Abstract
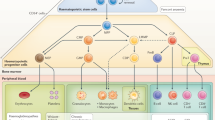
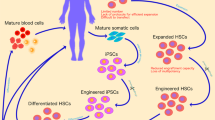
Hematopoietic stem cells (HSCs) are precursor cells that give rise to blood, immune and tissue-resident progeny in humans. Their position at the starting point of hematopoiesis offers a unique therapeutic opportunity to treat certain hematologic diseases by implementing corrective changes that are subsequently directed through to multiple cell lineages. Attempts to exploit HSCs clinically have evolved over recent decades, from initial approaches that focused on transplantation of healthy donor allogeneic HSCs to treat rare inherited monogenic hematologic disorders, to more contemporary genetic modification of autologous HSCs offering the promise of benefits to a wider range of diseases. We are on the cusp of an exciting new era as the transformative potential of HSC gene therapy to offer durable delivery of gene-corrected cells to a range of tissues and organs, including the central nervous system, is beginning to be realized. This article reviews the rationale for targeting HSCs, the approaches that have been used to date for delivering therapeutic genes to these cells, and the latest technological breakthroughs in manufacturing and vector design. The challenges faced by the biotechnology cell and gene therapy sector in the commercialization of HSC gene therapy are also discussed.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Cheng H, Zheng Z, Cheng T. New paradigms on hematopoietic stem cell differentiation. Protein Cell. 2020;11:34–44.
Morgan RA, Gray D, Lomova A, Kohn DB. Hematopoietic stem cell gene therapy: progress and lessons learned. Cell Stem Cell. 2017;21:574–90.
Blaese RM, Culver KW, Miller AD, Carter CS, Fleisher T, Clerici M, et al. T lymphocyte-directed gene therapy for ADA- SCID: initial trial results after 4 years. Science. 1995;270:475–80.
Bordignon C, Notarangelo LD, Nobili N, Ferrari G, Casorati G, Panina P, et al. Gene therapy in peripheral blood lymphocytes and bone marrow for ADA- immunodeficient patients. Science. 1995;270:470–5.
Kohn DB, Shaw KL, Garabedian E, Carbonaro-Sarracino DA, Moore TB, De Oliveira SN, et al. Lentiviral gene therapy with autologous hematopoietic stem and progenitor cells (HSPCs) for the treatment of severe combined immune deficiency due to adenosine deaminase deficiency (ADA-SCID): two year follow-up results. Mol Ther. 2020;28:554.
Fumagalli F, Calbi V, Sessa M, Zambon A, Baldoli C, Cugnata F, et al. Lentiviral hematopoietic stem and progenitor cell gene therapy (HSPC-GT) for metachromatic leukodystrophy (MLD): clinical outcomes from 33 patients. Mol Genet Metab. 2020;129:S59.
Scaramuzza S, Marktel S, Giglio F, Cicalese M, Lidonnici M, Rossi C, et al. Clinical outcomes from a phase I/II gene therapy trial for patients affected by severe transfusion dependent beta-thalassemia: two years’ follow up. Mol Ther. 2020;28:169.
Thompson AA, Walters MC, Kwiatkowski J, Rasko JEJ, Ribeil JA, Hongeng S, et al. Gene therapy in patients with transfusion-dependent β-thalassemia. N Engl J Med. 2018;378:1479–93.
Huang X, Cho S, Spangrude GJ. Hematopoietic stem cells: generation and self-renewal. Cell Death Differ. 2007;14:1851–9.
Crisan M, Dzierzak E. The many faces of hematopoietic stem cell heterogeneity. Development. 2016;143:4571–81.
Seita J, Weissman IL. Hematopoietic stem cell: self-renewal versus differentiation. Wiley Interdiscip Rev Syst Biol Med. 2010;2:640–53.
Sidney LE, Branch MJ, Dunphy SE, Dua HS, Hopkinson A. Concise review: evidence for CD34 as a common marker for diverse progenitors. Stem Cells. 2014;32:1380–9.
Zonari E, Desantis G, Petrillo C, Boccalatte FE, Lidonnici MR, Kajaste-Rudnitski A, et al. Efficient ex vivo engineering and expansion of highly purified human hematopoietic stem and progenitor cell populations for gene therapy. Stem Cell Rep. 2017;8:977–90.
