Abstract
Dedicated control of oxygen vacancies is an important route to functionalizing complex oxide films. It is well-known that tensile strain significantly lowers the oxygen vacancy formation energy, whereas compressive strain plays a minor role. Thus, atomic reconstruction by extracting oxygen from a compressive-strained film is challenging. Here we report an unexpected LaCoO2.5 phase with a zigzag-like oxygen vacancy ordering through annealing a compressive-strained LaCoO3 in vacuum. The synergetic tilt and distortion of CoO5 square pyramids with large La and Co shifts are quantified using scanning transmission electron microscopy. The large in-plane expansion of CoO5 square pyramids weaken the crystal field splitting and facilitated the ordered high-spin state of Co2+, which produces an insulating ferromagnetic state with a Curie temperature of ~284 K and a saturation magnetization of ~0.25 μB/Co. These results demonstrate that extracting targeted oxygen from a compressive-strained oxide provides an opportunity for creating unexpected crystal structures and novel functionalities.
Similar content being viewed by others
Introduction
Dedicated control of oxygen defects has been considered an important route to functionalizing complex oxide thin films. The nature of oxygen vacancies (Vo) in thin films has been utilized in the redox chemistry and energy applications1,2,3,4. Recently, it has been recognized that the formation and thermodynamically motion of Vo in oxide thin films can be effectively controlled by epitaxial strain. Vo related fascinating properties by utilizing epitaxial tensile strain have been widely reported such as in the nickelates5, manganites6, and ferrites7. Tensile strain would lower the Vo formation energy in most oxides, in favor of a high Vo concentration8,9,10. It can create Vo even in a highly oxidizing environment, resulting in the enhancement of catalytic activity by up to an order of magnitude11. However, strain effects on the Vo formation are nonlinear but highly anisotropic; the compressive strain would not play a significant role in engineering the oxygen content of oxide thin films. As a matter of fact, it strongly limits the possibility of tailoring the physical properties by manipulating their oxygen stoichiometry in a compressively strained film. Many fascinating oxides have had their performance enhanced or have been enticed into a ferroic ground state with compressive strain. For instance, the record-high ferroelectric polarization up to ~130 μC/cm2 achieved in the tetragonal-like BiFeO3 can be stabilized under an extremely large compressive strain greater than −4.5%12. Compressively strained EuTiO3 with enhanced spin-lattice coupling exhibits emergent multiferroicity with the strongest ferroelectric and ferromagnetic properties known today13,14,15. Therefore, understanding how the kinetic behavior of oxygen loss affects the structure of compressively strained oxides is critical for identifying the true nature of physical properties in a multitude of functional oxide films.
An excellent example of strain-mediated physical property can be found in studies of lanthanum cobaltite thin films, LaCoO3 (LCO3), in which the active spin state transition and intriguing magnetic ground state vary dramatically with lattice distortion16,17,18,19,20. The spin state of Co ions is known to be reversibly controlled by the delicate competition between crystal field splitting and Hund’s exchange coupling16. Tensile-strained LCO3 films exhibit an insulating behavior and a robust ferromagnetic ordering17,18,19,20, suggesting potential applications toward low-power spintronic devices (e.g., spin filters and spin-based logics), in which the spin current carries the encoded information without mobile charges21,22. However, the Curie temperature (TC) of tensile-strained LCO3 films is independent as tensile strain increases and keeps a constant value of ~80 K, limiting their practical applications. Thus, there is an urgent need to increase TC of LCO3 in order to achieve high-temperature ferromagnetic insulating (FMI). In the case of LCO3 films under compressive strain, a general consensus is that the exchange between different Co ions is suppressed by compression and restrains the long-range magnetic ordering. Meanwhile, the stoichiometry and lattice structure of a compressively strained LCO3 film does not change at ambient conditions.
