Abstract
The surface frustrated Lewis pairs (SFLPs) on defect-laden metal oxides provide catalytic sites to activate H2 and CO2 molecules and enable efficient gas-phase CO2 photocatalysis. Lattice engineering of metal oxides provides a useful strategy to tailor the reactivity of SFLPs. Herein, a one-step solvothermal synthesis is developed that enables isomorphic replacement of Lewis acidic site In3+ ions in In2O3 by single-site Bi3+ ions, thereby enhancing the propensity to activate CO2 molecules. The so-formed BixIn2-xO3 materials prove to be three orders of magnitude more photoactive for the reverse water gas shift reaction than In2O3 itself, while also exhibiting notable photoactivity towards methanol production. The increased solar absorption efficiency and efficient charge-separation and transfer of BixIn2-xO3 also contribute to the improved photocatalytic performance. These traits exemplify the opportunities that exist for atom-scale engineering in heterogeneous CO2 photocatalysis, another step towards the vision of the solar CO2 refinery.
Similar content being viewed by others
Introduction
The increasing energy demands of civil society have accelerated the consumption of coal, oil and natural gas, and associated greenhouse gas emissions. This situation is tipping the delicate balance of CO2 in our atmosphere, leading to global warming. To this end, the photocatalytic hydrogenation of CO2 into value-added chemicals and fuels has attracted global attention, touted a promising means of achieving a carbon-neutral economy1,2,3. Although materials such as Pd/Nb2O54, Ru/Al2O35, LDH nanosheets6, and Co-PS@SiO27 have been successfully employed as photocatalysts for CO2 hydrogenation, a photocatalyst does not currently exist that can meet all the stringent requirements for practical application, including a broad solar response, high conversion efficiency, robust stability and low cost. This renders the design of a practical photocatalyst for CO2 hydrogenation a challenge.
Besides catalyst modifications designed to broaden spectral response and improve charge transfer efficiency, it is also important to accelerate conversion rates of H2 or CO2 on specially designed surface sites to boost photon and energy efficiency. Recently, surface frustrated Lewis pairs (SFLPs) have shown a propensity towards H2 dissociation and CO2 activation, a key enabler for many catalytic reactions, including hydrogenation, hydroamination and CO2 reduction8,9,10. Generally speaking, SFLPs comprise proximal Lewis acidic and Lewis basic sites providing synergetic activation of reactant molecules. For example, SFLPs sites in the In2O3–x(OH)y photocatalyst, comprises a coordinately unsaturated Lewis acidic In atom, proximal to an oxygen vacancy and an adjacent Lewis basic hydroxide group, enable the heterolysis of H2 and reaction with CO2 to form either CO or CH3OH11,12,13. As well, SFLPs involving coordinately unsaturated surface cobalt sites adjacent to surface hydroxides in the CoGeO2(OH)2 photocatalyst form CH4 from H2O and CO214. In addition, SFLPs in oxygen vacancy laden CeO2 bearing SFLPs catalyze the hydrogenation of alkenes and alkynes15. All of these cases utilize oxygen vacancies and hydroxides to engineer the catalytic activity of SFLPs. How to tailor the reactivity of the SFLPs themselves is rarely mentioned.
Indium sesquioxide (In2O3) is proving to be a promising catalyst for the thermal hydrogenation of CO2 to CH3OH or CO16,17,18. Experimental and computational studies of CO2 hydrogenation over oxygen vacancy laden In2O3 revealed that methanol formation was favored over the reverse water gas shift (RWGS) reaction19,20. Methanol production was remarkably enhanced when In2O3 was supported on ZrO2 arising from electronic support effects21. A bifunctional catalyst composed of partially reduced In2O3 supported on HZSM-5 could convert CO2 directly into gasoline-range hydrocarbons with a 78.6% selectivity due to the synergistic effects of these two components22. By controlling the degree of non-stoichiometry in In2O3–x, a black indium oxide catalyst, which utilized the entire solar spectrum, facilitated the photothermal RWGS reaction under ambient conditions with 100% selectivity23. Tailoring the electronic properties of In2O3 can also be achieved via replacement of an indium atom in the lattice with a H2 spillover palladium atom, although the rarity and cost of palladium could prove an issue for its practical implementation24.
Bismuth, regarded as a “green” element, has a long and fascinating history25. The Incas in sixteenth century South America, made corrosion resistant bronzes for their knives by mixing bismuth with tin26. Paracelsus in fifteenth century Germany, recognized bismuth as a non-toxic brother to lead27. Since, it is finding myriad eco-friendly uses from cosmetics and personal care products to medicine and lubricants. Most recently it has proved to be a serious contender for replacing toxic lead halide perovskite materials in solar cells with non-toxic bismuth oxyiodide, retaining a comparable energy conversion efficiency of 22%28. Contextually, bismuth materials with layered structures and visible light absorption properties, exemplified by BiOX (X=Cl, Br, I), Bi2MO6 (M=Mo, W), BiVO4 and Bi2S3, behave as photocatalysts to be applied in dye degradation, water and carbon dioxide reduction29.
Described herein, we developed a facile solvothermal route to achieve atom-precise substitution of Bi3+ for In3+ sites in In2O3 and realize the tailor of the reactivity of SFLPs, as well as the electronic properties of In2O3. To amplify, by substituting cheaper and safer bismuth for indium in UV absorbing In2O3, one can create the broad-spectrum UV–Vis light absorber BixIn2–xO3. Significantly, single-site Bi3+ substitution for In3+ provides strong Lewis acidic/basic Bi3+–O2− pairs that enhance CO2 adsorption and activation, while Bi 6s2 lone-pair electrons create mid-gap energy states. Atom-precise lattice engineering of this kind, boosts the reactivity of SFLPs and the harvesting efficiency of solar photons by BixIn2–xO3 compared to In2O3, which enables 1000 times photoactivity enhancement of the RWGS reaction together with a noticeable increase in the production of solar methanol.