Baldwin K, Urbinati F, Romero Z, Campo-Fernandez B, Kaufman ML, Cooper AR, et al. Enrichment of human hematopoietic stem/progenitor cells facilitates transduction for stem cell gene therapy. Stem Cells. 2015;33:1532–42.
Radtke S, Adair JE, Giese MA, Chan YY, Norgaard ZK, Enstrom M, et al. A distinct hematopoietic stem cell population for rapid multilineage engraftment in nonhuman primates. Sci Transl Med. 2017;9:eaan1145.
Gordon PR, Leimig T, Babarin-Dorner A, Houston J, Holladay M, Mueller I, et al. Large-scale isolation of CD133+ progenitor cells from G-CSF mobilized peripheral blood stem cells. Bone Marrow Transplant. 2003;31:17–22.
Negrin RS, Atkinson K, Leemhuis T, Hanania E, Juttner C, Tierney K, et al. Transplantation of highly purified CD34+Thy-1+ hematopoietic stem cells in patients with metastatic breast cancer. Biol Blood Marrow Transplant. 2000;6:262–71.
Pellin D, Loperfido M, Baricordi C, Wolock SL, Montepeloso A, Weinberg OK, et al. A comprehensive single cell transcriptional landscape of human hematopoietic progenitors. Nat Commun. 2019;10:2395.
Gatti RA, Meuwissen HJ, Allen HD, Hong R, Good RA. Immunological reconstitution of sex-linked lymphopenic immunological deficiency. Lancet. 1968;2:1366–9.
Vossen JM, de Koning J, van Bekkum DW, Dicke KA, Eysvoogel VP, Hijmans W, et al. Successful treatment of an infant with severe combined immunodeficiency by transplantation of bone marrow cells from an uncle. Clin Exp Immunol. 1973;13:9–20.
Boelens JJ, Aldenhoven M, Purtill D, Ruggeri A, Defor T, Wynn R, et al. Outcomes of transplantation using various hematopoietic cell sources in children with Hurler syndrome after myeloablative conditioning. Blood. 2013;121:3981–7.
Walters MC. Update of hematopoietic cell transplantation for sickle cell disease. Curr Opin Hematol. 2015;22:227–33.
Cooke KR, Luznik L, Sarantopoulos S, Hakim FT, Jagasia M, Fowler DH, et al. The biology of chronic graft-versus-host disease: a Task Force Report from the National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease. Biol Blood Marrow Transplant. 2017;23:211–34.
Hatzimichael E, Tuthill M. Hematopoietic stem cell transplantation. Stem Cells Cloning. 2010;3:105–17.
Frangoul H, Altshuler D, Cappellini MD, Chen Y-S, Domm J, Eustace BK, et al. CRISPR-Cas9 gene editing for sickle cell disease and β-thalassemia. N Engl J Med. 2020;384:252–60.
Esrick EB, Lehmann LE, Biffi A, Achebe M, Brendel C, Ciuculescu MF, et al. Post-transcriptional genetic silencing of BCL11A to treat sickle cell disease. N Engl J Med. 2020;384:205–15.
Chang AH, Sadelain M. The genetic engineering of hematopoietic stem cells: the rise of lentiviral vectors, the conundrum of the ltr, and the promise of lineage-restricted vectors. Mol Ther. 2007;15:445–56.
Lundstrom K. Viral vectors in gene therapy. Diseases. 2018;6:42.
Dunbar CE, High KA, Joung JK, Kohn DB, Ozawa K, Sadelain M. Gene therapy comes of age. Science. 2018;359:eaan4672.
Cavazzana-Calvo M, Hacein-Bey S, de Saint Basile G, Gross F, Yvon E, Nusbaum P, et al. Gene therapy of human severe combined immunodeficiency (SCID)-X1 disease. Science. 2000;288:669–72.
Neelapu SS, Locke FL, Bartlett NL, Lekakis LJ, Miklos DB, Jacobson CA, et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med. 2017;377:2531–44.
Cicalese MP, Ferrua F, Castagnaro L, Pajno R, Barzaghi F, Giannelli S, et al. Update on the safety and efficacy of retroviral gene therapy for immunodeficiency due to adenosine deaminase deficiency. Blood. 2016;128:45–54.
Goswami R, Subramanian G, Silayeva L, Newkirk I, Doctor D, Chawla K, et al. Gene therapy leaves a vicious cycle. Front Oncol. 2019;9:297.
Yamashita M, Emerman M. Retroviral infection of non-dividing cells: old and new perspectives. Virology. 2006;344:88–93.
