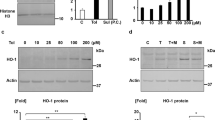
Abstract
A close relationship between angiotensin II (ANG II) and the renal dopaminergic system (RDS) has been reported. Our aim was to study whether renal dopamine and ANG II can interact to modify renal sodium handling and then to elucidate the related mechanism. Anesthetized male Sprague–Dawley rats were used in experiments. ANG II, exogenous dopamine, and decynium-22 (or D-22, an isocyanine that specifically blocks electrogenic organic cation transporters, OCTs), were infused in vivo for 120 min. We analyzed renal and hemodynamic parameters, renal Na+, K+-ATPase levels, OCT activity, and urinary dopamine concentrations. We also evaluated the expression of D1 receptor, electroneutral organic cation transporters (OCTNs), and OCTs. ANG II decreased renal excretion of sodium in the presence of exogenous dopamine, increased Na+, K+-ATPase activity, and decreased the urinary dopamine concentration. D-22 treatment exacerbated the ANG II-mediated decrease in renal excretion of sodium and dopamine urine excretion but did not modify ANG II stimulation of Na+, K+-ATPase activity. The infusion of ANG II did not affect the expression of D1 receptor, OCTs, or OCTNs. However, the activity of OCTs was diminished by the presence of ANG II. Although ANG II did not alter the expression of D1 receptor, OCTs, and OCTNs in renal tissues, it modified the activity of OCTs and thereby decreased the urinary dopamine concentration, showing a novel mechanism by which ANG II decreases dopamine transport and its availability in the tubular lumen to stimulate D1 receptor. This study demonstrates a relationship between ANG II and dopamine, where both agents counteract their effects on sodium excretion.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Cuevas S, Villar VA, Jose PA, Armando I. Renal dopamine receptors, oxidative stress, and hypertension. Int J Mol Sci. 2013;14:17553–72. https://doi.org/10.3390/ijms140917553
Pinho MJ, Serrão MP, Soares-da-Silva P. High-salt intake and the renal expression of amino acid transporters in spontaneously hypertensive rats. Am J Physiol Ren Physiol. 2007;292:F1452–1463. https://doi.org/10.1152/ajprenal.00465.2006
del Amo EM, Urtti A, Yliperttula M. Pharmacokinetic role of L-type amino acid transporters LAT1 and LAT2. Eur J Pharm Sci. 2008;35:161–74. https://doi.org/10.1016/j.ejps.2008.06.015
Jonker JW, Schinkel AH. Pharmacological and physiological functions of the polyspecific organic cation transporters: OCT1, 2, and 3 (SLC22A1-3). J Pharm Exp Ther. 2004;308:2–9. https://doi.org/10.1124/jpet.103.053298
Koepsell H, Lips K, Volk C. Polyspecific organic cation transporters: structure, function, physiological roles, and biopharmaceutical implications. Pharm Res. 2007;24:1227–51. https://doi.org/10.1007/s11095-007-9254-z
Wright SH, Dantzler WH. Molecular and cellular physiology of renal organic cation and anion transport. Physiol Rev. 2004;84:987–1049. https://doi.org/10.1152/physrev.00040.2003
Graefe KH, Friedgen B, Wölfel R, Bossle F, Russ H, Schömig E. 1,1’-Diisopropyl-2,4’-cyanine (disprocynium24), a potent uptake2 blocker, inhibits the renal excretion of catecholamines. Naunyn Schmiedebergs Arch Pharm. 1997;356:115–25. https://doi.org/10.1007/pl00005018
Hussain T, Lokhandwala MF. Renal dopamine receptors and hypertension. Exp Biol Med Maywood NJ. 2003;228:134–42.
Jose PA, Eisner GM, Felder RA. Role of dopamine receptors in the kidney in the regulation of blood pressure. Curr Opin Nephrol Hypertens. 2002;11:87–92.
Jose PA, Eisner GM, Felder RA. Dopamine and the kidney: a role in hypertension? Curr Opin Nephrol Hypertens. 2003;12:189–94. https://doi.org/10.1097/01.mnh.0000058800.51455.7e
Zeng C, Wang D, Asico LD, Welch WJ, Wilcox CS, Hopfer U, et al. Aberrant D1 and D3 dopamine receptor transregulation in hypertension. Hypertension. 2004;43:654–60. https://doi.org/10.1161/01.HYP.0000114601.30306.bf
Aperia A, Holtbäck U, Syrén ML, Svensson LB, Fryckstedt J, Greengard P. Activation/deactivation of renal Na+,K(+)-ATPase: a final common pathway for regulation of natriuresis. FASEB J. 1994;8:436–9.