Results
Structural characterizations of single-site BixIn2–xO3
BixIn2–xO3 nanocrystals were prepared via a one-step solvothermal route, in which the molar ratio of Bi could be controlled by adjusting the concentration of Bi(NO3)3 and In(NO3)3 precursors. The mole percent Bi content of each sample in the BixIn2–xO3 series of nanocrystals was determined using inductively coupled plasma mass spectrometry (ICP-MS, Supplementary Table 1). Transmission electron microscopy (TEM) shows that the pristine In2O3 nanocrystals are flower-like agglomerates of small nanocrystals with an average size of 3.7 nm (Supplementary Fig. 1). Bi3+ substitution results in similarly sized BixIn2–xO3 nanocrystals (3.5 nm) that show lattice fringes with a spacing of 2.92 Å, corresponding to the (222) plane of bcc In2O3 (Supplementary Fig. 2). The obtained selected area electron diffraction pattern shown no evidence of any metallic Bi or Bi2O3. Most significantly, spherical aberration-corrected scanning transmission electron microscopy (STEM) images provide an insightful and distinct result, in which atomically dispersed single-site Bi atoms are revealed under these high-resolution imaging conditions as bright dots (Fig. 1a, b). Energy-dispersive X-ray spectroscopy (EDS) line scans and elemental mapping (Fig. 1c and Supplementary Fig. 3) provided further evidence for the homogeneous distribution of elemental Bi in these BixIn2–xO3 nanocrystals.
a Aberration-corrected STEM image of 1.0% BixIn2–xO3 nanocrystals. b Aberration-corrected STEM image of 5.0% BixIn2–xO3 nanocrystals. c EDS mapping profiles of 5.0% BixIn2–xO3 along the indicated red line. d PXRD patterns of BixIn2–xO3 nanocrystals and pristine In2O3. e Normalized Bi L3-edge XANES spectra of 1.0% and 5.0% BixIn2–xO3, as well as Bi foil and Bi2O3 references. f k3-Weighted Bi L3-edge Fourier-transform EXAFS spectra of 1.0% and 5.0% BixIn2–xO3, as well as Bi foil and Bi2O3 references.
The phase structure of the obtained BixIn2–xO3 nanocrystals was studied by powder X-ray diffraction (PXRD, Fig. 1d). All the BixIn2–xO3 nanocrystals displayed nearly identical XRD patterns diagnostic of face-centered cubic In2O3 except that the diffraction peaks were shifted to lower 2θ values relative to those of pristine In2O3. This result indicates In3+ were isomorphously substituted by Bi3+, which has a larger ionic radius than In3+ (i.e., 0.96 Å versus 0.81 Å). This conclusion is supported by the In 3d peaks in the corresponding X-ray photoelectron spectroscopy (XPS) spectra (Supplementary Fig. 4a), which exhibited a gradual positive energy shift for BixIn2–xO3 nanocrystals relative to pure In2O3, that is attributed to the higher electronegativity of Bi3+ compared to In3+. The spin–orbit coupled doublet of Bi 4f XPS peaks at 158.6 eV and 163.9 eV define the oxidation state of bismuth as Bi3+ rather than Bi0 (Supplementary Fig. 4b), following the isomorphous substitution of In3+ by Bi3+. The electron paramagnetic resonance (EPR) spectra of BixIn2–xO3 nanocrystals at both room temperature and 77 K revealed the absence of paramagnetic species, thereby providing further evidence for the lack of Bi0 (Supplementary Fig. 5). Possibly EPR for semiconductors with high populations of [O]v occupied by electrons either does not exist or the existence of electronically degenerate ground states with fast electron relaxation and line broadening creates EPR silence. Maybe also the [O]v are devoid of trapped electrons or are doubly filled and hence diamagnetic and EPR silent. Further studies, such as 4 probe Van Der Paaw electrical conductivity measurements, are necessary to fully elucidate the conduction electron model.
Synchrotron radiation-based X-ray absorption spectroscopy (XAS) was further used to obtain information regarding the local structural environment of these distributed Bi sites. The Bi L3−edge X-ray absorption near-edge structure (XANES) spectra in Fig. 1e reveal visible similarities between the 1.0% and 5.0% BixIn2–xO3 sample spectra and that of the Bi2O3 reference. These similarities are to be expected, given that Bi atoms in both Bi2O3 and BixIn2–xO3 lattices are expected to be octahedrally coordinated by oxygen atoms, leading to similarities in their structural and electronic properties. The Fourier-transformed Bi L3-edge extended X-ray absorption fine structure (EXAFS) spectra are presented in Fig. 1f. The similar positions of the Bi–O peak positions suggest that these bonds lengths in the BixIn2–xO3 samples are similar to those in the Bi2O3 reference. In stark contrast, though, the observed Bi–M peaks appear at distinctly different positions in the BixIn2–xO3 spectra, revealing a distinct structural difference relative to the Bi2O3 reference. In order to more accurately quantify these differences in bond length and structure, the spectra were also fitted to extract key structural parameter values (Supplementary Fig. 6 and Supplementary Table 2). The resulting Bi–In bond lengths in the BixIn2–xO3 samples are shorter than those found in the pristine Bi2O3 lattice, though slightly longer than those observed in the pristine In2O3 lattice. Relatively larger Debye-Waller coefficient values for the Bi–O peaks in BixIn2–xO3 samples were also observed, reflecting a broader range of constituent Bi–O bond lengths.
CO2 hydrogenation performance
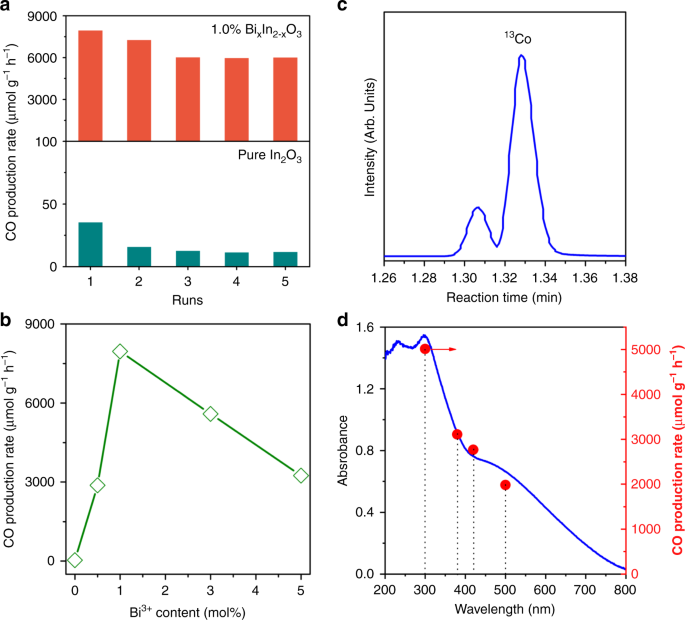
The photocatalytic CO2 hydrogenation activity of BixIn2–xO3 nanocrystals was evaluated in a batch reactor under simulated solar light irradiation and using a 1:1 ratio of CO2 and H2 gases. In these experiments, the RWGS reaction (i.e., CO2 + H2 → CO + H2O) led to CO being the sole product detected. The CO production rates revealed that 1.0% BixIn2–xO3 was more active than In2O3 by approximately three orders of magnitude (Fig. 2a), with an impressive peak rate of 8000 μmol g−1 h−1 for its first run, as compared to just 35 μmol g−1 h−1 for pristine In2O3. The estimated turnover frequency (TOF) of In2O3 and 1.0% BixIn2–xO3 is 0.42 h−1 and 93.6 h−1, respectively (Supplementary Note), implying that substituting Bi atoms into In2O3 nanocrystals significantly enhanced the photocatalytic activity towards CO2 hydrogenation. Remarkably, this boost in catalytic activity was much more dramatic than that observed in analogous hydroxylated systems (i.e., BixIn2–xO3(OH)y)30, thereby suggesting a distinct and potent mechanism of catalytic activity enhancement in BixIn2–xO3. Furthermore, such a CO production rate is much higher than some of the most active noble metal decorated photocatalysts (Supplementary Table 3). In addition, the 1.0% BixIn2–xO3 was very stable, exhibiting a CO production rate that was still roughly 600 times greater than the 11 μmol g−1 h−1 exhibited by pristine In2O3 under the same experimental conditions. In both cases, the CO production rate was observed to decrease over the course of five consecutive runs.