Here we report a near-room temperature FMI phase stabilized in lanthanum cobaltite thin films with previously unknown lattice structure. The metastable phase with stoichiometry of LaCoO2.5 is naturally formed through vacuum annealing by extracting oxygen from a compressively strained LCO3 film. Compared with the antiferromagnetic brownmillerite (BM) LaCoO2.5, this unexplored LaCoO2.5 phase exhibits an ideal FMI behavior with TC of ~284 K and a saturation magnetization of ~0.25 μB/Co. It exhibits a zigzag-like Vo ordering and consists of CoO5 square pyramids with large atomic shifts of both La and Co ions. The unique Vo pattern mediated by compressive stress induces a large in-plane expansion of CoO5 square pyramids, which weakens crystal field splitting and leads to the high-spin filling of d orbitals, and produces the FMI state close to room temperature, which agrees with our first-principles calculations. We believe that the similar emergent phase mediated by compressive strain may also stimulate further studies on many other multifunctional oxides, such as nickelates and ferrites with relatively low oxygen vacancy formation energy.
Results
Vo orderings evolution and emergent near-room temperature ferromagnetism
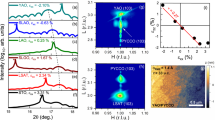
High-quality stoichiometric LCO3 films with a thickness of 200 unit cells (u.c.) were grown on (001)-oriented LaAlO3 (LAO) substrates using pulsed laser deposition (PLD) (see Methods). The LCO3 layer was subsequently capped with a 50-u.c.-thick SrTiO3 layer to prevent the intrinsic non-stoichiometry. We first investigated the microstructure of the LCO3 films on (001)-oriented LaAlO3 (LAO) substrates using scanning transmission electron microscopy (STEM) and selected-area electron diffraction (SAED). The cross-sectional STEM measurements illustrate both high crystallinity and an atomically sharp interface between the film and substrate (Fig. 1a). The SAED pattern in Fig. 1a confirms that LCO3 films are coherently strained. Structural inhomogeneities, such as dark stripes or Vo stripes19,23, were not detected in pristine LCO3, which indicates that the film was nearly stoichiometric without visible Vo. We performed in situ sample-annealing. After annealed 2 h in vacuum, the dark stripes in every third Co (001) plane with a diffraction vector of 1/3 (0, 0, 1) appear in the film, as labeled by small yellow arrows in Fig. 1b. This phase is recognized as LaCoO2.67 (LCO2.67) or La3Co3O824, which is in agreement with previous reports that indicated that compressive-strained LCO2.67 favored in-plane Vo stripes25. We continuously annealed the sample in vacuum for 4 h. Surprisingly, a previously unknown ordered dark stripe pattern with Vo ordering is aligned along the diagonal direction, i.e., [011] orientation appears, as shown in Fig. 1c. The SAED pattern of this structure possesses a diffraction vector of 1/3 (0, 1, 1). Compared to earlier works, the Vo ordering and SAED pattern are completely different from the BM-type LCO2.5 structure, in which the alternative tetrahedral CoO4 layers and octahedral CoO6 layers are stacked and a diffraction vector along the 1/2 (0, 0, 1)26. To our best knowledge, this type of Vo ordering pattern has never been reported in any oxygen-deficient perovskite oxides26. High magnification high-angle angular dark-field (HAADF)-STEM images and corresponding FFT patterns of these structures are summarized in Supplementary Fig. 1 for a close comparison. Both samples exhibit atomically sharp interfaces and highly coherent epitaxial films after the thermal processes (Supplementary Fig. 2).
Low magnification high-angle angular dark-field (HAADF) images of a pristine LCO3, b LCO2.67, and c nLCO2.5 films grown on LAO substrates. The corresponding selected-area electron diffraction (SAED) patterns are inserted on the right top corner of each panel, where the yellow arrows indicate the positions of superstructure spots. The white bar represents 10 nm in scale. d M-T curves of LCO3 and nLCO2.5 films. The measurements were carried out during the sample warm-up under a magnetic field of 0.1 T. Solid and open symbols represent the field-cooled (FC) and zero-field-cooled (ZFC) data, respectively. e, f M-H curves of LCO3 and nLCO2.5 films at 10 and 200 K, respectively.
