Abstract
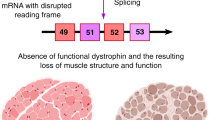
Duchenne muscular dystrophy (DMD) is one of the most common lethal muscle-wasting disorders affecting young boys caused by mutations in the DMD gene. Exon skipping has emerged as a promising therapy for DMD. Antisense oligonucleotides (AONs) are designed to induce the skipping of exon(s), in order to restore the reading frame, and therefore, allow for dystrophin expression. Eteplirsen and golodirsen, AONs for DMD exons 51 and 53 skipping, have been recently approved by the FDA. Viltolarsen, an AON for DMD exon 53 skipping, was approved in Japan earlier this year. Although promising, the efficacy of eteplirsen and AON sequence employed remain controversial. In addition, exon skipping faces challenges including the applicability and delivery. This article reviews and discusses exon skipping and the current advances being made in the field, on drugs, multi-exon skipping, sequence design, and applicability. We also discuss challenges and future directions that will facilitate the development of exon skipping therapy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Moat SJ, Bradley DM, Salmon R, Clarke A, Hartley L. Newborn bloodspot screening for Duchenne muscular dystrophy: 21 years experience in Wales (UK). Eur J Hum Genet. 2013;21:1049–53.
Nakamura A, Takeda S. Mammalian models of Duchenne muscular dystrophy: pathological characteristics and therapeutic applications. J Biomed Biotechnol. 2011;2011:1–9.
Roberts RG, Coffey AJ, Bobrow M, Bentley DR. Exon structure of the human dystrophin gene. Genomics. 1993;2:536–8.
Juan-Mateu J, Gonzalez-Quereda L, Rodriguez MJ, Baena M, Verdura E, Nascimento A, et al. DMD mutations in dystrophinopathy families: a step forward in genotype-phenotype correlations. PLoS One. 2015;10:1–21.
Okubo M, Goto K, Komaki H, Nakamura H, Mori-Yoshimura M, Hayashi YK, et al. Comprehensive analysis for genetic diagnosis of dystrophinopathies in Japan. Orphanet J Rare Dis. 2017;12:1–7.
Muntoni F, Torelli S, Ferlini A. Dystrophin and mutations: one gene, several proteins, multiple phenotypes. Lancet Neurol. 2003;2:731–40.
Monaco AP, Bertelson CJ, Liechti-Gallati S, Moser H, Kunkel LM. An explanation for the phenotypic differences between patients bearing partial deletions of the DMD locus. Genomics. 1988;1:90–95.
Gao Q, McNally EM. The dystrophin complex: structure, function and implications for therapy. Compr Physiol. 2016;5:1223–39.
Uchino M, Teramoto H, Naoe H, Yoshioka K, Miike T, Ando M. Localization and characterization of dystrophin in the central nervous system of controls and patients with Duchenne muscular dystrophy. J Neurol Neurosurg Psychiatry. 1994;57:426–9.
Campbell KP. Three muscular dystrophies: loss of cytoskeleton-extracellular matrix linkage. Cell. 1995;80:675–9.
Wagner KR, Lechtzin N, Judge DP. Current treatment of adult Duchenne muscular dystrophy. Biochimica et Biophys Acta. 1772;2007:229–37.
Backman E, Henriksson KG. Low-dose prednisolone treatment in Duchenne and Becker muscular dystrophy. Neuromuscul Disord. 1995;5:233–41.
Biggar WD, Gingras M, Fehlings DL, Harris VA, Steele CA. Deflazacort treatment of Duchenne muscular dystrophy. J Pediatrics. 2001;138:45–50.
Drachman DB, Toyka KV, Myer E. Prednisone in Duchenne muscular dystrophy. Lancet. 1974;304:1409–12.
Manzur AY, Kuntzer T, Pike M, Swan A. Glucocorticoid corticosteroids for Duchenne muscular dystrophy. Cochrane Database Syst Rev. 2008;1:1–33.
Nakamura A, Takeda S. Exon-skipping therapy for Duchenne muscular dystrophy. Neuropathology. 2009;29:494–501.
Heemskerk H, Winter CL, Ommen GJB, Deutekom JCT, Aartsma-Rus A. Development of antisense-mediated exon skipping as a treatment for Duchenne muscular dystrophy. Oligonucleotide Therap. 2009;1175:71–79.
Aartsma-Rus A, Janson AAM, Kaman WE, Bremmer-Bout M, Dunnen JTD, Baas F, et al. Therapeutic antisense-induced exon skipping in cultured muscle cells from six different DMD patients. Hum Mol Genet. 2003;12:907–14.
Yokota T, Lu QL, Partridge T, Kobayashi M, Nakamura A, Takeda S, et al. Efficacy of systemic morpholino exon-skipping in Duchenne dystrophy dogs. Ann Neurol. 2009;65:667–76.
Mann CJ, Honeyman K, McClorey G, Fletcher S, Wilton SD. Improved antisense oligonucleotide induced exon skipping in the mdx mouse model of muscular dystrophy. J Gene Med. 2002;4:644–54.