a CO rate of pristine In2O3 (down) and 1.0% BixIn2–xO3 nanocrystals (up) in catalyzing hydrogenation of CO2 under illumination. b CO rate as a function of Bi3+ content for various BixIn2–xO3 nanocrystals. c GC-MS plot of 13CO produced from 13CO2 over 1.0% BixIn2–xO3 nanocrystals. d CO rate as a function of absorption cutoff filter wavelength for 1.0% BixIn2–xO3 nanocrystals.
The actual bulk reaction temperature for In2O3 and BixIn2–xO3 tested by infrared camera is about 70 and 115 °C (Supplementary Fig. 7), respectively. The relatively low thermal energy supplied by solar light indicates the limited contribution of photothermal effect on the photocatalytic RWGS reaction. As an endothermic reaction, the rate of the RWGS is expected increase quite rapidly as a function of temperature. According to the Arrhenius Law, the rate should approximately double for every 10 °C increase in temperature. Thus, we would expect the reaction rate to increase by a factor of ~22.5, assuming that the bulk temperature of the catalyst accurately reflects the temperature of the catalytically active sites. Based on the rate of pristine In2O3 (i.e., 35 μmol g−1 h−1), this would result in a rate increase to about 788 μmol g−1 h−1 and account for about 10 % of the observed activity increase, thereby suggesting the existence of a significant photochemical effect. Notably, the marked increase in CO production rate upon substituting single-site Bi atoms into In2O3 was highly dependent on the concentration of Bi atoms (Fig. 2b). Meanwhile, isotopically labeled 13CO2 experiment confirmed that CO was the unequivocal product from photocatalytic CO2 hydrogenation (Fig. 2c).
We also investigated the dependence of the CO production rate on the wavelength of light, to demonstrate the CO2 hydrogenation proceeds mainly through a photocatalytic process. As seen in the action spectra shown in Fig. 2d and Supplementary Fig. 8, the production rate of CO monotonically decreased with longer wavelengths of the light, which correlates with the optical absorption spectrum of pristine In2O3 and BixIn2–xO3 catalysts. It should also be mentioned that the CO production rate remained at about 2000 μmol g−1 h−1 on 1.0% BixIn2–xO3, even when a 500 nm cutoff filter was applied, thus implying that BixIn2–xO3 nanocrystals can function as broadband, green photocatalysis for harvesting solar energy.
In light of the promising performance of BixIn2–xO3 nanocrystals towards gas-phase CO2 hydrogenation, this new catalyst was also studied for solar methanol production in a flow reactor at 230 °C, both with and without light irradiation. As shown in Supplementary Fig. 9, all catalysts exhibited similarly low CO and CH3OH production rates under purely thermal conditions; however, a remarkable enhancement in the production rates of CO and CH3OH was obtained on changing from dark to light conditions. In this case, pristine In2O3 exhibited CO and CH3OH production rates of 312 and 82 μmol g−1 h−1, respectively (Fig. 3a, b). In comparison, the single-site BixIn2–xO3 samples exhibited much better activities for both CO and CH3OH production, with 1.0% BixIn2–xO3 exhibiting the highest CO and CH3OH production rates of 918 μmol g−1 h−1 and 158 μmol g−1 h−1, respectively. Overall, the measured activities of the BixIn2–xO3 samples were highly dependent on Bi content and showed a volcano-shaped trend. The volcano trend of the activity towards CO2 hydrogenation versus the extent of In3+ substitution by the larger, more electronegative, 6s2 stereochemically active lone-pair containing Bi3+, can be attributed to a subtle interplay of numerous and competing intertwined properties: chemical effects (e.g., influence of surface Lewis acidity and basicity of In-O-In, In-O-Bi, Bi-O-Bi sites on CO2-H2 adsorption, activation, reaction processes) and physical effects (e.g., photogenerated electron and hole charge-separation and charge-trapping by bismuth and oxygen vacancy mid-gap states).
a CH3OH production rates as a function of Bi3+ content under light irradiation and at 230 °C. b CO production rates as a function of Bi3+ content under light irradiation and at 230 °C. c Arrhenius plots for CH3OH production rates of 1.0% BixIn2–xO3 with and without solar irradiation. d Arrhenius plots for CO production rates of 1.0% BixIn2–xO3 with and without solar irradiation.
The CH3OH and CO production rates of BixIn2–xO3 nanocrystals showed negligible deactivation, even after 50 h of continuous testing under light irradiation at 230 °C (Supplementary Fig. 10a), suggesting their excellent catalytic stability. The recorded XRD patterns, TEM images and XPS spectra (Supplementary Fig. 10b–d) for the spent BixIn2–xO3 photocatalysts after 50 h of reaction demonstrate that, except for a slight increase in particle size, the phase and oxidation states of the nanocrystals were well maintained, confirming their favorable structural stability.
To obtain more information on the origin of the activity enhancement under flow reaction conditions, activity tests were also conducted at lower reaction temperatures, beginning where products can be observed (130, 195, and 210 °C), with and without light irradiation (Supplementary Fig. 11). The drastic activity difference between dark and light conditions lend further support confirmed the contribution of the photochemical effect on CO and CH3OH production. Moreover, based on the Arrhenius plots for 1.0% BixIn2–xO3, the apparent activation energy for the CO and CH3OH photochemical processes are much lower than the thermochemical ones (Fig. 3c, d), reflecting the solar advantage for the excited-state reaction pathway relative to the ground state pathway31.