We performed electron energy loss spectra (EELS) measurements of both O K-edge and Co L-edges to quantify the oxygen concentration of these structures (Supplementary Fig. 3). The significant decrease of pre-peak’s intensity at the O K-edge and concomitant chemical shift at Co L-edges suggest that the number of Vo increases with doubling of the annealing time. Quantified analysis of the O/Co atomic ratio by the standard procedure as implemented in DigitalMicrographTM allows us to estimate the oxygen content of ~2.70 ± 0.07 and ~2.53 ± 0.07 for LCO2.67 and LCO2.5 phase, respectively. To differentiate it from the BM LCO2.5 phase, this unexplored phase is hereafter referred to as nLCO2.5. Macroscopic structure and topography characterizations confirm the highly epitaxial, coherent growth, smooth surface, and single-phase of pristine LCO3 and nLCO2.5 films (Supplementary Fig. 4). X-ray absorption spectroscopy measurements demonstrate that the Co3+ ions in LCO3 transit into Co2+ ions in nLCO2.5 due to the formation of Vo. Transport measurements demonstrate that both LCO3 and nLCO2.5 films exhibit insulating behavior (Supplementary Fig. 5).
The magnetic properties were examined in LCOx samples with different annealing times. The temperature (T)- and magnetic field (H)-dependent magnetization (M) of pristine LCO3 and nLCO2.5 samples, respectively, is shown in Fig. 1d–f. Pristine LCO3 film exhibits the typical diamagnetic or paramagnetic behavior, which is in agreement with earlier reports27. However, nLCO2.5 exhibits a clear magnetic phase transition at the Curie temperature (TC) of ~284 K and square-like hysteresis loops, which indicates a ferromagnetic character in nLCO2.5. At 10 K, the continuous increase of magnetization with increasing magnetic field suggests a small portion of paramagnetic component in the nLCO2.5, consistent with a large upturn in M-T curve at low temperatures. The saturation magnetic moment (MS) of nLCO2.5 reaches ~35 emu/cm3 (~0.25 μB/Co) at 200 K. Furthermore, we also measured the magnetic properties of LCO2.67. TC of LCO2.67 is similar to that of nLCO2.5, but MS of LCO2.67 is an order of magnitude smaller than that of nLCO2.5 (Supplementary Fig. 6). We believe that the small magnetization in LCO2.67 may be attributed to the presence of small amount nLCO2.5 phase in LCO2.67 films.
Atomic-scale lattice and EELS analysis of two different Vo orderings
The atomistic details of LCO2.67 and nLCO2.5 with two different Vo orderings are examined via atomic-resolved HAADF images and EELS spectra. The HAADF image of the LaCoO2.67 lattice with Vo stripes in every third Co (001) plane is shown in Fig. 2a, which is also manifested by the periodic chemical expansion (~4.53 Å) of the out-of-plane La–La distances (Fig. 2b) and the periodic intensity decrease of O K-edges (Fig. 2c, d and Supplementary Fig. 7) in the oxygen-deficient tetrahedral CoO4 layer. We extract the fine structures of the O K-edge and Co L-edges in both tetrahedral and octahedral layers, respectively. A clear broadness of the main peak in the O K-edge and a narrowing of Co L3- and L2-edges in the tetrahedral CoO4 layer can be clearly visualized. Furthermore, a chemical shift toward lower energy and increased Co L3/L2 ratio in the tetrahedral CoO4 layer also indicates the reduced valence state of Co ions28. These results provide atomic-scale evidence for the alternative stacking of one tetrahedral CoO4 layer (yellow polyhedral) with two octahedral CoO6 layers (green polyhedral), which is consistent with a previous report24. In contrast, a highly distorted lattice with Vo stripes in every third O (011)p plane appears in the nLCO2.5 film (Fig. 2g), which is characterized by alternative chemical expansion (~4.60 Å) between out-of-plane La–La distances (Fig. 2h), resulting in the concomitant in-plane wave-like atomic arrangement of La. The respective upward and downward shifts of two La that are vertically close to Vo sites lead to the dark contrast; the ordering of which produces dark stripes along the (011) plane (Fig. 2g). Meanwhile, the periodic intensity decreases of O K-edges (Fig. 2i, j) confirms Vo sites. Similar peak positions and intensity distribution in the fine structures of Co L-edges on each Co sites are observed (Supplementary Fig. 8), which suggests the same type of [CoOx] polyhedra. Combining with the EELS quantification analysis shown in Supplementary Fig. 3, the nLCO2.5 phase should consist of all [CoO5] square pyramids.
