulating GTPase activity37,46,47,48. Interestingly, the Rag GTPases of the mTOR pathway are composed of a small GTPase domain and a C-terminal roadblock domain and form heterodimers using their roadblock domains49. These heterodimers are recruited to lysosomes by the Ragulator complex, which contains two roadblock heterodimers that interact head-to-tail forming a tetrameric complex49. The Rag GTPase/Ragulator interaction occurs via the roadblock domains, resulting in three layers of heterodimeric roadblock domains49. Intriguingly, Rag heterodimers’ GTP/GDP state allosterically regulates their binding to Ragulator by tuning the interaction between pairs of roadblock heterodimers50,51. This mechanism is conceptually remarkably similar to the GTP/GDP state of MglA regulating the interaction between the MglC/MglB homodimers, supporting that this regulatory mechanism is evolutionary conserved. We suggest that the presence of MglC in the RomR/MglC/MglB positive feedback reflects an ancient regulatory mechanism in which the GTP/GDP state of a partner GTPase can modulate the interaction between pairs of roadblock dimers.
Methods
Cell growth and construction of strains
Strains, plasmids and primers used in this work are listed in Supplementary Table 1, 2 and 3, respectively. All M. xanthus strains are derivatives of the DK1622 WT strain52. M. xanthus was grown at 32 °C in 1% CTT broth53 or on 1.5% agar supplemented with 1% CTT and kanamycin (50 µg mL-1) or oxytetracycline (10 µg mL-1) as appropriate. In-frame deletions were generated as described54. Plasmids were introduced in M. xanthus by electroporation and integrated by homologous recombination at the endogenous locus or at the mxan18-19 locus or by site-specific recombination at the Mx8 attB site. All in-frame deletions and plasmid integrations were verified by PCR. Plasmids were propagated in Escherichia coli TOP10 (F-, mcrA, Δ(mrr-hsdRMS-mcrBC), φ80lacZΔM15, ΔlacX74, deoR, recA1, araD139, Δ(ara-leu)7679, galU, galK, rpsL, endA1, nupG). E. coli was grown in LB or on plates containing LB supplemented with 1.5% agar at 37 °C with added antibiotics when appropriate55. All DNA fragments generated by PCR were verified by sequencing.
Motility assays and determination of reversal frequency
Population-based motility assays were done as described56. Briefly, M. xanthus cells from exponentially growing cultures were harvested at 4000× g for 10 min at room temperature (RT) and resuspended in 1% CTT to a calculated density of 7×109 cells mL-1. 5 µL aliquots of cell suspensions were placed on 0.5% agar plates supplemented with 0.5% CTT for T4P-dependent motility and 1.5% agar plates supplemented with 0.5% CTT for gliding motility and incubated at 32 °C. After 24 h, colony edges were visualized using a Leica M205FA stereomicroscope and imaged using a Hamamatsu ORCA-flash V2 Digital CMOS camera (Hamamatsu Photonics) using the LASX software (Leica Microsystems). For higher magnifications of cells at colony edges on 1.5% agar, cells were visualized using a Leica DMi8 inverted microscope and imaged with a Leica DFC9000 GT camera.
Individual cells were tracked as described29. Briefly, for T4P-dependent motility, 5 µL of exponentially growing cultures were spotted into a 24-well polystyrene plate (Falcon). After 10 min at RT, cells were covered with 500 µL 1% methylcellulose in MMC buffer (10 mM MOPS (3-(N-morpholino) propanesulfonic acid) pH 7.6, 4 mM MgSO4, 2 mM CaCl2), and incubated at RT for 30 min. Subsequently, cells were visualized for 10 min at 20 sec intervals at RT using a Leica DMi8 inverted microscope and a Leica DFC9000 GT camera and the LASX software (Leica Microsystems).. Individual cells were tracked using Metamorph 7.5 (Molecular Devices) and ImageJ 1.52b57 and then the speed of individual cells per 20 sec interval as well as the number of reversals per cell per 10 min calculated. For gliding, 5 µL of exponentially growing cultures were placed on 1.5% agar plates supplemented with 0.5% CTT, covered by a cover slide and incubated at 32 °C. After 4 to 6 h, cells were observed for 15 min at 30 sec intervals at RT as described, speed per 30 sec interval as well as the number of reversals per 15 min calculated.