Montini E, Cesana D, Schmidt M, Sanvito F, Bartholomae CC, Ranzani M, et al. The genotoxic potential of retroviral vectors is strongly modulated by vector design and integration site selection in a mouse model of HSC gene therapy. J Clin Investig. 2009;119:964–75.
Hacein-Bey-Abina S, Garrigue A, Wang GP, Soulier J, Lim A, Morillon E, et al. Insertional oncogenesis in 4 patients after retrovirus-mediated gene therapy of SCID-X1. J Clin Investig. 2008;118:3132–42.
Boztug K, Schmidt M, Schwarzer A, Banerjee PP, Diez IA, Dewey RA, et al. Stem-cell gene therapy for the Wiskott-Aldrich syndrome. N Engl J Med. 2010;363:1918–27.
Ott MG, Schmidt M, Schwarzwaelder K, Stein S, Siler U, Koehl U, et al. Correction of X-linked chronic granulomatous disease by gene therapy, augmented by insertional activation of MDS1-EVI1, PRDM16 or SETBP1. Nat Med. 2006;12:401–9.
Orchard Therapeutics. 2020. Press release: Orchard statement on Strimvelis®, a gammaretroviral vector-based gene therapy for ADA-SCID. https://ir.orchard-tx.com/news-releases/news-release-details/orchard-statement-strimvelisr-gammaretroviral-vector-based-gene.
Zufferey R, Dull T, Mandel RJ, Bukovsky A, Quiroz D, Naldini L, et al. Self-inactivating lentivirus vector for safe and efficient in vivo gene delivery. J Virol. 1998;72:9873–80.
Cante-Barrett K, Mendes RD, Smits WK, van Helsdingen-van Wijk YM, Pieters R, Meijerink JP. Lentiviral gene transfer into human and murine hematopoietic stem cells: size matters. BMC Res Notes. 2016;9:312.
Kafri T. Gene delivery by lentivirus vectors an overview. Methods Mol Biol. 2004;246:367–90.
Case SS, Price MA, Jordan CT, Yu XJ, Wang L, Bauer G, et al. Stable transduction of quiescent CD34(+)CD38(−) human hematopoietic cells by HIV-1-based lentiviral vectors. Proc Natl Acad Sci USA. 1999;96:2988–93.
Mazurier F, Gan OI, McKenzie JL, Doedens M, Dick JE. Lentivector-mediated clonal tracking reveals intrinsic heterogeneity in the human hematopoietic stem cell compartment and culture-induced stem cell impairment. Blood. 2004;103:545–52.
Yu SF, von Ruden T, Kantoff PW, Garber C, Seiberg M, Ruther U, et al. Self-inactivating retroviral vectors designed for transfer of whole genes into mammalian cells. Proc Natl Acad Sci USA. 1986;83:3194–8.
Nienhuis AW, Dunbar CE, Sorrentino BP. Genotoxicity of retroviral integration in hematopoietic cells. Mol Ther. 2006;13:1031–49.
Mitchell RS, Beitzel BF, Schroder AR, Shinn P, Chen H, Berry CC, et al. Retroviral DNA integration: ASLV, HIV, and MLV show distinct target site preferences. PLoS Biol. 2004;2:E234.
Schroder AR, Shinn P, Chen H, Berry C, Ecker JR, Bushman F. HIV-1 integration in the human genome favors active genes and local hotspots. Cell. 2002;110:521–9.
Biffi A, Bartolomae CC, Cesana D, Cartier N, Aubourg P, Ranzani M, et al. Lentiviral vector common integration sites in preclinical models and a clinical trial reflect a benign integration bias and not oncogenic selection. Blood. 2011;117:5332–9.
Zychlinski D, Schambach A, Modlich U, Maetzig T, Meyer J, Grassman E, et al. Physiological promoters reduce the genotoxic risk of integrating gene vectors. Mol Ther. 2008;16:718–25.
Modlich U, Navarro S, Zychlinski D, Maetzig T, Knoess S, Brugman MH, et al. Insertional transformation of hematopoietic cells by self-inactivating lentiviral and gammaretroviral vectors. Mol Ther. 2009;17:1919–28.
Cui Y, Golob J, Kelleher E, Ye Z, Pardoll D, Cheng L. Targeting transgene expression to antigen-presenting cells derived from lentivirus-transduced engrafting human hematopoietic stem/progenitor cells. Blood. 2002;99:399–408.