HAADF images of a LCO2.67 and g nLCO2.5 phase with overlaid structural model, where green spheres represent La, purple and yellow spheres represent Co in octahedral and tetrahedral layers, the O are omitted for clarity. Dark blue and yellow lines indicated the acquired positions of EELS spectra. Periodic change of out-of-plane La–La distances in the b LCO2.67 phase and h nLCO2.5 phase, the 3.79 Å is indicated by dotted line. Local HAADF images overlaid with projected polyhedra and corresponding periodic change of intensity of ADF signal and O K-edge in LCO2.67 phase (c, d) and nLCO2.5 phase (i, j). Red double arrows indicate the correspondence between the HAADF image and EELS results. The normalized O K-edge and Co L-edges of the LCO2.67 phase (e, f) and the nLCO2.5 phase (k, l).
Quantitative analysis of atomic shifts and tilt of CoO5 square pyramids
To determine the exact structure of the nLCO2.5 phase, we used a recently developed integrated differential phase contrast (iDPC) imaging technique29,30,31 to identify atomic positions of oxygen and Vo. The iDPC-STEM enables the linear imaging of the projected electrostatic potential of atomic columns, resulting in contrast mechanism nearly proportional to the atomic number Z instead of its square in the HAADF-STEM. This method is extremely sensitive to light elements (e.g., O) because of the linear phase contrast mechanism, which provides better signal-to-noise ratio and enhanced accuracy of atomic positions30,31. As shown in Fig. 3a and Supplementary Fig. 9, the wave-like atomic shifts of La (big green circles), cooperative displacement of O (small red spheres), and Co (pink spheres) can be directly identified. The remarkable absence of image contrast in some oxygen sites manifests itself as Vo rows along the viewing direction. By overlaying pink polygon, the tilt and distortion of CoO5 square pyramids can be clearly visualized (Fig. 3b), i.e., the clockwise rotation in the projected triangles of [Co1–O5] and [Co1′–O5] pyramids close to Vo sites and the counterclockwise rotation in the projected quadrilateral of [Co2–O5] pyramids.
a The iDPC image of the nLCO2.5 phase. The structural model is overlaid to show the contrast assignment of La (green), Co (pink), and O (red) atoms. The scale bar is 1 nm. b Overlaid iDPC image in a by circles and polyhedra, where La, Co, and O atom columns are outlined by blue, pink, and red circles, respectively; projected CoO5 square pyramids are labeled by pink polyhedral; three kinds of Co sites are also indicated by red arrows. c Periodic out-of-plane atomic shifts of La, where the HAADF image is inserted on the top to demonstrate the correspondence relationship. d Periodic in-plane Co–Co distances, where relatively small Co1′-Co1 and large Co1-Co2, Co2-Co1′ distances are indicated by blue and yellow columns, respectively. e Tilt angles of CoO5 square pyramids on Co1, Co2, and Co1′ sites, which are indicated by brown, pink, and green columns, respectively.
We conduct quantitative analysis of the lattice distortion and CoO5 square pyramid tilting on the iDPC images by extracting the atomic positions of La, Co, and O using the Calatom software32. The vertical La shifts caused by Vo, with the magnitude of ~0.3 Å, are identified in Fig. 3c. The in-plane projected Co–Co distance exhibits a distinctive reduction in every third unit cell, decreasing from 3.90 to 3.49 Å. This reduction is unusual because the atomic distances across Vo sites always increase because of chemical expansion, e.g., La–La distances, as shown in Figs. 2a, g and 3a. Most likely the abnormal contraction of the in-plane Co–Co distance is the result of the interplay between this zigzag-like Vo and compressive strain state, which leads to the highly elongated lattice along the out-of-plane direction for better misfit accommodation. Besides the large displacement of La and Co ions, oxygen positions are also quantitatively extracted, which allows to perform accurate statistics on CoO5 square pyramids. As shown in Fig. 3e, the statistical rotation of [Co1–O5], [Co2–O5], and [Co1′–O5] square pyramids are 6.0 ± 0.6°, −17.4 ± 0.8°, and 15.8 ± 1.1°, respectively, which considerably changes the in-plane Co–O–Co bond angle.