Immunoblot analysis
Immunoblot analysis was done as described55. Rabbit polyclonal α-MglA27 (dilution 1:5000), α-MglB27 (dilution 1:5000), α-RomR32 (1:5000), α-PilC58 (dilution 1:5000), α-PilO59 (dilution 1:2000), α-PilT60 (dilution 1:2000), α-mCherry (Biovision, dilution 1:15000) and α-MglC antibodies (dilution 1:5000) were used together with horseradish peroxidase-conjugated goat anti-rabbit immunoglobulin G (Sigma) as a secondary antibody (dilution 1:10000). Mouse α-GFP (Sigma, dilution 1:2000) and α-EF-Tu (HycultBiotech, dilution 1:5000) antibodies were used together with horseradish peroxidase conjugated sheep anti-mouse immunoglobulin G (GE Healthcare) as a secondary antibody (dilution 1:2000). To generate rabbit polyclonal α-MglC antibodies, His6-MglC was purified as described (see below) and used for immunization as described55. Blots were developed using Luminata Crescendo Western HRP substrate (Millipore) and visualized using a LAS-4000 luminescent image analyzer (Fujifilm). Proteins were separated by SDS-PAGE as described55.
Fluorescence microscopy
For fluorescence microscopy, exponentially growing cells were placed on slides containing a thin pad of 1% SeaKem LE agarose (Cambrex) with TPM buffer (10 mM Tris-HCl pH 7.6, 1 mM KH2PO4 pH 7.6, 8 mM MgSO4) and 0.2% CTT, and covered with a coverslip. After 30 min at 32˚C, cells were visualized using a temperature-controlled Leica DMi8 inverted microscope and phase contrast and fluorescence images acquired using a Hamamatsu ORCA-flash V2 Digital CMOS camera and the LASX software (Leica Microsystems). For time-lapse recordings, cells were imaged for 15 min using the same conditions. To induce expression of genes from the vanillate inducible promoter61, cells were treated as described in the presence of 300 μM vanillate and imaged for 6 h. To precisely quantify the localization of fluorescently labelled proteins, we used an established analysis pipeline17 in which the output for each cell is total cellular fluorescence, total fluorescence in clusters at each pole and the mean fractions of fluorescence at each of the two poles. Data points for individual cells were plotted in scatterplots in which the poles with the highest and lowest polar fraction of fluorescence are defined as pole 1 and pole 2, respectively. Dispersion of the single-cell measurements is calculated and shown by error bars and ellipses in which the direction and length of error bars are defined by the eigenvectors and square root of the corresponding eigenvalues of the polar fraction covariance matrix for each strain. For calculating mean fraction of fluorescence at the poles, cells with and without clusters were included. The quantification of fluorescence signals is included in Supplementary Table 4.
Image analysis
Microscope images were processed with Fiji62 and cell masks determined using Oufti63 and manually corrected when necessary. Fluorescence was quantified in Matlab R2020a (The MathWorks) as described17.
In vivo fluorescence recovery after photobleaching (FRAP)
FRAP experiments were performed as described64 with a temperature-controlled Nikon Ti-E microscope with Perfect Focus System and a CFI PL APO 100x/1.45 Lambda oil objective at 32 °C with a Hamamatsu Orca Flash 4.0 camera using NIS Elements AR 2.30 software (Nikon) in the dark. Photobleaching was performed using a single circular shaped region with 20% laser power (561 nm) and a 500µsec dwelling time. For every image, integrated fluorescence intensities of a whole cell and the bleached region of interest (ROI) were measured. After background correction, the corrected fluorescence intensity of the bleached ROI was divided by total corrected cellular fluorescence, correcting for bleaching effects during picture acquisition. Cell segmentation and background correction was performed with Oufti. This normalized fluorescence was correlated to the initial fluorescence in the ROI. The mean relative fluorescence of cells was plotted as a function of time. The recovery rate for a given fluorescent protein was determined by fitting the plotted data to a single exponential equation with Matlab R2020a (The MathWorks).