Lois C, Hong EJ, Pease S, Brown EJ, Baltimore D. Germline transmission and tissue-specific expression of transgenes delivered by lentiviral vectors. Science. 2002;295:868–72.
Pfeifer A, Ikawa M, Dayn Y, Verma IM. Transgenesis by lentiviral vectors: lack of gene silencing in mammalian embryonic stem cells and preimplantation embryos. Proc Natl Acad Sci USA. 2002;99:2140–5.
Lai Z, Brady RO. Gene transfer into the central nervous system in vivo using a recombinanat lentivirus vector. J Neurosci Res. 2002;67:363–71.
Higashimoto T, Urbinati F, Perumbeti A, Jiang G, Zarzuela A, Chang LJ, et al. The woodchuck hepatitis virus post-transcriptional regulatory element reduces readthrough transcription from retroviral vectors. Gene Ther. 2007;14:1298–304.
Merten OW, Hebben M, Bovolenta C. Production of lentiviral vectors. Mol Ther Methods Clin Dev. 2016;3:16017.
Dull T, Zufferey R, Kelly M, Mandel RJ, Nguyen M, Trono D, et al. A third-generation lentivirus vector with a conditional packaging system. J Virol. 1998;72:8463–71.
Humbert O, Gisch DW, Wohlfahrt ME, Adams AB, Greenberg PD, Schmitt TM, et al. Development of third-generation cocal envelope producer cell lines for robust lentiviral gene transfer into hematopoietic stem cells and t-cells. Mol Ther. 2016;24:1237–46.
Finkelshtein D, Werman A, Novick D, Barak S, Rubinstein M. LDL receptor and its family members serve as the cellular receptors for vesicular stomatitis virus. Proc Natl Acad Sci USA. 2013;110:7306–11.
Babic A & Trigoso E. Cell source and apheresis. In: Kenyon M, Babic A, editors. The European Blood and Marrow Transplantation Textbook for Nurses: under the Auspices of EBMT. Cham (CH):Springer; 2018. p. 71–87.
Krause DS, Fackler MJ, Civin CI, May WS. CD34: structure, biology, and clinical utility. Blood. 1996;87:1–13.
Biasco L, Pellin D, Scala S, Dionisio F, Basso-Ricci L, Leonardelli L, et al. In vivo tracking of human hematopoiesis reveals patterns of clonal dynamics during early and steady-state reconstitution phases. Cell Stem Cell. 2016;19:107–19.
Six E, Guilloux A, Denis A, Lecoules A, Magnani A, Vilette R, et al. Clonal tracking in gene therapy patients reveals a diversity of human hematopoietic differentiation programs. Blood. 2020;135:1219–31.
Masiuk KE, Brown D, Laborada J, Hollis RP, Urbinati F, Kohn DB. Improving gene therapy efficiency through the enrichment of human hematopoietic stem cells. Mol Ther. 2017;25:2163–75.
Bernardo ME, Aiuti A. The role of conditioning in hematopoietic stem-cell gene therapy. Hum Gene Ther. 2016;27:741–8.
Cartier N, Hacein-Bey-Abina S, Bartholomae CC, Veres G, Schmidt M, Kutschera I, et al. Hematopoietic stem cell gene therapy with a lentiviral vector in X-linked adrenoleukodystrophy. Science. 2009;326:818–23.
Cavazzana-Calvo M, Payen E, Negre O, Wang G, Hehir K, Fusil F, et al. Transfusion independence and HMGA2 activation after gene therapy of human beta-thalassaemia. Nature. 2010;467:318–22.
Fischer A. Severe combined immunodeficiencies (SCID). Clin Exp Immunol. 2000;122:143–9.
European Medicines Agency. 2016. Strimvelis summary of product characteristics. https://www.ema.europa.eu/en/medicines/human/EPAR/strimvelis.
Aiuti A, Roncarolo MG, Naldini L. Gene therapy for ADA-SCID, the first marketing approval of an ex vivo gene therapy in Europe: paving the road for the next generation of advanced therapy medicinal products. EMBO Mol Med. 2017;9:737–40.
Garcia-Perez L, van Eggermond M, van Roon L, Vloemans SA, Cordes M, Schambach A, et al. Successful preclinical development of gene therapy for recombinase-activating gene-1-deficient SCID. Mol Ther Methods Clin Dev. 2020;17:666–82.