Discussion
To further clarify the origin of FMI properties, we employ first-principles calculations on the nLCO2.5 phase using the Heyd–Scuseria–Ernzerhof (HSE06) hybrid functional. According to the distribution of Vo from STEM, a √5 × 2√2 × 2 supercell is adopted (Supplementary Fig. 10), which is optimized with fixed lattice parameters based on the LaAlO3 substrate (Fig. 4a and Supplementary Table 1). Three distinct types of [CoO5] pyramids, named [Co1–O5], [Co1′–O5], and [Co2–O5], were identified with different Co–O coordination, which are distributed in the nearest-neighbor and next nearest-neighbor sites of the Vo channels. The Co–O bonds in the subface (xy plane) of [Co2–O5] pyramids clearly expand compared with [Co1–O5] and [Co1′–O5] pyramids, as shown in Fig. 4b and Supplementary Table 2. The electrostatic field of [CoO5] square pyramids cause 3d orbitals to split into a doubly degenerate pair (\(d_{xz}\), \(d_{yz}\)) and three singly degenerate \(d_{xy}\), \(d_{z^2}\), and \(d_{x^2 - y^2}\) levels33. As shown in Fig. 4, the higher \(d_{x^2 - y^2}\) level due to short Co–O bonds in the xy plane accounts for the low-spin state in [Co1–O5] and [Co1′–O5] pyramids. By contrast, the expansion in the xy plane of [Co2–O5] pyramids result in a weaker crystal field splitting and lowers the \(d_{x^2 - y^2}\) level, which leads to electron filling and the high-spin state of Co2 ions.
a The optimized structures of nLCO2.5 phase. b Schematic diagram of Co 3d orbits splitting in [Co1–O5] and [Co2–O5] tetragonal pyramid, including the low spin of Co1 and high-spin of Co2. c Projected density of states of Co1, Co1′, and Co2. d Integrated spin as a function of the radius around Co1, Co1′, Co2, La, and O ions. e Projected density of states of nLCO2.5 structure.
The density of states (DOS) is used to elucidate spin states of Co1, Co1′, and Co2 originating from distorted crystalline field, where the obvious asymmetric spin occupation of Co2 promises a high-spin state (Fig. 4c). The integrated spin of Co1, Co1′, and Co2 is −0.77, −0.91, and 2.54 μB, respectively, as shown in Fig. 4d. It produces the total net magnetic moment determined by the following equation:
which is in good agreement with experimental magnetic moment (0.25 μB/Co).
The insulating nature of the nLCO2.5 phase is attributed to the ordering of Vo19, which is confirmed by the total DOS result. The occupied states closer to the Fermi energy is primarily composed of O-p and Co-d states. There exists a slightly different DOS distribution between Co1 and Co1′, as shown in Fig. 4c, which may be attributed to the different rotation magnitude of CoO5 square pyramids, as shown in the iDPC image (Fig. 3e). Except for a small contribution near the Fermi level from the high-spin state of Co1, a bandgap of 1.1 eV is obtained, as displayed in Fig. 4c, e. Thus, oxygen ordering and compressive strain work synergistically to lower the crystal field splitting energy and produce the FMI behavior.
In summary, we report a high-temperature FMI state in a previously unknown lattice structure of LaCoO2.5 films by gently extracting oxygen from a compressively strained LaCoO3 film. Our result challenges the present hypothesis on the indispensability of tensile stress in the origin of ferromagnetism in LaCoO3 films. More importantly, the compressive-strain-induced peculiar Vo ordering causes synergetic tilt and distortion of CoO5 square pyramids, where the large in-plane expansion weakened the crystal field splitting and facilitated the ordered high-spin state of Co2+ and an insulating ferromagnetic state near-room temperature. These results suggest that compressive stress can be reconsidered as being important for the reconstruction of Vo ordering pattern in other oxygen-deficient functional oxide films, and emergent crystal structures and promising functionalities can be expected even in the non-perovskite structural families that are relevant include chrysoberyl34, pyrochlore35, and delafossite36.