Protein purification
All proteins were expressed in E. coli Rosetta 2(DE3) (F− ompT hsdSB (rB − mB −) gal dcm (DE3 pRARE2) at 18 °C or 37 °C. To purify His6-tagged proteins, Ni-NTA affinity purification was used. Briefly, cells were washed in buffer A (50 mM Tris-HCl pH 7.5, 150 mM NaCl, 10 mM imidazole, 5% glycerol, 5 mM MgCl2) and resuspended in lysis buffer A (50 mL of wash buffer A supplemented with 1 mM DTT, 100 µg ml−1 phenylmethylsulfonylfluoride (PMSF), 10U ml−1 DNase 1 and Complete Protease Inhibitor Cocktail Tablet (Roche)). Cells were lysed by sonication and cell debris was removed by centrifugation (48,000× g, 4 °C, 30 min) and filtration through a 0.45 µm filter (Sarsted). The cleared cell lysate was loaded onto a 5 mL HiTrap Chelating HP column (Cytiva) preloaded with NiSO4 as described by the manufacturer and pre-equilibrated in buffer A. The column was washed with 20 column volumes of column wash buffer (buffer A with 20 mM imidazole). Proteins were eluted with elution buffer (buffer A with 500 mM imidazole) using a linear imidazole gradient from 20 to 500 mM. Fractions containing purified His6-tagged proteins were combined and loaded onto a HiLoad 16/600 Superdex 75 pg (GE Healthcare) gel filtration column that was equilibrated with buffer 1 (50 mM Tris-HCl pH 7.5, 150 mM NaCl, 1 mM DTT, 5 mM MgCl2, 5% glycerol). Fractions containing His6-tagged proteins were pooled, frozen in liquid nitrogen and stored at −80 °C.
To purify MalE-tagged proteins (MalE-RomR, MalR-RomR1–368, MalR-RomRC and MalE-MglC), maltose-binding protein (MBP) affinity purification was used. Briefly, cells were washed in buffer B (50 mM Tris-HCl pH 7.5, 150 mM NaCl, 1 mM EDTA, 1 mM DTT) and resuspended in 50 mL lysis buffer B (50 mL buffer B supplemented with PMSF 100 µg mL−1, DNase 1 10U mL−1 and Complete Protease Inhibitor Cocktail Tablet (Roche)). Cells were lysed and cleared cell lysates prepared as described and loaded onto a 5 mL MBPTrapHP (Cytiva) column equilibrated with buffer B. The column was washed with 20 column volumes of buffer B. Proteins were eluted with elution buffer B (buffer B with 10 mM maltose). Eluted fractions containing the relevant MalE-tagged protein were loaded onto a 5 mL HiTrap Q HP ion exchange column (Cytiva) equilibrated with buffer C (50 mM Tris-HCl pH 7.5, 50 mM NaCl, 5 mM MgCl2, 1 mM DTT, 5% glycerol). The column was washed with 20 column volumes of buffer C. The Mal-tagged proteins were eluted with buffer C using a linear gradient of NaCl from 50 to 500 mM. Fractions containing the MalE-tagged proteins were loaded onto a HiLoad 16/600 Superdex 200 pg (GE Healthcare) gel filtration column that was equilibrated with buffer 1. Fractions with MalE-tagged proteins were pooled, frozen in liquid nitrogen and stored at −80 °C.
To purify Strep-tagged proteins, biotin affinity purification was used. Briefly, cells were washed in buffer C (100 mM Tris-HCl pH 8.0, 150 mM NaCl, 1 mM EDTA, 1 mM DTT) and resuspended in lysis buffer C (50 mL of wash buffer C supplemented with 100 µg mL−1 PMSF, 10U mL−1 DNase 1 and Complete Protease Inhibitor Cocktail Tablet (Roche)). Cells were lysed and cleared lysate prepared as described and loaded onto a 5 mL Strep Trap HP (Cytiva) column, equilibrated with buffer C. The column was washed with 20 column volumes of buffer C. Protein was eluted with elution buffer C (buffer C with 2.5 mM desthiobiotin). Elution fractions containing Strep-tagged proteins were loaded onto a HiLoad 16/600 Superdex 75 pg (GE Healthcare) gel filtration column that was equilibrated with buffer 1. Fractions with Strep-tagged proteins were pooled, frozen in liquid nitrogen and stored at −80 °C.