Photocatalytic reaction pathway
The photocatalytic CO2 hydrogenation reaction involves photon-absorption, electron-hole separation, and CO2 adsorption/activation processes. The first two steps are closely related to the intrinsic nature of the photocatalyst, while the third is highly dependent on the gas-solid interface. A significant red shift occurs in the absorption edge of the UV–Vis spectra for all BixIn2–xO3 samples (Fig. 4a), along with an enhanced tail above 440 nm, which grows with Bi3+ content and is accompanied by a change in color from cream to rust (Supplementary Fig. 12). This is consistent with the simulated band structures that show the introduction of Bi3+ can leads to the formation of mid-gap states in the bandgap of In2O3 (Fig. 4b, c). The total density of states (DOS) and partial density of states (PDOS) (Supplementary Fig. 13) can further reveal that the substitution of Bi at the In site induces mid-gap energy states with Bi 6s states below the conduction band edge of In2O3 and is consistent with the reported results in this paper32,33,34.
a Diffuse reflectance spectra of pristine In2O3 and various BixIn2–xO3 nanocrystals. b The simulated band structure and DOS plots of pristine In2O3. c The simulated band structure and DOS plots of BixIn2–xO3 nanocrystals. d Room-temperature PL spectra of pristine In2O3 and 1.0% BixIn2–xO3 nanocrystals using an excitation wavelength of 325 nm. e Schematic illustrating charge carrier recombination pathways on surface defects states (SFLPs) and enabling CO2 hydrogenation reactions.
To understand the photogenerated charge transfer mechanism, the room-temperature photoluminescence (PL) spectra of pristine In2O3 and 1.0% BixIn2–xO3 nanocrystals are shown in Fig. 4d. The pristine In2O3 nanocrystals exhibited a strong green emission peak centered at ca. 440 nm, originating from the radiative recombination of photo-excited electrons trapped in mid-gap oxygen vacancy states with photogenerated holes in the valence band35. The existence of oxygen vacancies is further evidenced by the O 1 s core level XPS spectra (Supplementary Fig. 14). In contrast, the incorporation of single-site Bi into In2O3 leads to weakening and broadening of the PL emission peak. This can be explained in two ways. One is that the substitutional Bi3+ slightly decreases the concentration of oxygen vacancies (Supplementary Fig. 15), which would decrease the intensity due to electron-hole radiative recombination. Alternatively, the substituted Bi3+ states lying below the conduction band of In2O3 could also act as traps capturing photo-excited electrons and inhibiting electron-hole recombination emission, resulting in lower PL emission. Thus, as illustrated in Fig. 4e, the substituted Bi3+ sites (denoted as Bi’), oxygen vacancies [O], coordinately unsaturated indium In’ sites and oxygen O’ sites, exist as mid-gap defect states (comprising surface frustrated Lewis pairs, SFLPs) in the bandgap of BixIn2–xO3, can function as traps for photogenerated electrons and holes enabling the reaction between CO2/H236,37. This results in the quenching of steady-state PL emission as well as a slight shortening of the average fluorescence lifetime from 120 to 110 ps, probed by time-resolved PL spectroscopy (Supplementary Fig. 16). Albeit small, this reduction of the fluorescence lifetime, suggests that, relative to In2O3, single-site Bi atoms can increase the occurrence of competitive non-radiative relaxation processes in BixIn2–xO338,39,40.
Apart from its effect on the electronic structure and charge transfer, single-site Bi3+ substitution is also expected to strengthen the adsorption-bonding-activating ability of BixIn2–xO3 toward CO2. The textural structure of BixIn2–xO3 including surface area, pore volume and pore size show obvious improvements and could favor the adsorption of CO2 reactants (Supplementary Table 4 and Supplementary Fig. 17). With respect to the surface chemistry, CO2 can bond through its carbon atom and oxygen atoms to either the surface oxygen atoms, metal sites, or directly with the oxygen vacancies of metal oxides (Supplementary Fig. 18)41,42,43. To investigate the effect of Bi3+ substitution on the interaction between CO2 and BixIn2–xO3 or pristine In2O3 nanocrystals, CO2 temperature-programmed desorption (CO2-TPD) measurements were initially performed. As shown in Fig. 5a, one broad desorption peak at around 100 °C, corresponding to physically adsorbed CO2, is observed for all BixIn2–xO3 nanocrystals and pristine In2O3. A significant desorption peak is clearly observed at 256 °C for pristine In2O3 and can be attributed to the chemical desorption of CO2 that is binding with oxygen vacancies to form bent CO2δ− species44. Since Bi3+ substitution results in fewer oxygen vacancies, this peak intensity gradually decreases with increased Bi3+ doping of BixIn2–xO3 nanocrystals and shifts slightly to higher temperatures (as high as 275 °C for 5.0 % BixIn2–xO3), implying that the binding strength of CO2 and oxygen vacancies is remarkably enhanced. Moreover, weak desorption peaks at higher temperatures (300 to 600 °C) were clearly observed for In2O3, and can be assigned to the decomposition of surface HCO3− and CO32− species45. After single-site Bi3+ substitution, typical desorption peaks can also be clearly identified and show a slight shift to higher temperatures, again indicating that these surface species are binding more strongly to the surface.
In situ diffuse reflectance infrared Fourier-transform spectroscopy (DRIFTS) experiments were further carried out to identify surface species. Figure 5b show the transient evolution of the surface species during CO2 adsorption over 1.0% BixIn2–xO3 nanocrystals. The bands at 1510 and 1372 cm−1 are assigned to the asymmetric and symmetric OCO stretching modes of monodentate carbonates (m-CO32−). The features at 1549 and 1330 cm−1 are attributed to the asymmetric and symmetric OCO stretching modes of bidentate carbonates (b-CO32−). The bent CO2δ− species adsorbed at oxygen vacancy sites can be identified by two bands at 1596 and 1348 cm−1, corresponding to the asymmetric and symmetric stretching modes, respectively. The appearance of bands at 1625, 1437, 1390, and 1222 cm−1 indicates the formation of bicarbonate species (HCO3−). Moreover, a small amount of a linearly adsorbed CO2 species with bands appearing between 1000 and 1100 cm−1 can also be observed. Thus, via DRIFTS measurements, all the surface species observed during CO2-TPD measurements, CO32−, HCO3− and CO2δ−, were observed and identified on BixIn2–xO3 nanocrystals. All these surface species can be observed on pristine In2O3; however, the peak intensities of these species are weaker than that on BixIn2–xO3 nanocrystals, suggesting the improved CO2 adsorption-bonding-activating capacity after Bi3+ substitution.
Density functional theory (DFT) slab calculations were further carried out to unravel the promotion effect of Bi3+ substitution on In2O3. The perfect In2O3 (110) surface was initially selected as it has proven to be most thermodynamically stable20,46. The defective In2O3 (110) surface with an oxygen vacancy at the O4 site was then created owing to the more favorable ability for CO2 activation and hydrogenation19. Following then, we examined the possibility of Bi3+ substitution at the In site.