Pike-Overzet K, Rodijk M, Ng YY, Baert MR, Lagresle-Peyrou C, Schambach A, et al. Correction of murine Rag1 deficiency by self-inactivating lentiviral vector-mediated gene transfer. Leukemia. 2011;25:1471–83.
Nienhuis AW, Nathan DG. Pathophysiology and clinical manifestations of the beta-thalassemias. Cold Spring Harb Perspect Med. 2012;2:a011726.
Kato GJ, Piel FB, Reid CD, Gaston MH, Ohene-Frempong K, Krishnamurti L, et al. Sickle cell disease. Nat Rev Dis Primers. 2018;4:18010.
bluebirdbio. 2021. bluebird bio announces temporary suspension on phase 1/2 and phase 3 studies of LentiGlobin gene therapy for sickle cell disease (bb1111). https://investor.bluebirdbio.com/news-releases/news-release-details/bluebird-bio-announces-temporary-suspension-phase-12-and-phase-3.
Hsieh MM, Bonner M, Pierciey FJ Jr, Uchida N, Rottman J, Demopoulos L, et al. Myelodysplastic syndrome unrelated to lentiviral vector in a patient treated with gene therapy for sickle cell disease. Blood Adv. 2020;4:2058–63.
Brunson A, Keegan THM, Bang H, Mahajan A, Paulukonis S, Wun T. Increased risk of leukemia among sickle cell disease patients in California. Blood. 2017;130:1597–9.
Seminog OO, Ogunlaja OI, Yeates D, Goldacre MJ. Risk of individual malignant neoplasms in patients with sickle cell disease: English national record linkage study. J R Soc Med. 2016;109:303–9.
Wilcox WR. Lysosomal storage disorders: the need for better pediatric recognition and comprehensive care. J Pediatr. 2004;144:S3–14.
Puhl DL, D’Amato AR, Gilbert RJ. Challenges of gene delivery to the central nervous system and the growing use of biomaterial vectors. Brain Res Bull. 2019;150:216–30.
Biffi A, Capotondo A, Fasano S, del Carro U, Marchesini S, Azuma H, et al. Gene therapy of metachromatic leukodystrophy reverses neurological damage and deficits in mice. J Clin Investig. 2006;116:3070–82.
Biffi A, De Palma M, Quattrini A, Del Carro U, Amadio S, Visigalli I, et al. Correction of metachromatic leukodystrophy in the mouse model by transplantation of genetically modified hematopoietic stem cells. J Clin Investig. 2004;113:1118–29.
Capotondo A, Milazzo R, Politi LS, Quattrini A, Palini A, Plati T, et al. Brain conditioning is instrumental for successful microglia reconstitution following hematopoietic stem cell transplantation. Proc Natl Acad Sci USA. 2012;109:15018–23.
Bernardo E, Gentner B, Tucci F, Fumagalli F, Ciotti F, Sarzana M, et al. First-in-human phase I/II clinical trial of hematopoietic stem cell-gene therapy for mucopolysaccharidosis type I, Hurler (MPS-IH): preliminary evidence of extensive metabolic correction. HemaSphere. 2020;4:S281.
Kinsella J, Jones S, Church H, Jones C, Ellison S, Booth C, et al. Ex-vivo autologous haematopoietic stem cell gene therapy in mucopolysaccharidosis type IIIa. Bone Marrow Transplant. 2020;55:320.
Biffi A, Montini E, Lorioli L, Cesani M, Fumagalli F, Plati T, et al. Lentiviral hematopoietic stem cell gene therapy benefits metachromatic leukodystrophy. Science. 2013;341:1233158.
Sessa M, Lorioli L, Fumagalli F, Acquati S, Redaelli D, Baldoli C, et al. Lentiviral haemopoietic stem-cell gene therapy in early-onset metachromatic leukodystrophy: an ad-hoc analysis of a non-randomised, open-label, phase 1/2 trial. Lancet. 2016;388:476–87.
Eichler F, Duncan C, Musolino PL, Orchard PJ, De Oliveira S, Thrasher AJ, et al. Hematopoietic stem-cell gene therapy for cerebral adrenoleukodystrophy. N Engl J Med. 2017;377:1630–8.
Gleitz HF, Liao AY, Cook JR, Rowlston SF, Forte GM, D'Souza Z, et al. Brain-targeted stem cell gene therapy corrects mucopolysaccharidosis type II via multiple mechanisms. EMBO Mol Med. 2018;10:e8730.