Pull-down experiments
To test for interactions with MglA, MglB and RomR variants, Strep-MglC (final concentration 10 µM) was incubated with MglA-His6, His6-MglB, MalE-RomR, MalE-RomR1–368 or MalE-RomRC (final concentration 10 µM) in buffer 1 (50 mM Tris-HCl pH 7.5, 150 mM NaCl, 1 mM DTT, 5 mM MgCl2, 5% glycerol) for 30 min at RT. Subsequently, 10 µL of Strep-Tactin MagStrep’ type3’ XT beads (IBA Lifesciences) previously equilibrated with buffer 1 was added for 30 min at RT. Then beads were washed 10 times with 1 mL buffer 1. Proteins were eluted with 200 µL elution buffer (100 mM Tris-HCl pH 8.0, 150 mM NaCl, 1 mM EDTA, 50 mM biotin). To test for interactions with MglC, MglA and MglB, MalE-RomR (final concentration 10 µM) was incubated with Strep-MglC, MglA-His6 and/or His6-MglB (final concentration 10 µM) in buffer 1 for 30 min at RT. Subsequently, the mixture was added to 200 µL of Amylose Resin, previously equilibrated with buffer 1, and incubated for 30 min at RT. The resin was then washed 10 times with 1 mL buffer 1. Proteins were eluted with 200 µL elution buffer (100 mM Tris-HCl pH 8.0, 150 mM NaCl, 1 mM EDTA, 10 mM amylose). To test for interactions with MglC, MglA and RomR, His6-MglB (final concentration 10 µM) was incubated with Strep-MglC, MglA-His6 and/or MalE-RomR (final concentration 10 µM) in buffer 1 for 30 min at RT. Subsequently, 20 µL of Amintra Nickel Magnetic beads (Expedeon), previously equilibrated with buffer 1, was added to the mixture and incubated for 30 min at RT. Beads were then washed 10 times with 1 mL buffer 2 (buffer 1 with 50 mM imidazole). Proteins were eluted with 200 µL elution buffer (buffer 1 with 500 mM imidazole). In experiments involving MglA-His6, MglA-His6 (final concentration 10 µM) was preloaded with GTP, GDP or GppNHp (final concentration 40 µM) for 30 min at RT in buffer 1 and all buffers contained 40 µM of the relevant nucleotide.
GTPase assays
GTP-hydrolysis by MglA-His6 was measured using a continuous, regenerative coupled GTPase assay65 in reaction buffer (50 mM Tris-HCl pH 7.5, 150 mM NaCl, 5% glycerol, 1 mM DTT, 7.5 mM MgCl2) supplemented with 495 µM NADH (Sigma), 2 mM phosphoenolpyruvate (Sigma), 18-30U mL-1 pyruvate kinase (Sigma) and 27-42 U mL-1 lactate dehydrogenase (Sigma). For all assays, MglA-His6 (final concentration 2 µM) was preloaded with GTP (final concentration 3.3 mM) for 30 min at RT in reaction buffer. In parallel, MglB was preincubated with Strep-MglC, MalE-RomR and/or Strep-RomY for 10 min at RT in reaction buffer. GTPase reactions were performed in 96-well plates (Greiner Bio-One) and initiated by adding His6-MglB, Strep-MglC, MalE-RomR and/or Strep-RomY to the MglA/GTP mixture. Final concentration, MglA-His6: 2 µM, His6-MglB: 4 µM, Strep-MglC: 4 µM, MalE-RomR: 2 µM, Strep-RomY: 2 µM, GTP: 1 mM. Absorption was measured at 340 nm for 60 min at 37 °C using an Infinite M200 Pro plate-reader (Tecan) and the amount of hydrolyzed GTP per hour per molecule of MglA-His6 calculated. For each reaction, background subtracted GTPase activity was calculated as the mean of three technical replicates.
Solubility test
To test the solubility of MglC-mVenus, MglCFDI-mVenus and MglCKRR-mVenus in M. xanthus cells were fractionated into fractions enriched for soluble and insoluble proteins, including inner membrane and outer membrane proteins as described66. Briefly, exponentially growing cultures were harvested at 11,000 g for 10 min at RT and the cell pellet resuspended in Lysis Buffer D (50 mM Tris-HCl pH 7.5, 150 mM NaCl, 5 mM MgCl2, supplemented with Complete Protease Inhibitor Cocktail Tablet (Roche)). Cells were lysed using sonication and lysates cleared by centrifugation at 8000 g for 5 min, RT. The cleared lysate was subjected to ultra-centrifugation using an Air-Fuge (Beckman) at ~150,000 g for 1 hr. The resulting pellet contains insoluble proteins and was separated from the supernatant, which contains soluble proteins, and resuspended in Lysis Buffer D. Both fractions were mixed with SDS-lysis buffer and analyzed by by SDS-PAGE and immunoblotting. As a control for a protein that forms inclusion bodies, His6-PilT60 expressed in E. coli was used. EF-Tu was used as a control for a soluble E. coli protein. E. coli cells were treated as M. xanthus cells.