As shown in Fig. 6a, the perfect In2O3 (110) surface consists of chains of In and O atoms, with the numbering In and O atoms along the chain as repeating unit. The surface In and O in the chain are adjacent and contiguous to each other, forming a classic Lewis acid-base adjunct, whereas the unbonded In3 and In4 in the chain and O in the top layer show a distance of 4.106 and 4.312 Å, respectively, which may deliver SFLPs-like activity (Fig. 6d). However, the electronic interactions between In3 or In4 and its neighboring O4 will block the function of In3-O or In4-O pairs. Therefore, the removal of oxygen atom at the O4 site is the prerequisite to construct a pair of unbonded Lewis acid and base sites. When the O4 atom is removed, two In atoms (In3 and In4) are coordinatively unsaturated and one oxygen vacancy (OV4) is produced (Fig. 6b). However, in this case, only one In atom (In4) locates at the surface while the other one (In3) moves to the inner atomic layer. The surface In4 atom is found to be surrounded by two adjacent oxygen atoms, of which the In4-O with a distance of 4.222 Å can construct a SFLPs site (Fig. 6e). We further investigated the effect of Bi3+ substitution at In4 site on the configuration and charge population (Fig. 6c, f). As compared to In4-O configuration, Bi4-O shows a shorter distance (3.821 Å) but can still fall in the domain of solid SFLPs. On the other hand, the Bader charge calculations show that the related Lewis acid Bi3+ and Lewis base O2− involve atomic local charges of +1.500 e and −0.910 e, respectively, which is higher than that of the In3+ and O2− pair (+1.300 e and −0.900 e). The larger charge difference between the Lewis acid and Lewis base pairs in the BixIn2–xO3 compared with that of defective In2O3 would form more active Lewis acid-base pairs than the In2O3 pair can muster, and therefore could deliver a higher capability to activate CO2 molecules, consistent well with the DRIFTs and CO2-TPD results.
a Optimized structure of perfect In2O3 (110). b Optimized structure of defective In2O3 (110) with one oxygen vacancy. c Optimized structure of BixIn2–xO3 (110) with Bi3+ substitution. d Electron-density isosurface of perfect In2O3 (110). e Electron-density isosurface of defective In2O3 (110) with one oxygen vacancy. f Electron-density isosurface of BixIn2–xO3 (110) with Bi3+ substitution.
In the photo-excited state of a SFLPs system, the Lewis acidity and Lewis basicity have been shown to increase as compared with the ground state, thereby facilitating the photochemical CO2 hydrogenation, with a decrease in activation energy. To get more insight into the improved activity from Bi3+ substitution, in situ DRIFTS experiments were further performed under reaction conditions to detect the reaction intermediates and uncover the photocatalytic pathway in the CO2 hydrogenation process. As shown in Fig. 5c, when the BixIn2–xO3 nanocrystals were exposed to the mixture of CO2 and H2 gases, bidentate formate (*HCOO), methoxy (*H3CO) and carboxylate (*CO2)47, were the three principal intermediates observed from the transformation of bicarbonate and carbonate species, as evidenced by the decrease and disappearance of characteristic bands at 1510, 1390, and 1222 cm−1. The *HCOO species can be linked to fingerprint modes at 2973 and 2731 cm−1, which correspond to a combination of the CH bending and OCO stretching modes47,48. The bands at 1592 and 1370 cm−1 can be assigned to the asymmetric and symmetric OCO stretching modes while that at 2868 cm−1 is attributed to the CH stretching mode of the same species47,49,50. The *H3CO species is signaled by diagnostic modes at 2941 and 2838 cm−1 that are assigned to the CH3 stretching modes and the band at 1182 cm−1 is attributed to the CO stretching mode of bridged methoxide species47,48,51. In addition to *HCOO and *H3CO, *CO2 species were also observed, with bands at 1567 and 1379 cm−1 that can be associated with the OCO stretching modes. Under light irradiation, all intermediates showed an increase in band intensity (Supplementary Fig. 19), thereby confirming the photochemical effect of CO2 hydrogenation, and is consistent with the activity results. From these DRIFT results, CO2 hydrogenation over BixIn2–xO3 may proceed via two major reaction pathways featured by both formate intermediate and CO intermediate, which has been well established for the In2O3-x(OH)y systems12. In the case of pristine In2O3 (Fig. 5c and Supplementary Fig. 20), *HCOO and *H3CO species of virtually insignificant intensity were observed for CO2 hydrogenation, and light irradiation resulted in much noisier peaks. This further indicates the moderate catalytic performance of pristine In2O3 and the significant promotion effect resulting from single-site Bi3+ substitution. To corroborate these experimental observations, free energy profiles for CO2 hydrogenation via the proposed RWGS pathway over BixIn2–xO3 and pristine In2O3 were calculated (Supplementary Fig. 21). It can be seen that the H2 dissociation into H* on pristine In2O3 (defective type with one oxygen vacancy) is the rate-limiting step and endothermic with an activation energy barrier of 1.47 eV. Importantly, compared with pristine In2O3, the BixIn2–xO3 exhibits a negative ΔG value of −0.05 eV for the H2 dissociation, which implies that the H2 dissociation into H* on the surface of BixIn2–xO3 is energetically favorable. This result indicates that the single-site Bi3+ substituted nanostructure has more active Lewis acid-base pairs than the In2O3 pair can muster, and therefore can strongly polarize H–H bonds and dissociate H2 molecules into *H. The proceeding hydrogenation reactions of H* with CO2 on the surface of In2O3 and BixIn2–xO3 are similar. However, benefiting from the favorable H2 dissociation, BixIn2–xO3 shows a much-lowered reaction energy profile for CO and H2O formation than pristine In2O3.
Discussion
In summary, we have demonstrated a one-step solvothermal route towards atom-precise isomorphic substitution of In3+ in In2O3 by Bi3+ to generate BixIn2–xO3 materials with broad-spectrum UV–Vis absorption. The incorporation of single-site Bi atoms in the In2O3 host lattice provides strong Lewis acid-base Bi3+–O2− pairs to enhance CO2 adsorption and activation, resulting in distinctly enhanced reaction rates relative to those observed for pristine In2O3 and other indium oxide-based catalysts. The Bi 6s2 lone pairs create mid-gap energy states, which can increase the harvesting of solar photons and favor the generation and separation of photo-induced charge carriers. Remarkably, single-site Bi3+-substituted BixIn2–xO3 proves to be a highly efficient and stable photocatalyst, achieving an impressive CO production rate three orders of magnitude greater than that of pristine In2O3, with notable photoactivity towards solar methanol. In addition to increased activity catalytic sites, the greening of indium oxide by single-site bismuth atom substitution represents a new approach to CO2 photocatalyst engineering and is a further step towards the vision of a solar CO2 refinery.