Holley RJ, Ellison SM, Fil D, O’Leary C, McDermott J, Senthivel N, et al. Macrophage enzyme and reduced inflammation drive brain correction of mucopolysaccharidosis IIIB by stem cell gene therapy. Brain. 2018;141:99–116.
Penna S, Crippaa S, Capoa V, Santia L, Bosottia R, Riminuccic M, et al. Investigation of the bone damage in mucopolysaccharidosis type I Hurler syndrome: pathophysiological mechanisms and the impact of ex vivo gene therapy. Bone Rep. 2020;13(suppl):P309.
Ng AP, Alexander WS. Haematopoietic stem cells: past, present and future. Cell Death Discov. 2017;3:17002.
Xu W, Han SD, Zhang C, Li JQ, Wang YJ, Tan CC, et al. The FAM171A2 gene is a key regulator of progranulin expression and modifies the risk of multiple neurodegenerative diseases. Sci Adv. 2020;6:eabb3063.
Kohn DB, Booth C, Kang EM, Pai SY, Shaw KL, Santilli G, et al. Lentiviral gene therapy for X-linked chronic granulomatous disease. Nat Med. 2020;26:200–6.
McCarron A, Donnelley M, McIntyre C, Parsons D. Challenges of up-scaling lentivirus production and processing. J Biotechnol. 2016;240:23–30.
Chames P, Van Regenmortel M, Weiss E, Baty D. Therapeutic antibodies: successes, limitations and hopes for the future. Br J Pharmacol. 2009;157:220–33.
Shukla AA, Wolfe LS, Mostafa SS, Norman C. Evolving trends in mAb production processes. Bioeng Transl Med. 2017;2:58–69.
Boitano AE, Wang J, Romeo R, Bouchez LC, Parker AE, Sutton SE, et al. Aryl hydrocarbon receptor antagonists promote the expansion of human hematopoietic stem cells. Science. 2010;329:1345–8.
Schott JW, Leon-Rico D, Ferreira CB, Buckland KF, Santilli G, Armant MA, et al. Enhancing lentiviral and alpharetroviral transduction of human hematopoietic stem cells for clinical application. Mol Ther Methods Clin Dev. 2019;14:134–47.
Tomas HA, Rodrigues AF, Carrondo MJT, Coroadinha AS. LentiPro26: novel stable cell lines for constitutive lentiviral vector production. Sci Rep. 2018;8:5271.
Throm RE, Ouma AA, Zhou S, Chandrasekaran A, Lockey T, Greene M, et al. Efficient construction of producer cell lines for a SIN lentiviral vector for SCID-X1 gene therapy by concatemeric array transfection. Blood. 2009;113:5104–10.
Stewart HJ, Fong-Wong L, Strickland I, Chipchase D, Kelleher M, Stevenson L, et al. A stable producer cell line for the manufacture of a lentiviral vector for gene therapy of Parkinson’s disease. Hum Gene Ther. 2011;22:357–69.
Manceur AP, Kim H, Misic V, Andreev N, Dorion-Thibaudeau J, Lanthier S, et al. Scalable lentiviral vector production using stable HEK293SF producer cell lines. Hum Gene Ther Methods. 2017;28:330–9.
Hu P, Li Y, Sands MS, McCown T, Kafri T. Generation of a stable packaging cell line producing high-titer PPT-deleted integration-deficient lentiviral vectors. Mol Ther Methods Clin Dev. 2015;2:15025.
Jang Y, Kim YS, Wielgosz MM, Ferrara F, Ma Z, Condori J, et al. Optimizing lentiviral vector transduction of hematopoietic stem cells for gene therapy. Gene Ther. 2020;27:545–56.
Heffner GC, Bonner M, Christiansen L, Pierciey FJ, Campbell D, Smurnyy Y, et al. Prostaglandin E2 increases lentiviral vector transduction efficiency of adult human hematopoietic stem and progenitor cells. Mol Ther. 2018;26:320–8.
Wurm M, Schambach A, Lindemann D, Hanenberg H, Standker L, Forssmann WG, et al. The influence of semen-derived enhancer of virus infection on the efficiency of retroviral gene transfer. J Gene Med. 2010;12:137–46.
Lewis G, Christiansen L, McKenzie J, Luo M, Pasackow E, Smurnyy Y, et al. Staurosporine increases lentiviral vector transduction efficiency of human hematopoietic stem and progenitor cells. Mol Ther Methods Clin Dev. 2018;9:313–22.