Mass photometry (MP)
MP was performed using a TwoMP mass photometer (Refeyn Ltd, Oxford, UK). Data acquisition was performed using AcquireMP (Refeyn Ltd. v2.3). MP movies were recorded at 1 kHz, with exposure times varying between 0.6 and 0.9 ms, adjusted to maximize camera counts while avoiding saturation. Microscope slides (1.5 H, 24×50 mm, Carl Roth) and CultureWellTM Reusable Gaskets were cleaned with three consecutive rinsing steps of double-distilled H2O and 100% isopropanol and dried under a stream of pressurized air. For measurements, gaskets were assembled on coverslips and placed on the stage of the mass photometer with immersion oil. Assembled coverslips were held in place using magnets. For measurements, gasket wells were filled with 10 µL of 1× phosphate-buffered saline (137 mM NaCl, 2.7 mM KCl, 8 mM Na2HPO4, 2 mM KH2PO4) to enable focusing of the glass surface. After focusing, 10 µL sample were added, rapidly mixed while keeping the focus position stable and measurements started. MP contrast values were calibrated to molecular masses using an in-house standard. For each sample, three separate measurements were performed. The data were analyzed using the DiscoverMP software (Refeyn Ltd, v. 2022 R1). MP image analysis was done as described67.
AlphaFold model generation
AlphaFold-multimer (version 2.3.1) structure prediction was done with the ColabFold pipeline43,44,45. ColabFold was executed with default settings where multiple sequence alignments were generated with MMseqs268 and HHsearch69. The ColabFold pipeline generates five model ranks. Predicted Local Distance Difference Test (pLDDT) and alignment error (pAE) graphs were generated for each rank with a custom Matlab R2020a (The MathWorks) script. Ranking of the models was performed based on combined pLDDT and pAE values, with the best-ranked models used for further analysis and presentation. Per residue model accuracy was estimated based on pLDDT values (>90, high accuracy; 70-90, generally good accuracy; 50-70, low accuracy; <50, should not be interpreted)45. Relative domain positions were validated by pAE45. Only models of the highest confidence, based on combined pLDDT and pAE values, were used for further investigation. For all models, sequences of full-length proteins were used.
Bioinformatics
Sequence alignments were generated using ClustalOmega70 with default parameters and alignments were visualized with Jalview71. Protein domains were identified using Interpro72. Charge score was calculated using the Protein-sol tool73. Structural alignments and calculation of electrostatic surface potential were done in Pymol (The PyMOL Molecular Graphics System, Version 1.2r3pre, Schrödinger, LLC).
Statistics
Statistics were performed using a two-tailed Student’s t-test for samples with unequal variances.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The structural data mentioned in this study is available in the Protein Data Bank database under the accession codes 6HJM (MglB), 7CY1 (MglC) and 6IZW (MglA bound to GTP-ɣ-S and MglB). The authors declare that all data supporting this study are available within the article, its Supplementary Information file or in the source data file. Source data are provided with this paper.
Code availability
Custom MATLAB scripts used for analysis of fluorescence data and FRAP data are available from the corresponding author upon request.
References
Treuner-Lange, A. & Søgaard-Andersen, L. Regulation of cell polarity in bacteria. J. Cell Biol. 206, 7–17 (2014).
Rafelski, S. & Marshall, W. Building the cell: design principles of cellular architecture. Nat. Rev. Mol. Cell Biol. 9, 593–602 (2008).
Surovtsev, I. V. & Jacobs-Wagner, C. Subcellular organization: A critical feature of bacterial cell replication. Cell 172, 1271–1293 (2018).
Halatek, J., Brauns, F. & Frey, E. Self-organization principles of intracellular pattern formation. Philos. Trans. R. Soc. Lond. B Biol. Sci. 373, 20170107 (2018).
Chau, A. H., Walter, J. M., Gerardin, J., Tang, C. & Lim, W. A. Designing synthetic regulatory networks capable of self-organizing cell polarization. Cell 151, 320–332 (2012).
Ridley, A. J. et al. Cell migration: Integrating signals from front to back. Science 302, 1704–1709 (2003).
Buckley, C. E. & St Johnston, D. Apical–basal polarity and the control of epithelial form and function. Nat. Rev. Mol. Cell Biol. 23, 559–577 (2022).
Gerland, U. & Hwa, T. Evolutionary selection between alternative modes of gene regulation. Proc. Natl. Acad. Sci. USA 106, 8841–8846 (2009).
Chiou, J.-G., Balasubramanian, M. K. & Lew, D. J. Cell polarity in yeast. Ann. Rev. Cell Dev. Biol. 33, 77–101 (2017).
Etienne-Manneville, S. & Hall, A. Rho GTPases in cell biology. Nature 420, 629–635 (2002).