Guncay A, Yokota T. Antisense oligonucleotide drugs for Duchenne muscuklar dystrophy: how far have we come and what does the future hold. Future Med Chem. 2015;7:1631–5.
Flanigan KM, Dunn DM, von Niederhausern A, Soltanzadeh P, Gappmaier E, Howard MT, et al. Mutational spectrum of DMD mutations in dystrophinopathy patients: application of modern diagnostic techniques to a large cohort. Hum Mutat. 2009;30:1657–66.
Syed YY. Eteplirsen: first global approval. Drugs. 2016;76:1699–704.
Lim KRQ, Maruyama R, Yokota T. Eteplirsen in the treatment of Duchenne muscular dystrophy. Drug Des Dev Ther. 2017;11:533–45.
U.S National Library of Medicine. Study of SRP-4045 and SRP-4053 in DMD Patients (ESSENCE). 2019. https://clinicaltrials.gov/ct2/show/NCT02500381.
Nippon Shinyaku Co. LTD. Marketing authorization in Japan of VILTEPSO intravenous infusion 250 mg for the treatment of Duchenne muscular dystrophy. 2020. https://www.nippon-shinyaku.co.jp.
Komaki H, Takeshima Y, Matsumura T, Ozasa S, Funato M, Egawa Y, et al. (n.d.). A Japanese Phase I/II study of NS-065/NCNP-01 (Viltolarsen), Exon 52 skipping drug, in patients with Duchenne muscular dystrophy—a dose-finding study. Mendoza: Poster presented at the 23rd International Annual Congress of the World Muscle Society; 2018.
Yokota T, Duddy W, Echigoya Y, Kolski H. Exon skipping for nonsense mutations in Duchenne muscular dystrophy: too many mutations, too few patients? Expert Opin Biol Ther. 2012;12:1141–52.
Wood MJA, Gait MJ, Yin H. RNA-targeted splice-correction therapy for neuromuscular disease. Brain. 2010;133:957–72.
Siva K, Covello G, Denti MA. Exon-skipping antisense oligonucleotides to correct missplicing in neurogenetic diseases. Nucleic Acid Therap. 2014;24:69–86.
Wilton SD, Fall AM, Harding PL, McClorey G, Coleman C, Fletcher S. Antisense oligonucleotide-induced exon skipping across the human dystrophin gene transcript. Mol Ther. 2007;15:1288–96.
Dominski A, Kole R. Restoration of correct splicing in thalassemic pre-mRNA by antisense oligonucleotides. Proc. Natl Acad Sci. 1993;90:8673–7.
Slijkerman RW, Vache C, Dona M, Garcia-Garcia G, Claustres M, Hetterschijt L, et al. Antisense oligonucleotide-based splice correction for ush2a-associated retinal degeneration caused by a frequent deepintronic mutation. Mol Ther Nucleic Acids. 2016;5:1–10.
Taylor J, Zhang Q, Wyatt J, Dean NM. Induction of endogenous Bcl-xS through the control of Bcl-x pre-mRNA splicing by antisense oligonucleotides. Nat Biotechnol. 1999;17:1097–1100.
Arthur GK, Cruse G. Exon skipping of FcεRIβ for allergic diseases. Methods Mol Biol. 2018;1828:503–18.
Rigo F, Hua Y, Krainer AR, Bennett CF. Antisense-based therapy for the treatment of spinal muscular atrophy. J Cell Biol. 2012;199:21–25.
Aartsma-Rus A, Singh K, Fokkema IFAC, Ginjaar LB, Ommen GJV, Dunnen JTV. et al. Therapeutic exon skipping for dysferlinopathies?. Eur J Hum Genet. 2010;18:889–94.
Summerton JE. Morpholino, siRNA, and S-DNA compared: impact of structure and mechanism of action on off-target effects and sequence specificity. Curr Topics Med Chem. 2007;7:651–60.
Heemskerk H, Winter C, Kuik P, Heuvelmans N, Sabatelli P, Rimessi P, et al. Preclinical PK and PD studies on 2′-O-Methyl-phosphorothioate RNA Antisense Oligonucleotides in the mdx Mouse Model. Mol Ther. 2010;18:1210–7.
Kole R, Krieg AM. Exon skipping therapy for Duchenne muscular dystrophy. Adv Drug Deliv Rev. 2015;87:104–7.
Hilhorst N, Spanoudi-Kitrimi I, Goemans N, Morren MA. Injection site reactions after long-term subcutaneous delivery of drisapersen: a retrospective study. Eur J Pediatrics. 2019;178:253–8.
Kesselheim AS, Avorn J. Approving a problematic muscular dystrophy drug: implications for FDA policy. JAMA. 2016;316:2357–8.
Yokota T, Duddy W, Partridge T. Optimizing exon skipping therapies for DMD. Acta Myol. 2007;26:179–84.
Alter J, Lou F, Rabinowitz A, Yin H, Rosenfeld J, Wilton SD. Systemic delivery of morpholino oligonucleotide restores dystrophin expression bodywide and improves dystrophic pathology. Nat Med. 2006;12:175–7.