Delville M, Soheili T, Bellier F, Durand A, Denis A, Lagresle-Peyrou C, et al. A nontoxic transduction enhancer enables highly efficient lentiviral transduction of primary murine T cells and hematopoietic stem cells. Mol Ther Methods Clin Dev. 2018;10:341–7.
Moutsatsou P, Ochs J, Schmitt RH, Hewitt CJ, Hanga MP. Automation in cell and gene therapy manufacturing: from past to future. Biotechnol Lett. 2019;41:1245–53.
U.S. Department of Health and Human Services FaDA, Center for Drug Evaluation and Research (CDER), Center for Biologics Evaluation and Research (CBER) 2014. Guidance for Industry. Expedited programs for serious conditions – drugs and biologics. https://www.fda.gov/files/drugs/published/Expedited-Programs-for-Serious-Conditions-Drugs-and-Biologics.pdf.
European Medicines Agency. 2016. Guideline on the scientific application and the practical arrangements necessary to implement the procedure for accelerated assessment pursuant to Article 14(9) of Regulation (EC) No 726/2004. https://www.ema.europa.eu/en/guideline-scientific-application-practical-arrangements-necessary-implement-procedure-accelerated.
Orchard Therapeutics. 2020. Orchard Therapeutics Corporate Presentation. https://ir.orchard-tx.com/static-files/91cc703c-7cb1-413f-af3a-42c64fe5d580.
Cowan MJ, Yu J, Facchino J, Chag S, Fraser-Browne C, Long-Boyle J, et al. Early outcome of a phase I/II clinical trial (NCT03538899) of gene-corrected autologous CD34+ hematopoietic cells and low-exposure busulfan in newly diagnosed patients with artemis-deficient severe combined immunodeficiency (ART-SCID). Biol Blood Marrow Transplant. 2020;26:S88.
De Ravin SS, Anaya O’Brien S, Kwatemaa N, Theobald N, Liu S, Lee J, et al. Enhanced transduction lentivector gene therapy for treatment of older patients with X-linked severe combined immunodeficiency. Blood. 2019;134:608.
De Ravin SS, Wu X, Moir S, Anaya-O’Brien S, Kwatemaa N, Littel P, et al. Lentiviral hematopoietic stem cell gene therapy for X-linked severe combined immunodeficiency. Sci Transl Med. 2016;8:335ra57.
Mamcarz E, Zhou S, Lockey T, Abdelsamed H, Cross SJ, Kang G, et al. Lentiviral gene therapy combined with low-dose busulfan in infants with SCID-X1. N Engl J Med. 2019;380:1525–34.
Ferrua F, Cicalese MP, Galimberti S, Giannelli S, Dionisio F, Barzaghi F, et al. Lentiviral haemopoietic stem/progenitor cell gene therapy for treatment of Wiskott-Aldrich syndrome: interim results of a non-randomised, open-label, phase 1/2 clinical study. Lancet Haematol. 2019;6:e239–253.
Ferrua F, Cicalese MP, Galimberti S, Giannelli S, Dionisio F, Barzaghi F, et al. Lentiviral hematopoietic stem and progenitor cell gene therapy for Wiskott-Aldrich Syndrome (WAS): up to 8 years of follow up in 17 subjects treated since 2010. Blood. 2019;134:3346. (abstract)
Kohn DB, Rao GR, Almarza E, Terrazas D, Nicoletti E, Fernandes A, et al. A Phase 1/2 study of lentiviral-mediated ex-vivo gene therapy for pediatric patients with severe leukocyte adhesion deficiency-I (LAD-I): results from Phase 1. Blood. 2020;136(suppl 1):15.
Volck B, Thomas MAB, Carnely BP, Golipour A & C. M. Gb3 substrate in endothelial cells of renal peritubular capillaries was reduced in a previously untreated classic fabry male patient treated with AVR-RD-01 investigational lentiviral gene therapy. Mol Ther. 2020;28:234.
AVROBIO Inc. 2020. ASGCT 2020 Fabry & cystinosis data update. https://investors.avrobio.com/static-files/1367a559-cc62-4d0c-bc21-df77bc5143ec.
Barshop B, Ball E, Dohil M, Kohn D, Dohil R, Benador N, et al. Hematopoietic stem cell gene therapy for cystinosis: Initial results from a phase I/II clinical trial. Mol Ther. 2020;28:233.