Schumacher, D. & Søgaard-Andersen, L. Regulation of cell polarity in motility and cell division in Myxococcus xanthus. Annu. Rev. Microbiol. 71, 61–78 (2017).
Seetharaman, S. & Etienne-Manneville, S. Cytoskeletal crosstalk in cell migration. Trends Cell Biol. 30, 720–735 (2020).
Wittinghofer, A. & Vetter, I. R. Structure-function relationships of the G domain, a canonical switch motif. Ann. Rev. Biochem. 80, 943–971 (2011).
Cherfils, J. & Zeghouf, M. Regulation of small GTPases by GEFs, GAPs, and GDIs. Physiol. Rev. 93, 269–309 (2013).
Wu, C.-F. & Lew, D. J. Beyond symmetry-breaking: competition and negative feedback in GTPase regulation. Trends Cell Biol. 23, 476–483 (2013).
Martin, S. G. Spontaneous cell polarization: Feedback control of Cdc42 GTPase breaks cellular symmetry. BioEssays 37, 1193–1201 (2015).
Carreira, L. A. M., Tostevin, F., Gerland, U. & Søgaard-Andersen, L. Protein-protein interaction network controlling establishment and maintenance of switchable cell polarity. PLOS Genet. 16, e1008877 (2020).
Zhang, Y., Ducret, A., Shaevitz, J. & Mignot, T. From individual cell motility to collective behaviors: insights from a prokaryote, Myxococcus xanthus. FEMS Microbiol. Rev. 36, 149–164 (2012).
Carreira, L. A. M., Szadkowski, D., Müller, F. & Søgaard-Andersen, L. Spatiotemporal regulation of switching front–rear cell polarity. Curr. Opin. Cell Biol. 76, 102076 (2022).
Blackhart, B. D. & Zusman, D. R. “Frizzy” genes of Myxococcus xanthus are involved in control of frequency of reversal of gliding motility. Proc. Natl. Acad. Sci. USA 82, 8771–8774 (1985).
Sun, H., Zusman, D. R. & Shi, W. Type IV pilus of Myxococcus xanthus is a motility apparatus controlled by the frz chemosensory system. Curr. Biol. 10, 1143–1146 (2000).
Mignot, T., Shaevitz, J. W., Hartzell, P. L. & Zusman, D. R. Evidence that focal adhesion complexes power bacterial gliding motility. Science 315, 853–856 (2007).
Mercier, R. et al. The polar Ras-like GTPase MglA activates type IV pilus via SgmX to enable twitching motility in Myxococcus xanthus. Proc. Natl. Acad. Sci. USA 117, 28366–28373 (2020).
Potapova, A., Carreira, L. A. M. & Søgaard-Andersen, L. The small GTPase MglA together with the TPR domain protein SgmX stimulates type IV pili formation in M. xanthus. Proc. Natl. Acad. Sci. USA 117, 23859–23868 (2020).
Treuner-Lange, T. et al. The small G-protein MglA connects to the MreB actin cytoskeleton at bacterial focal adhesions. J. Cell Biol. 210, 243–256 (2015).
Zhang, Y., Franco, M., Ducret, A. & Mignot, T. A bacterial Ras-like small GTP-binding protein and its cognate GAP establish a dynamic spatial polarity axis to control directed motility. PLOS Biol. 8, e1000430 (2010).
Leonardy, S. et al. Regulation of dynamic polarity switching in bacteria by a Ras-like G-protein and its cognate GAP. EMBO J. 29, 2276–2289 (2010).
Szadkowski, D., Carreira, L. A. M. & Søgaard-Andersen, L. A bipartite, low-affinity roadblock domain-containing GAP complex regulates bacterial front-rear polarity. PLOS Genet. 18, e1010384 (2022).
Szadkowski, D. et al. Spatial control of the GTPase MglA by localized RomR/RomX GEF and MglB GAP activities enables Myxococcus xanthus motility. Nat. Microbiol. 4, 1344–1355 (2019).
Keilberg, D., Wuichet, K., Drescher, F. & Søgaard-Andersen, L. A response regulator interfaces between the Frz chemosensory system and the MglA/MglB GTPase/GAP module to regulate polarity in Myxococcus xanthus. PLOS Genet 8, e1002951 (2012).
Zhang, Y., Guzzo, M., Ducret, A., Li, Y.-Z. & Mignot, T. A dynamic response regulator protein modulates G-protein–dependent polarity in the bacterium Myxococcus xanthus. PLOS Genet 8, e1002872 (2012).