Methods
Sample preparation
PLD was used to fabricate single-crystalline LCO films by ablating a stoichiometric ceramic target. The LCO films with a thickness of 200 u.c. were grown on LAO substrates at a substrate temperature of 700 °C and under an oxygen partial pressure of 100 mTorr. During the deposition, the laser frequency and energy density were kept as 5 Hz and 1.5 J/cm2, respectively. A 50-u.c.-thick SrTiO3 layer was subsequently capped on top of LCO layer to prevent formation of Vo at the surfaces. After the film growth, the pristine LCO3 samples were cooled down to room temperature in an oxygen environment of 100 Torr to avoid Vo. The LCO2.67 and nLCO2.5 samples were formed by annealing in vacuum (P ~1 × 10−7 Torr) at 600 °C for 2 and 4 h, respectively. X-ray reflectivity, diffraction measurements, and reciprocal space mapping were conducted on D8 Discovery diffractometer. The thickness of each layer was calibrated by x-ray reflectivity measurements. The magnetic properties of all samples were probed using SQUID. M-T curves were recorded during the warming up under a small field of 0.1 T after the samples were either zero-field-cooled (ZFC) or field-cooled (FC).
STEM characterizations
Sample was prepared by using focused ion beam (FIB) milling. Cross-sectional lamellas were thinned down to 100 nm thick at an accelerating voltage of 30 kV with a decreasing current from the maximum 2.5 nA, followed by fine polish at an accelerating voltage of 2 kV with a small current of 40 pA. The atomic structures of the LCO, LCO2.67, and nLCO2.5 films was characterized using an ARM 200CF (JEOL, Tokyo, Japan) transmission electron microscope operated at 200 kV and equipped with double spherical aberration (Cs) correctors. HAADF images were acquired at acceptance angle of 90–370 mrad. The iDPC-STEM imaging was conducted using a Cs-corrected (S)TEM (FEI Titan Cubed Themis G2 300) with a convergence semi-angle of 15 mrad, operated at a voltage of 300 kV. The collection angle for the iDPC-STEM imaging is 4–20 mrad. The STEM was equipped with a DCOR+ spherical aberration corrector for the electron probe which was aligned using a standard gold sample before observations. Four images used for 2D integration were acquired by a 4-quadrant DF4 detector with an optional high-pass filter applied to reduce the low frequency information in the image.
Calculation details
All first-principles calculations were performed within the Vienna Ab Initio Simulation Package (VASP) based on the density functional theory (DFT). The projected augmented wave (PAW) potentials were used to deal with the electronic exchange-correlation interaction along with GGA functional in the parameterization of Perdew–Burke–Ernzerhof (PBE) pseudopotential. A plane wave representation for the wave function with a cut off energy of 500 eV was applied. Geometry optimizations were performed using a conjugate gradient minimization until all the forces acting on ions were less than 0.01 eV/Å per atom. The K-point mesh with a spacing of ca. 0.03 Å−1 was adopted. Crystal structures are built using VESTA software. The in-plane lattice parameters were fixed to the LaAlO3 substrate (3.79 Å), as experimental proved by x-ray diffraction measurements. The La12Co12O30 structure with a supercell of √5 × 2√2 × 2 was adopted for the structural simulation (Supplementary Fig. 7a). All calculations of electronic structure are based on the HSE06 hybrid functional with hybrid mixing parameters (α = 0.25).
Data availability
The data that support the findings of this study are available on request from the first author (Q.H.Z.) and the corresponding authors (E.J.G. and L.G.).
References
Schlom, D. et al. Strain tuning of ferroelectric thin films. Annu. Rev. Mater. Res. 37, 589–626 (2007).
Haeni, J. H. et al. Room-temperature ferroelectricity in strained SrTiO3. Nature 430, 758–761 (2004).