Boulad F, Wang X, Qu J, Taylor C, Ferro L, Karponi G, et al. Safe mobilization of CD34+ cells in adults with beta-thalassemia and validation of effective globin gene transfer for clinical investigation. Blood. 2014;123:1483–6.
Boulad F, Riviere I, Wang X, Bartido S, Prockop SE, Barone R, et al. First US phase I clinical trial of globin gene transfer for the treatment of beta-thalassemia major. Blood. 2013;122:716. (abstract)
Mapara MY, Tisdale JF, Kanter J, Kwiatkowski JL, Krishnamurti L, Schmidt M, et al. Lentiglobin gene therapy in patients with sickle cell disease: updated interim results from Hgb-206. Biol Blood Marrow Transplant. 2019;25:S64.
Negre O, Bartholomae C, Beuzard Y, Cavazzana M, Christiansen L, Courne C, et al. Preclinical evaluation of efficacy and safety of an improved lentiviral vector for the treatment of beta-thalassemia and sickle cell disease. Curr Gene Ther. 2015;15:64–81.
Esrick EB, Lehmann LE, Biffi A, Achebe M, Brendel C, Ciuculescu MF, et al. Post-transcriptional genetic silencing of BCL11A to treat sickle cell disease. N Engl J Med. 2021;384:205–15.
Grimley M, Asnani M, Shrestha A, Felker S, Lutzko C, Arumugam PI, et al. Early results from a phase 1/2 study of Aru-1801 gene therapy for sickle cell disease (SCD): manufacturing process enhancements improve efficacy of a modified gamma globin lentivirus vector and reduced intensity conditioning transplant. Blood. 2020;136(suppl 1):20–21.
Romero Z, Urbinati F, Geiger S, Cooper AR, Wherley J, Kaufman ML, et al. Beta-globin gene transfer to human bone marrow for sickle cell disease. J Clin Invest. 2013;123:3317–30.
Hacke K, Treger JA, Bogan BT, Schiestl RH, Kasahara N. Genetic modification of mouse bone marrow by lentiviral vector-mediated delivery of hypoxanthine-guanine phosphoribosyltransferase short hairpin RNA confers chemoprotection against 6-thioguanine cytotoxicity. Transplant Proc. 2013;45:2040–4.
Czechowicz A, Agarwal R, Sevilla J, Río P, Navarro S, Beard BC, et al. Gene therapy for Fanconi anemia, Complementation Group a: updated results from ongoing global clinical studies of RP-L102. Blood. 2020;136(suppl 1):14.
Gonzalez-Murillo A, Lozano ML, Alvarez L, Jacome A, Almarza E, Navarro S, et al. Development of lentiviral vectors with optimized transcriptional activity for the gene therapy of patients with Fanconi anemia. Hum Gene Ther. 2010;21:623–30.
Rio P, Navarro S, Wang W, Sanchez-Dominguez R, Pujol RM, Segovia JC, et al. Successful engraftment of gene-corrected hematopoietic stem cells in non-conditioned patients with Fanconi anemia. Nat Med. 2019;25:1396–1401.
López Lorenzo JL, Navarro S, Shah AJ, Roncarolo MG, Sevilla J, Llanos L et al. Lentiviral mediated gene therapy for pyruvate kinase deficiency: a global phase 1 study for adult and pediatric patients. Blood. 2020;136(suppl 1):47.
Acknowledgements
We would like to thank Robin LeWinter, PhD, Leslie Meltzer, PhD and Denise Sarracino, PhD from Orchard Therapeutics (Europe) Ltd for their help coordinating the writing of the paper. Editorial support, based on authors’ direction, was provided by Ben Drever PhD from Comradis, UK, and was paid for by Orchard Therapeutics Ltd.
Funding
This research was supported by Orchard Therapeutics Ltd and medical writing assistance was provided by Ben Drever, PhD, of Comradis, UK and funded by Orchard Therapeutics Ltd.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
PS and HBG are employees of Orchard Therapeutics Ltd.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sagoo, P., Gaspar, H.B. The transformative potential of HSC gene therapy as a genetic medicine. Gene Ther 30, 197–215 (2023). https://doi.org/10.1038/s41434-021-00261-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41434-021-00261-x
This article is cited by
-
Human Hematopoietic Stem Cells Co-cultured in 3D with Stromal Support to Optimize Lentiviral Vector-mediated Gene Transduction
Indian Journal of Hematology and Blood Transfusion (2023)