Leonardy, S., Freymark, G., Hebener, S., Ellehauge, E. & Søgaard-Andersen, L. Coupling of protein localization and cell movements by a dynamically localized response regulator in Myxococcus xanthus. EMBO J. 26, 4433–4444 (2007).
Kaimer, C. & Zusman, D. R. Phosphorylation-dependent localization of the response regulator FrzZ signals cell reversals in Myxococcus xanthus. Mol. Microbiol. 88, 740–753 (2013).
Guzzo, M. et al. A gated relaxation oscillator mediated by FrzX controls morphogenetic movements in Myxococcus xanthus. Nat. Microbiol. 3, 948–959 (2018).
Kapoor, S., Kodesia, A., Kalidas, N., Ashish & Thakur, K. G. Structural characterization of Myxococcus xanthus MglC, a component of the polarity control system, and its interactions with its paralog MglB. J. Biol. Chem. 296, 100308 (2021).
McLoon, A. L. et al. MglC, a paralog of Myxococcus xanthus GTPase-Activating Protein MglB, plays a divergent role in motility regulation. J. Bacteriol. 198, 510–520 (2016).
Wuichet, K. & Søgaard-Andersen, L. Evolution and diversity of the Ras superfamily of small GTPases in prokaryotes. Genome Biol. Evol. 7, 57–70 (2015).
McBride, M. J., Köhler, T. & Zusman, D. R. Methylation of FrzCD, a methyl-accepting taxis protein of Myxococcus xanthus, is correlated with factors affecting cell behaviour. J. Bacteriol. 174, 4246–4257 (1992).
Bustamante, V. H., Martinez-Flores, I., Vlamakis, H. C. & Zusman, D. R. Analysis of the Frz signal transduction system of Myxococcus xanthus shows the importance of the conserved C-terminal region of the cytoplasmic chemoreceptor FrzCD in sensing signals. Mol. Microbiol 53, 1501–1513 (2004).
Galicia, C. et al. MglA functions as a three-state GTPase to control movement reversals of Myxococcus xanthus. Nat. Commun. 10, 5300 (2019).
Baranwal, J. et al. Allosteric regulation of a prokaryotic small Ras-like GTPase contributes to cell polarity oscillations in bacterial motility. PLOS Biol. 17, e3000459 (2019).
Miertzschke, M. et al. Structural analysis of the Ras-like G protein MglA and its cognate GAP MglB and implications for bacterial polarity. EMBO J. 30, 4185–4197 (2011).
Mirdita, M. et al. ColabFold: making protein folding accessible to all. Nat. Methods 19, 679–682 (2022).
Evans, R. et al. Protein complex prediction with AlphaFold-Multimer. bioRxiv, 2021.2010.2004.463034 (2021).
Jumper, J. et al. Highly accurate protein structure prediction with AlphaFold. Nature 596, 583–589 (2021).
Levine, T. P. et al. Discovery of new Longin and Roadblock domains that form platforms for small GTPases in Ragulator and TRAPP-II. Small GTPases 4, 62–69 (2013).
Koonin, E. V. & Aravind, L. Dynein light chains of the Roadblock/LC7 group belong to an ancient protein superfamily implicated in NTPase regulation. Curr. Biol. 10, R774–R776 (2000).
Spang, A. et al. Complex archaea that bridge the gap between prokaryotes and eukaryotes. Nature 521, 173–179 (2015).
Cherfils, J. Encoding allostery in mTOR signaling: The structure of the Rag GTPase/Ragulator complex. Mol. Cell 68, 823–824 (2017).
Bar-Peled, L., Schweitzer, L. D., Zoncu, R. & Sabatini, D. M. Ragulator is a GEF for the Rag GTPases that signal amino acid levels to mTORC1. Cell 150, 1196–1208 (2012).
Lawrence, R. E. et al. A nutrient-induced affinity switch controls mTORC1 activation by its Rag GTPase–Ragulator lysosomal scaffold. Nat. Cell Biol. 20, 1052–1063 (2018).
Kaiser, D. Social gliding is correlated with the presence of pili in Myxococcus xanthus. Proc. Natl. Acad. Sci. USA 76, 5952–5956 (1979).
Hodgkin, J. & Kaiser, D. Cell-to-cell stimulation of movement in nonmotile mutants of Myxococcus. Proc. Natl. Acad. Sci. USA 74, 2938–2942 (1977).
Shi, X. et al. Bioinformatics and experimental analysis of proteins of two-component systems in Myxococcus xanthus. J. Bacteriol. 190, 613–624 (2008).