Stanimirovic J, Obradovic M, Panic A, Petrovic V, Alavantic D, Melih I, et al. Regulation of hepatic Na+/K+-ATPase in obese female and male rats: involvement of ERK1/2, AMPK, and Rho/ROCK. Mol Cell Biochem. 2018;440:77–88. https://doi.org/10.1007/s11010-017-3157-z
Kobori H, Nangaku M, Navar LG, Nishiyama A. The intrarenal renin-angiotensin system: from physiology to the pathobiology of hypertension and kidney disease. Pharm Rev. 2007;59:251–87. https://doi.org/10.1124/pr.59.3.3
Godin CM, Ferguson SSG. Biased agonism of the angiotensin II type 1 receptor. Mini Rev Med Chem. 2012;12:812–6.
Choi MR, Lee BM, Medici C, Correa AH, Fernández BE. Effects of angiotensin II on renal dopamine metabolism: synthesis, release, catabolism and turnover. Nephron Physiol. 2010;115:p1–7. https://doi.org/10.1159/000311522
Rosón MI, Cao G, Della Penna S, Gorzalczany S, Pandolfo M, Medici C, et al. Sodium load combined with low doses of exogenous angiotensin II upregulate intrarenal angiotensin II. Kidney Blood Press Res. 2009;32:334–41. https://doi.org/10.1159/000245036
Russ H, Friedgen B, Königs B, Schumacher C, Graefe KH, Schömig E. Pharmacokinetic and alpha 1-adrenoceptor antagonistic properties of two cyanine-type inhibitors of extraneuronal monoamine transport. Naunyn Schmiedebergs Arch Pharm. 1996;354:268–74. https://doi.org/10.1007/BF00171057
Doose H. Determination of phosphorus in the smallest blood sample; ultramicromodification of the Fiske-Subbarow method using amidol. Z Gesamt Exp Med. 1959;131:646–8.
Albers RW, Rodriguezde L, Derobertis E. Sodium-potassium activated ATPase and potassium-activated p-nitrophenylphosphatase: a comparison of their subcellular localizations in rat brain. Proc Natl Acad Sci USA. 1965;53:557–64.
Lowry OH, Lopez JA. The determination of inorganic phosphate in the presence of labile phosphate esters. J Biol Chem. 1946;162:421–8.
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–75.
Kouyoumdzian NM, Rukavina Mikusic NL, Kravetz MC, Lee BM, Carranza A, Del Mauro JS, et al. Atrial natriuretic peptide stimulates dopamine tubular transport by organic cation transporters: a novel mechanism to enhance renal sodium excretion. PLoS ONE. 2016;11:e0157487. https://doi.org/10.1371/journal.pone.0157487
Kouyoumdzian NM, Mikusic NR, Cao G, Choi MR, Penna SD, Fernández BE, et al. Adverse effects of tempol on hidrosaline balance in rats with acute sodium overload. Biotech Histochem. 2016;91:510–21. https://doi.org/10.1080/10520295.2016.1249029
Fernández BE, Correa AH, Choi MR. Atrial natriuretic factor stimulates renal dopamine uptake mediated by natriuretic peptide-type A receptor. Regul Pept. 2005;124:137–44. https://doi.org/10.1016/j.regpep.2004.07.006
Choi MR, Medici C, Gironacci MM, Correa AH, Fernández BE. Angiotensin II regulation of renal dopamine uptake and Na(+),K(+)-ATPase activity. Nephron Physiol. 2009;111:53–58. https://doi.org/10.1159/000209211
Iimura O, Shimamoto K. Salt and hypertension: water-sodium handling in essential hypertension. Ann NY Acad Sci. 1993;676:105–21. https://doi.org/10.1111/j.1749-6632.1993.tb38729.x
Li XC, Shull GE, Miguel-Qin E, Chen F, Zhuo JL. Role of the Na+/H+ exchanger 3 in angiotensin II-induced hypertension in NHE3-deficient mice with transgenic rescue of NHE3 in small intestines. Physiol Rep. 2015;3:e12605. https://doi.org/10.14814/phy2.12605
Ciarimboli G, Lancaster CS, Schlatter E, Franke RM, Sprowl JA, Pavenstädt H, et al. Proximal tubular secretion of creatinine by organic cation transporter OCT2 in cancer patients. Clin Cancer Res. 2012;18:1101–8. https://doi.org/10.1158/1078-0432.CCR-11-2503
Kajiwara M, Ban T, Matsubara K, Nakanishi Y, Masuda S. Urinary dopamine as a potential index of the transport activity of multidrug and toxin extrusion in the kidney. Int J Mol Sci. 2016;17:1228. https://doi.org/10.3390/ijms17081228
Choi MR, Correa AH, del Valle Turco V, Garcia FA, Fernández BE. Angiotensin II regulates extraneuronal dopamine uptake in the kidney. Nephron Physiol. 2006;104:136–43. https://doi.org/10.1159/000095856
Yingst DR, Massey KJ, Rossi NF, Mohanty MJ, Mattingly RR. Angiotensin II directly stimulates activity and alters the phosphorylation of Na-K-ATPase in rat proximal tubule with a rapid time course. Am J Physiol Ren Physiol. 2004;287:F713–721. https://doi.org/10.1152/ajprenal.00065.2004