Ahn, K. H., Lookman, T. & Bishop, A. R. Strain-induced metal–insulator phase coexistence in perovskite manganites. Nature 428, 401–404 (2004).
Cao, J. & Wu, J. Strain effects in low-dimensional transition metal oxides. Mater. Sci. Eng. R. 71, 35–52 (2001).
Petrie, J. R. et al. Enhanced bifunctional oxygen catalysis in strained LaNiO3 perovskites. J. Am. Chem. Soc. 138, 2488–2491 (2016).
Guo, H. et al. The origin of oxygen vacancies controlling La2/3Sr1/3MnO3 electronic and magnetic properties. Adv. Mater. Interfaces 3, 1500753 (2016).
Xie, Y. et al. Control of functional responses via reversible oxygen loss in La1-xSrxFeO3- delta films. Adv. Mater. 26, 1434–1438 (2014).
Mayeshiba, T. & Morgan, D. Strain effects on oxygen migration in perovskites. Phys. Chem. Chem. Phys. 17, 2715–2721 (2015).
Aschauer, U., Pfenninger, R., Selbach, S. M., Grande, T. & Spaldin, N. A. Strain-controlled oxygen vacancy formation and ordering in CaMnO3. Phys. Rev. B 88, 054111 (2013).
Gadre, M., Lee, Y. L., Swaminathan, N. & Morgan, D. Strain effects on defect chemistry in epitaxial perovskite thin films for solid oxide fuel cells. ECS Trans. 35, 2113–2118 (2011).
Petrie, J. R., Jeen, H., Barron, S. C., Meyer, T. L. & Lee, H. N. Enhancing perovskite electrocatalysis through strain tuning of the oxygen deficiency. J. Am. Chem. Soc. 138, 7252–7255 (2016).
Zhang, J. X. et al. Microscopic origin of the giant ferroelectric polarization in tetragonal-like BiFeO3. Phys. Rev. Lett. 107, 147602 (2011).
Fennie, CraigJ. & Rabe, KarinM. Magnetic and electric phase control in epitaxial EuTiO3 from first principles. Phys. Rev. Lett. 97, 267602 (2006).
Fennie, CraigJ. Ferroelectrically induced weak ferromagnetism by design. Phys. Rev. Lett. 100, 167203 (2008).
Lee, J. H. et al. A strong ferroelectric ferromagnet created by means of spin–lattice coupling. Nature 466, 954–958 (2010).
Fuchs, D. et al. Ferromagnetic order in epitaxially strained LaCoO3 thin films. Phys. Rev. B 75, 144402 (2007).
Choi, W. S. et al. Strain-induced spin states in atomically ordered cobaltites. Nano Lett. 12, 4966–4970 (2012).
Guo, E. J. et al. Nanoscale ferroelastic twins formed in strained LaCoO3 films. Sci. Adv. 5, eaav5050 (2019).
Li, S. et al. Maximization of ferromagnetism in LaCoO3 films by competing symmetry. Phys. Rev. Mater. 3, 114409 (2019).
Biskup, N. et al. Insulating ferromagnetic LaCoO3-delta films: a phase induced by ordering of oxygen vacancies. Phys. Rev. Lett. 112, 087202 (2014).
Jaworski, C. M. et al. Observation of the spin-Seebeck effect in a ferromagnetic semiconductor. Nat. Mater. 9, 898–903 (2010).
Uchida, K. et al. Spin Seebeck insulator. Nat. Mater. 9, 894–897 (2010).
Kwon, J.-H. et al. Nanoscale spin-state ordering in LaCoO3 epitaxial thin films. Chem. Mater. 26, 2496–2501 (2014).
Hansteen, O. H., Fjellvag, H. & Hauback, B. C. Crystal structure, thermal and magnetic properties of La3Co3O8. Phase relations for LaCoO3-delta (0.00 <=delta <= 0.50) at 673 K. J. Mater. Chem. 8, 2081–2088 (1998).
Zhang, N. et al. Oxygen vacancy ordering modulation of magnetic anisotropy in strained LaCoO3-x thin films. Acs Appl. Mate. Inter. 10, 38230–38238 (2018).