Methods
Synthesis of In2O3 and BixIn2–xO3
Pristine In2O3 nanocrystals were prepared via a simple solvothermal route. In a typical synthesis, 0.3 g of In(NO3)3•4.5H2O was dissolved in 17 mL anhydrous dimethylformamide solution. After stirring for 30 min, the obtained homogeneous solution was transferred into a Teflon-lined stainless steel autoclave and then heated at 150 °C for 24 h. After being cooled to room temperature, the light-yellow product was collected through centrifugation, washed with ethanol and water, and finally dried at 60 °C in vacuum. Bi3+-substituted In2O3 nanocrystals were prepared using the same method employed for pristine In2O3, except that various amounts of Bi(NO3)3•5H2O were added to the indium solution prior to solvothermal reaction.
Material characterizations
The content of Bi in BixIn2–xO3 was determined using an inductively coupled plasma mass spectroscopy (ICP-MS) instrument (Optima 7300 DV). Powder X-ray diffraction (PXRD) was performed on a Bruker D2-Phaser X-ray diffractometer, using Cu Kα radiation at 30 kV. X-ray photoelectron spectroscopy (XPS) was performed using a PerkinElmer Phi 5500 ESCA spectrometer in an ultrahigh vacuum chamber with a base pressure of 1 × 10−9 Torr. The spectrometer used an Al Kα X-ray source operating at 15 kV and 27 mA. The samples were coated onto carbon tape prior to analysis and all results were calibrated to C1s 284.5 eV. EPR spectra were obtained at room temperature and 77 K using a Bruker A-300-EPR X-band spectrometer. Transmission electron microscopy (TEM) measurements were conducted using a JEM–2010 microscope working at 200 kV. The double spherical aberration-corrected scanning transmission electron microscope (STEM) images were obtained on an FEI Themis Z instrument. X-ray absorption spectra were collected at the BL14W beamline of the Shanghai Synchrotron Radiation Facility (SSRF). The storage ring of the SSRF was operated at 3.5 GeV with a stable current of 200 mA. Using a Si(111) double-crystal monochromator, the data collection was carried out in fluorescence mode using Lytle detector. All spectra were collected under ambient conditions. Diffuse reflectance spectra (DRS) of the powders were obtained for dry-pressed disk samples using a Cary 500 Scan Spectrophotometer (Varian, USA) over a range of 200 to 800 nm. Barium sulfate (BaSO4) was used as a reflectance standard. Room-temperature photoluminescence (PL) spectra were measured on an FL/FS 920 (Edinburgh Instruments) system equipped with a 450 W Xe arc lamp as the excitation source and a red sensitive Peltier element-cooled Hamamatsu R2658 PMT as the detector. Time-resolved fluorescence decay spectra were recorded on the Delta Pro (HORIBA instruments) using a 357 nm laser as the excitation source. BET surface area analyses were performed on an ASAP2020 M apparatus (Micromeritics Instrument Corp., USA) with the samples degassing in vacuum at 110 °C for 10 h and then measuring at 77 K. The CO2 temperature-programmed desorption (CO2-TPD) measurements were carried out on AutoChem II 2920 Version. The density functional theory (DFT) calculations were performed using the Cambridge Sequential Total Energy Package (CASTEP) computational codes. During the geometry optimization, lattice parameters and atomic positions were optimized simultaneously. Based on the experimental data, we replaced one In with Bi in the cell as the In15BiO24 model and deleted one of the oxygen atom that were coordinating with Bi to establish one In15BiO23(OVacancy) model. For calculating the electronic structures and density of states, the geometry optimization of In2O3 and In15BiO23(OVacancy) were calculated by the PBE method within Generalized Gradient-corrected Approximation (GGA), using the exchange-correlation potential. The Vanderbilt ultrasoft pseudopotential with a cutoff energy of 380 eV was used to ensure the precision of the results. Brillouin zone integration was represented using the K-point sampling scheme of 3 × 3 × 3 Monkhorst–Pack scheme. The convergence tolerance for geometry optimization was selected with the differences in total energy (5.0 × 10−6 eV/atom), the maximal ionic Hellmann–Feynman force (1.0 × 10−2 eV Å−1), the stress tensor (2.0 × 10−2 GPa), and the maximal displacement (5.0 × 10−4 Å).
Gas-phase CO2 hydrogenation tests
Batch reactions were conducted in a custom-built 1.5 mL stainless steel batch reactor with a fused-silica viewport sealed with Viton O-rings. The reactor with ∼4.5 mg of catalyst on a borosilicate film support was evacuated using an Alcatel dry pump prior to being purged with the reactant high-purity H2 reactant gas. After purging the reactor, it was filled with a 1:1 stoichiometric mixture of H2 (99.9995%) and CO2 (99.999%) until the total pressure reached 30 psi. The reactor was irradiated with a 300 W Xe lamp for a duration of 1 h without external heating. Product gases were analyzed using flame ionization and thermal conductivity detectors installed in a SRI-8610 gas chromatograph equipped with 3 in. Mole Sieve 13a and 6 in. Haysep D column. Isotopically labeled tracing experiments were performed using 13CO2 (99.9 at%, Sigma-Aldrich). Isotope distributions in the product gases were measured using an Agilent 7890A gas chromatograph-mass spectrometer with a 60 m GS-carbon plot column, leading to the mass spectrometer. Flow experiments were carried out in a fixed-bed tubular reactor with ∼10 mg of catalyst material being packed into a quartz tube and immobilized at both ends with quartz wool. The quartz tube had an inner diameter of 2 mm with a wall thickness of 0.5 mm, and was placed into a groove carved out into a copper block. An OMEGA temperature controller was attached to two heating cartridges inserted into the copper block and a thermocouple was inserted into the quartz tube contacting the catalyst but covered by the quartz wool. A 300 W Xe arc lamp illuminated the catalyst plug at a measured intensity of 2 W cm−2. CO2 and H2 were flowed through with a 1:3 ratio (1 sccm CO2, 3 sccm H2). The amounts of CO and CH3OH produced were determined using gas chromatography-mass spectrometry (GC-MS, 7890B and 5977A, Agilent) using a He carrier gas.