Li D, Scott L, Crambert S, Zelenin S, Eklöf A-C, Di Ciano L, et al. Binding of losartan to angiotensin AT1 receptors increases dopamine D1 receptor activation. J Am Soc Nephrol. 2012;23:421–8. https://doi.org/10.1681/ASN.2011040344
Gildea JJ. Dopamine and angiotensin as renal counterregulatory systems controlling sodium balance. Curr Opin Nephrol Hypertens. 2009;18:28–32. https://doi.org/10.1097/MNH.0b013e32831a9e0b
Rukavina Mikusic NL, Kouyoumdzian NM, Uceda A, Del Mauro JS, Pandolfo M, Gironacci MM, et al. Losartan prevents the imbalance between renal dopaminergic and renin angiotensin systems induced by fructose overload. l-Dopa/dopamine index as new potential biomarker of renal dysfunction. Metabolism. 2018;85:271–85. https://doi.org/10.1016/j.metabol.2018.04.010
Ciarimboli G, Schlatter E. Regulation of organic cation transport. Pflug Arch. 2005;449:423–41. https://doi.org/10.1007/s00424-004-1355-5
Ciarimboli G, Koepsell H, Iordanova M, Gorboulev V, Dürner B, Lang D, et al. Individual PKC-phosphorylation sites in organic cation transporter 1 determine substrate selectivity and transport regulation. J Am Soc Nephrol. 2005;16:1562–70. https://doi.org/10.1681/ASN.2004040256
Pinto V, Pinho MJ, Soares-da-Silva P. Renal amino acid transport systems and essential hypertension. FASEB J. 2013;27:2927–38. https://doi.org/10.1096/fj.12-224998
Choi MR, Kouyoumdzian NM, Rukavina Mikusic NL, Kravetz MC, Rosón MI, Rodríguez Fermepin M, et al. Renal dopaminergic system: pathophysiological implications and clinical perspectives. World J Nephrol. 2015;4:196–212. https://doi.org/10.5527/wjn.v4.i2.196
Cao G, Della Penna SL, Kouyoumdzian NM, Choi MR, Gorzalczany S, Fernández BE, et al. Immunohistochemical expression of intrarenal renin angiotensin system components in response to tempol in rats fed a high salt diet. World J Nephrol. 2017;6:29–40. https://doi.org/10.5527/wjn.v6.i1.29
Rukavina Mikusic NL, Kouyoumdzian NM, Del Mauro JS, Cao G, Trida V, Gironacci MM, et al. Effects of chronic fructose overload on renal dopaminergic system: alteration of urinary L-dopa/dopamine index correlates to hypertension and precedes kidney structural damage. J Nutr Biochem. 2018;51:47–55. https://doi.org/10.1016/j.jnutbio.2017.09.005
Acknowledgements
We thank Dr. María C. Kravetz from Cátedra de Farmacología, Facultad de Farmacia y Bioquímica, Universidad de Buenos Aires, Buenos Aires, Argentina; Dr. Graciela L. Giardina from Hospital Alemán, Laboratorio de Medicina Experimental, Buenos Aires, Argentina; Dr. Mariela Gironacci from CONICET–Universidad de Buenos Aires, Instituto de Química y Fisicoquímica Biológicas, Buenos Aires, Argentina'; and Dr. Gabriel Cao, Dr. Silvana L. Della Penna, and Dr. María I. Rosón from IATIMET, Buenos Aires, Argentina, for their technical collaboration.
Grants
This work was supported by grants from the ANPCYT (PICT 2012–1775), Universidad de Buenos Aires (UBACYT20020110200048 and 2002013200105BA) and Sociedad Argentina de Hipertensión Arterial (Stimulus Grant for Research on Hypertension 2014–2015) and Instituto Universitario en Ciencias de la Salud, Fundación Héctor A. Barceló (2017–2019).
Author information
Authors and Affiliations
Contributions
NMK performed all the experiments, analyzed the results, and wrote the manuscript. NLRM performed all the experiments, analyzed the results, and revised the manuscript. GDR collaborated in the surgery of the animals and measured urinary dopamine concentration by HPLC. SBG performed the surgery of all the animals and measured the mean arterial pressure. AC collaborated in western blot analyses. VT measured urine and plasma parameters. BEF analyzed the results and collaborated with manuscript writing and revision. MRC analyzed immunohistochemistry images, planned and directed the project, procured funding, and revised manuscript writing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
In memory of Dr. Jorge E. Toblli who passed away before the submission of the manuscript.
Rights and permissions
About this article
Cite this article
Kouyoumdzian, N.M., Rukavina Mikusic, N.L., Robbesaul, G.D. et al. Acute infusion of angiotensin II regulates organic cation transporters function in the kidney: its impact on the renal dopaminergic system and sodium excretion. Hypertens Res 44, 286–298 (2021). https://doi.org/10.1038/s41440-020-00552-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41440-020-00552-7
Keywords:
This article is cited by
-
Annual reports on hypertension research 2020
Hypertension Research (2022)