Anderson, M. T., Vaughey, J. T. & Poeppelmeier, K. R. Structural similarities among oxygen-deficient perovskites. Chem. Mater. 5, 151–165 (1993).
Fuchs, D. et al. Tuning the magnetic properties of LaCoO3 thin films by epitaxial strain. Phys. Rev. B 77, 014434 (2008).
Wang, Z. L., Yin, J. S. & Jiang, Y. D. EELS analysis of cation valence states and oxygen vacancies in magnetic oxides. Micron 31, 571–580 (2000).
Rose, H. Nonstandard imaging methods in electron-microscopy. Ultramicroscopy 2, 251–267 (1977).
Lazic, I., Bosch, E. G. T. & Lazar, S. Phase contrast STEM for thin samples: integrated differential phase contrast. Ultramicroscopy 160, 265–280 (2016).
Yucelen, E., Lazic, I. & Bosch, E. G. T. Phase contrast scanning transmission electron microscopy imaging of light and heavy atoms at the limit of contrast and resolution. Sci. Rep. 8, 2676 (2018).
Zhang, Q. et al. CalAtom: a software for quantitatively analysing atomic columns in a transmission electron microscope image. Ultramicroscopy 202, 114–120 (2019).
Rossell, M. D. et al. Atomic structure of highly strained BiFeO3 thin films. Phys. Rev. Lett. 108, 047601 (2012).
Newnham, R. E., Kramer, J. J., Schulze, W. A. & Cross, L. E. Magnetoferroelectricity in Cr2BeO4. J. Appl. Phys. 49, 6088 (1978).
Harris, M. J., Bramwell, S. T., McMorrow, D. F., Zeiske, T. & Godfrey, K. W. Geometrical frustration in the ferromagnetic pyrochlore Ho2Ti2O7. Phys. Rev. Lett. 79, 2554 (1997).
Ye, F. et al. Spontaneous spin-lattice coupling in the geometrically frustrated triangular lattice antiferromagnet CuFeO2. Phys. Rev. B 73, 220404 (2006).
Acknowledgements
This work was supported by the National Key Basic Research Program of China (Grant Nos. 2020YFA0309100 and 2019YFA0308500), Beijing Natural Science Foundation (Z190010 and 2202060), the Strategic Priority Research Program of Chinese Academy of Sciences (Grant Nos. XDB07030200 and XDB33030200), the National Natural Science Foundation of China (Grant Nos. 51672307, 51991344, 11974390, 52025025, and 52072400), and the Beijing Nova Program of Science and Technology (Grant No. Z191100001119112). XAS experiments were conducted at the beamline 4B9B of Beijing Synchrotron Radiation Facility (BSRF) of the Institute of High Energy Physics, Chinese Academy of Sciences were conducted via user proposals.
Author information
Authors and Affiliations
Contributions
These samples were grown and processed by Q.J. under the guidance of E.J.G. and K.J.J; TEM lamellas were fabricated with FIB milling by F.Q.M. and D.D.X.; TEM experiments were performed by Q.H.Z. and F.Q.M., analyzed by Q.H.Z., X.F.W., and D.S.; The first-principles calculations were performed by A.G., Q.J., and C.W. worked on the magnetic measurement. Q.H.Z. and E.J.G. initiated the research and L.G. supervised the work. All authors participated in writing the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Communications thanks the anonymous reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhang, Q., Gao, A., Meng, F. et al. Near-room temperature ferromagnetic insulating state in highly distorted LaCoO2.5 with CoO5 square pyramids. Nat Commun 12, 1853 (2021). https://doi.org/10.1038/s41467-021-22099-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-021-22099-y
This article is cited by
-
Orbital elasticity control of phase diagram for La0.67Sr0.33MnO3 films
Science China Materials (2024)
-
A small-dataset-trained deep learning framework for identifying atoms on transmission electron microscopy images
Scientific Reports (2023)
-
Emergent and robust ferromagnetic-insulating state in highly strained ferroelastic LaCoO3 thin films
Nature Communications (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.