In situ DRIFT studies
The in situ DRIFTS measurements were performed to detect the surface intermediates over pristine In2O3 and BixIn2–xO3 nanocrystals under reaction conditions. The spectra were collected using a Fourier-transform infrared spectroscopy spectrometer (Thermo, Nicolet 6700) equipped with an MCT detector. Before measurement, the catalyst was purged with He at 250 °C for 2 h. The catalyst was subsequently cooled down to 230 °C. The background spectrum with a resolution of 4 cm−1 was obtained at 230 °C in He flow. Then the catalyst was exposed to a mixture of CO2, H2, and He (1 sccm CO2, 3 sccm H2, and 16 sccm He, respectively) in dark and light conditions for different times. The in situ DRIFT spectra were recorded by collecting 32 scans at 4 cm−1 resolutions.
References
Dang, S. S. et al. Rationally designed indium oxide catalysts for CO2 hydrogenation to methanol with high activity and selectivity. Sci. Adv. 6, eaaz2060 (2020).
Wang, M. et al. Oxygen vacancy generation and stabilization in CeO2-x by Cu introduction with improved CO2 photocatalytic reduction activity. ACS Catal. 9, 4573–4581 (2019).
Wang, W., Wang, S. P., Ma, X. B. & Gong, J. L. Recent advances in catalytichydrogenation of carbon dioxide. Chem. Soc. Rev. 40, 3703–3727 (2011).
Jia, J. et al. Visible and near-infrared photothermal catalyzed hydrogenation of gaseous CO2 over nanostructured Pd@Nb2O5. Adv. Sci. 3, 1600189 (2016).
Meng, X. G. et al. Photothermal conversion of CO2 into CH4 with H2 over group VIII nanocatalysts: an alternative approach for solar fuel production. Angew. Chem. Int. Ed. 53, 11478–11482 (2014).
Chen, G. B. et al. Alumina-supported CoFe alloy catalysts derived from layered-double-hydroxide nanosheets for efficient photothermal CO2 hydrogenation to hydrocarbons. Adv. Mater. 29, 1704663 (2017).
Feng, K. et al. Cobalt plasmonic superstructures enable almost 100% broadband photon efficient CO2 photocatalysis. Adv. Mater. 32, 2000014 (2020).
Stephan, D. W. Frustrated Lewis pairs. J. Am. Chem. Soc. 137, 10018–10032 (2015).
Stephan, D. W. & Erker, G. Frustrated Lewis pair chemistry: development and perspectives. Angew. Chem. Int. Ed. 54, 6400–6441 (2015).
Ma, Y. Y. et al. Semi-solid and solid frustrated Lewis pair catalysts. Chem. Soc. Rev. 47, 5541–5553 (2018).
Ghuman, K. K. et al. Illuminating CO2 reduction on frustrated Lewis pair surfaces: investigating the role of surface hydroxides and oxygen vacancies on nanocrystalline In2O3-x(OH)y. Phys. Chem. Chem. Phys. 17, 14623–14635 (2015).
Yan, T. J. et al. Polymorph selection towards photocatalytic gaseous CO2 hydrogenation. Nat. Commun. 10, 2521–2530 (2019).
Wang, L. et al. Room-temperature activation of H2 by a surface frustrated Lewis pair. Angew. Chem. Int. Ed. 58, 9501–9505 (2019).
Wang, X. H. et al. Frustrated Lewis pairs accelerating CO2 reduction on oxyhydroxide photocatalysts with surface lattice hydroxyls as a solid-state proton donor. Adv. Funct. Mater. 28, 1804191–1804199 (2018).
Zhang, S. et al. Solid frustrated-Lewis-pair catalysts constructed by regulations on surface defects of porous nanorods of CeO2. Nat. Commun. 8, 15266–15277 (2017).
Wang, J. Y. et al. Variation in the In2O3 crystal phase alters catalytic performance toward the reverse water gas shift reaction. ACS Catal. 10, 3264–3273 (2020).
Wang, L. R. et al. In2O3 nanocrystals for CO2 fixation: atomic-level insight into the role of grain boundaries. iScience 16, 390–398 (2019).
Sun, K. H. et al. Hydrogenation of CO2 to methanol over In2O3 catalyst. J. CO2 Utiliz. 12, 1–6 (2015).
Ye, J. Y., Liu, C. J., Mei, D. H. & Ge, Q. F. Active oxygen vacancy site for methanol synthesis from CO2 hydrogenation on In2O3(110): A DFT study. ACS Catal. 3, 1296–1306 (2013).
Ye, J. Y., Liu, C. J. & Ge, Q. F. DFT study of CO2 adsorption and hydrogenation on the In2O3 surface. J. Phys. Chem. C. 116, 7817–7825 (2012).
Martin, O. et al. Indium oxide as a superior catalyst for methanol synthesis by CO2 hydrogenation. Angew. Chem. Int. Ed. 55, 6261–6265 (2016).
Gao, P. et al. Direct conversion of CO2 into liquid fuels with high selectivity over a bifunctional catalyst. Nat. Chem. 9, 1019–1024 (2017).
Wang, L. et al. Black indium oxide a photothermal CO2 hydrogenation catalyst. Nat. Commun. 11, 2432–2439 (2020).
Frei, M. S. et al. Atomic-scale engineering of indium oxide promotion by palladium for methanol production via CO2 hydrogenation. Nat. Commun. 10, 3377–3387 (2019).
Mohan, R. Green bismuth. Nat. Chem. 2, 336–336 (2010).
Gordon, R. B. & Rutledge, J. W. Bismuth bronze from Machu Picchu, Peru. Science 223, 585–586 (1984).
Rohr, O. Bismuth-the new ecologically green metal for modern lubricating engineering. Ind. Lubr. Tribol. 54, 153–164 (2002).
Leng, M. Y. et al. Lead-free, blue emitting bismuth halide perovskite quantum dots. Angew. Chem. Int. Ed. 55, 1–6 (2016).
Ye, L. Q., Deng, Y., Wang, L., Xie, H. Q. & Su, F. Y. Bismuth-based photocatalysts for solar photocatalytic carbon dioxide Conversion. ChemSusChem 12, 3671–3701 (2019).
Dong, Y. C. et al. Tailoring surface frustrated Lewis pairs of In2O3−x(OH)y for gas-phase heterogeneous photocatalytic reduction of CO2 by isomorphous substitution of In3+ with Bi3+. Adv. Sci. 5, 1700732–1700742 (2018).
Ghoussoub, M., Xia, M. K., Duchesne, P. N., Segal, D. & Ozin, G. A. Principles of photothermal gas-phase heterogeneous CO2 catalysis. Energ. Environ. Sci. 12, 1122–1142 (2019).
Kanhere, P. D., Zheng, J. W. & Chen, Z. Site specific optical and photocatalytic properties of Bi-doped NaTaO3. J. Phys. Chem. C. 115, 11846–11853 (2011).