Sambrook, J. & Russell, D. W. Molecular Cloning: A Laboratory Manual, Edn. 3rd. (Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.; 2001).
Shi, W. & Zusman, D. R. The two motility systems of Myxococcus xanthus show different selective advantages on various surfaces. Proc. Natl. Acad. Sci. USA 90, 3378–3382 (1993).
Schneider, C. A., Rasband, W. S. & Eliceiri, K. W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 9, 671–675 (2012).
Bulyha, I. et al. Regulation of the type IV pili molecular machine by dynamic localization of two motor proteins. Mol. Microbiol 74, 691–706 (2009).
Friedrich, C., Bulyha, I. & Søgaard-Andersen, L. Outside-in assembly pathway of the type IV pili system in Myxococcus xanthus. J. Bacteriol. 196, 378–390 (2014).
Jakovljevic, V., Leonardy, S., Hoppert, M. & Søgaard-Andersen, L. PilB and PilT are ATPases acting antagonistically in type IV pili function in Myxococcus xanthus. J. Bacteriol. 190, 2411–2421 (2008).
Iniesta, A. A., García-Heras, F., Abellón-Ruiz, J., Gallego-García, A. & Elías-Arnanz, M. Two systems for conditional gene expression in Myxococcus xanthus inducible by isopropyl-thiogalactopyranoside or vanillate. J. Bacteriol. 194, 5875–5885 (2012).
Schindelin, J. et al. Fiji: an open-source platform for biological-image analysis. Nat. Methods 9, 676–682 (2012).
Paintdakhi, A. et al. Oufti: an integrated software package for high-accuracy, high-throughput quantitative microscopy analysis. Mol. Microbiol. 99, 767–777 (2016).
Schumacher, D. et al. The PomXYZ proteins self-organize on the bacterial nucleoid to stimulate cell division. Dev. Cell 41, 299–314.e213 (2017).
Ingerman, E. & Nunnari, J. A continuous, regenerative coupled GTPase assay for dynamin‐related proteins. Methods Enzymol. 404, 611–619 (2005).
Herfurth, M. et al. A noncanonical cytochrome c stimulates calcium binding by PilY1 for type IVa pili formation. Proc. Natl. Acad. Sci. USA 119, e2115061119 (2022).
Sonn-Segev, A. et al. Quantifying the heterogeneity of macromolecular machines by mass photometry. Nat. Commun. 11, 1772 (2020).
Mirdita, M., Steinegger, M. & Söding, J. MMseqs2 desktop and local web server app for fast, interactive sequence searches. Bioinformatics 35, 2856–2858 (2019).
Steinegger, M. et al. HH-suite3 for fast remote homology detection and deep protein annotation. BMC Bioinforma. 20, 473 (2019).
Madeira, F. et al. Search and sequence analysis tools services from EMBL-EBI in 2022. Nucl. Acids Res. 50, W276–W279 (2022).
Waterhouse, A. M., Procter, J. B., Martin, D. M. A., Clamp, M. & Barton, G. J. Jalview Version 2—a multiple sequence alignment editor and analysis workbench. Bioinformatics 25, 1189–1191 (2009).
Blum, M. et al. The InterPro protein families and domains database: 20 years on. Nucl. Acids Res. 49, D344–D354 (2020).
Hebditch, M. & Warwicker, J. Web-based display of protein surface and pH-dependent properties for assessing the developability of biotherapeutics. Sci. Rep. 9, 1969 (2019).
Acknowledgements
We thank Dr. Anna McLoon for the generation of the α-MglC antibodies. G.K.A.H. and L.S.-A. gratefully acknowledge the financial support by the Max Planck Society.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
Conceptualization: L.A.M.C. and L.S.-A. Experimental work: L.A.M.C., D.S. and S.L. Analysis of experimental data: L.A.M.C. and S.L. Writing – original draft: L.A.M.C. and L.S.-A. Writing – editing of draft: L.A.M.C., D.S., S.L., G.K.A.H. and L.S.-A. Supervision: G.K.A.H. and L.S.-A. Funding acquisition: G.K.A.H. and L.S.-A.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Beiyan Nan, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Carreira, L.A.M., Szadkowski, D., Lometto, S. et al. Molecular basis and design principles of switchable front-rear polarity and directional migration in Myxococcus xanthus. Nat Commun 14, 4056 (2023). https://doi.org/10.1038/s41467-023-39773-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-023-39773-y
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.