Mizoguchi, H. et al. New mixed-valence oxides of bismuth: Bi1-xYxO1.5+δ (x = 0.4). J. Mater. Chem. 7, 943–946 (1997).
Liang, C. H., Meng, G. W., Lei, Y., Phillipp, F. & Zhang, L. D. Catalytic growth of semiconducting In2O3 nanofibers. Adv. Mater. 13, 1330–1333 (2001).
Saison, T. et al. Bi2O3, BiVO4, and Bi2WO6: impact of surface properties on photocatalytic activity under visible light. J. Phys. Chem. C. 115, 5657–5666 (2011).
Lei, F. C. et al. Oxygen vacancies confined in ultrathin indium oxide porous sheets for promoted visible-light water splitting. J. Am. Chem. Soc. 136, 6826–6829 (2014).
Hoch, L. B. et al. Carrier dynamics and the role of surface defects: Designing a photocatalyst for gas-phase CO2 reduction. Proc. Natl Acad. Sci. USA 113, E8011–E8020 (2016).
Hao, X. Q. et al. Zn-vacancy mediated electron-hole separation in ZnS/g-C3N4 heterojunction for efficient visible-light photocatalytic hydrogen production. Appl. Catal. B 229, 41–51 (2018).
Lu, Y. H. et al. A facile green antisolvent approach to Cu2+-doped ZnO nanocrystals with visible-light-responsive Photoactivities. Nanoscale 6, 8796–8803 (2014).
Dong, F. et al. An advanced semimetal-organic Bi spheres/g-C3N4 nanohybrid with SPR enhanced visible-light photocatalytic performance for NO purification. Environ. Sci. Technol. 49, 12432–12440 (2015).
Wang, Y. et al. CO2 photoreduction with H2O vapor on highly dispersed CeO2/TiO2 catalysts: Surface species and their reactivity. J. Catal. 337, 293–302 (2016).
Jia, J. et al. Heterogeneous catalytic hydrogenation of CO2 by metal oxides: defect engineering–perfecting Imperfection. Chem. Soc. Rev. 46, 4631–4644 (2017).
Pokrovski, K., Jung, K. T. & Bell, A. T. Investigation of CO and CO2 adsorption on tetragonal and monoclinic zirconia. Langmuir 17, 4297–4303 (2001).
Li, S. W. et al. Tuning the selectivity of catalytic carbon dioxide hydrogenation over iridium/cerium oxide catalysts with a strong metal–support interaction. Angew. Chem. Int. Ed. 56, 10761–10765 (2017).
Luo, C. et al. Photocatalytic CO2 reduction over SrTiO3: Correlation between surface structure and activity. Appl. Surf. Sci. 447, 627–635 (2018).
Walsh, A. & Catlow, C. R. A. Structure, stability and work functions of the low index surfaces of pure indium oxide and Sn-doped indium oxide (ITO) from density functional theory. J. Mater. Chem. 20, 10438–10444 (2010).
Kattel, S., Yan, B., Yang, Y., Chen, J. G. & Liu, P. Optimizing binding energies of key intermediates for CO2 hydrogenation to methanol over oxide-supported copper. J. Am. Chem. Soc. 138, 12440–12450 (2016).
Fisher, I. A. & Bell, A. T. In-situ infrared study of methanol synthesis from H2/CO2 over Cu/SiO2 and Cu/ZrO2/SiO2. J. Catal. 172, 222–237 (1997).
Bianchi, D., Chafik, T., Khalfallah, M. & Teichner, S. J. Intermediate species on zirconia supported methanol aerogel catalysts. IV. Adsorption of carbon dioxide. Appl. Catal. 105, 219–235 (1994).
Guglielminotti, E. Infrared study of syngas adsorption on zirconia. Langmuir 6, 1455–1460 (1990).
Rhodes, M., Pokrovski, K. & Bell, A. The effects of zirconia morphology on methanol synthesis from CO and H2 over Cu/ZrO2 catalysts Part II. Transient-response infrared studies. J. Catal. 233, 210–220 (2005).
Acknowledgements
T.Y. is thankful for financial support from the National Natural Science Foundation of China (21872081), Taishan Scholars Program of Shandong Province, and Youth Innovation and Technology Project of Shandong Province (2020KJC010). G.A.O. acknowledges the financial support of the Ontario Ministry of Research and Innovation (MRI), the Ministry of Economic Development, Employment and Infrastructure (MEDI), the Ministry of the Environment and Climate Change’s (MOECC) and the Best in Science (BIS) Award. Also acknowledged is additional support from the Ontario Center of Excellence (OCE) Solutions 2030 Challenge Fund, the Low Carbon Innovation Fund (LCIF), Imperial Oil, the University of Toronto Connaught Innovation Fund (CIF), the Connaught Global Challenge (CGC) Fund, and the Natural Sciences and Engineering Research Council of Canada (NSERC). P.N.D. acknowledges personal funding providing by the NSERC PDF program.
Author information
Authors and Affiliations
Contributions
T.Y. and G.A.O. conceived and designed the experiments. T.Y., N.L., and Linlin Wang prepared the materials for characterizations. T.Y. and N.T. carried out the batch and flow experiments for CO2 hydrogenation. W.R. and N.L. carried out the DFT calculation. T.Y. performed the in situ DRIFTS study and CO2-TPD test. Lu Wang performed the XPS characterization. M.X. and Linlin Wang carried out the ICP-AES study. T.Y and Lili Wan performed the MS test using 13C. P.D. performed the XAFS XANES experiments. T.Y., N.L., and G.A.O. co-wrote the manuscript. All authors discussed the results and commented on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Communications thanks Zhi-rong Geng, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yan, T., Li, N., Wang, L. et al. Bismuth atom tailoring of indium oxide surface frustrated Lewis pairs boosts heterogeneous CO2 photocatalytic hydrogenation. Nat Commun 11, 6095 (2020). https://doi.org/10.1038/s41467-020-19997-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-020-19997-y
This article is cited by
-
A nonmetallic plasmonic catalyst for photothermal CO2 flow conversion with high activity, selectivity and durability
Nature Communications (2024)
-
Progress in design and preparation of multi-atom catalysts for photocatalytic CO2 reduction
Science China Materials (2024)
-
Understanding the progress and challenges in the fields of thermo-catalysis and electro-catalysis for the CO2 conversion to fuels
Emergent Materials (2024)
-
Cu-based high-entropy two-dimensional oxide as stable and active photothermal catalyst
Nature Communications (2023)
-
Photocatalytic CO2 reduction
Nature Reviews Methods Primers (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.