Amantana A, Iversen PL. Pharmacokinetics and biodistribution of phosphorodiamidate morpholino antisense oligomers. Curr Opin Pharmacol. 2005;5:550–5.
Gebski BL, Mann CJ, Fletcher S, Wilton SD. Morpholino antisense oligonucleotide induced dystrophin exon 23 skipping in mdx mouse muscle. Hum Mol Genet. 2003;12:1801–11.
Dietz GPH, Bähr M. Delivery of bioactive molecules into the cell: the Trojan horse approach. Mol Cell Neurosci. 2004;27:85–131.
Copolovici DM, Langel K, Eriste E, Langel U. Cell-penetrating peptides: design, synthesis, and applications. ACS Nano. 2014;8:1972–94.
Blain AM, Greally E, McClorey G, Manzano R, Betts CA, Godfrey C, et al. Peptide-conjugated phosphodiamidate oligomer-mediated exon skipping has benefits for cardiac function in mdx and Cmah-/-mdx mouse models of Duchenne muscular dystrophy. PLoS ONE. 2018;13:1–22.
Echigoya Y, Nakamura A, Nagata T, Urasawa N, Lim KRQ, Trieu N. Effects of systemic multiexon skipping with peptide-conjugated morpholinos in the heart of a dog model of Duchenne muscular dystrophy. PNAS. 2017;114:4213–8.
Wu B, Lu P, Cloer C, Shaban M, Grewal S, Milazi S, et al. Long-term rescue of dystrophin expression and improvement in muscle pathology and function in dystrophic mdx mice by peptide-conjugated morpholino. Am J Pathol. 2012;181:392–400.
Li YF, Marcos PA. Design and synthesis of dendritic molecular transporter that achieves efficient in vivo delivery of morpholino antisense oligo. Bioconjugate Chem. 2008;19:1464–70.
U.S National Library of Medicine. A phase 2 study for dose determination of SRP-5051, then dose expansion in patients with Duchenne muscular dystrophy amenable to exon 51-skipping treatment. 2019. https://clinicaltrials.gov/ct2/show/NCT04004065.
Kang JK, Malerba A, Popplewell L, Foster K, Dickson G. Antisense-induced myostatin exon skipping leads to muscle hypertrophy in mice following octa-guanidine morpholino oligomer treatment. Mol Ther. 2010;19:159–64.
Wu B, Li Y, Morcos PA, Doran TJ, Lu P, Lu QL. Octa-guanidine morpholino restores dystrophin expression in cardiac and skeletal muscles and ameliorates pathology in dystrophic mdx mice. Mol Ther. 2009;17:864–71.
Cirak S, Arechavala-Gomza V, Guglieri M, Feng L, Torelli S, Anthony K, et al. Exon skipping and dystrophin restoration in patients with Duchenne muscular dystrophy after systemic phosphorodiamidate morpholino oligomer treatment: an open-label, phase 2, dose-escalation study. Lancet. 2011;378:595–605.
Goyenvalle A, Griffith G, Babbs A, El Andaloussi S, Ezzat K, Avril A, et al. Functional correction in mouse models of muscular dystrophy using exon-skipping tricyclo-DNA oligomers. Na Med. 2015;21:270–5.
Echevarría L, Aupy P, Relizani K, Bestetti T, Griffith G, Blandel F, et al. Evaluating the impact of variable phosphorothioate content in tricyclo-dna antisense oligonucleotides in a duchenne muscular dystrophy mouse model. Nucl Acid Ther. 2019;29:148–60.
U.S Food and Drug Administration. FDA grants accelerated approval to first targeted treatment for rare Duchenne muscular dystrophy mutation. 2019. https://www.fda.gov/news-events/press-announcements/fda-grants-accelerated-approval-first-targeted-treatment-rare-duchenne-muscular-dystrophy-mutation.
Echigoya Y, Lim KRQ, Trieu N, Bao B, Miskew Nichols B, Vila M, et al. Quantitative antisense screening and optimization for exon 51 skipping in Duchenne muscular dystrophy. Mol Ther. 2017;25:2561–72.
Aoki Y, Nakamura A, Yokota T, Saito T, Okazawa H, Nagata T, et al. In-frame dystrophin following exon 51-skipping improves muscle pathology and function in the exon 52-deficient mdx mouse. Mol Ther. 2010;18:1995–2005.
Mendell JR, Goemans N, Lowes LP, Alfano LN, Berry K, Shao J, et al. Longitudinal effect of eteplirsen versus historical control on ambulation in Duchenne muscular dystrophy. Ann Neurol. 2016;79:257–71.
Refusal of marketing authorization for Exondys (eteplirsen). Eur Med Agency. 2018;1:1–2.
Muntoni F, Frank D, Sardone V, Morgan J, Schnell F, Charleston J, et al. Golodirsen induces exon skipping leading to Sarcolemmal dystrophin expression in Duchenne muscular dystrophy patients with mutations amenable to exon 53 skipping. Neurology. 2018;90:S22.001.
U.S. National Library of Medicine. Phase I/II study of SRP-4053 in DMD patients. 2019. https://clinicaltrials.gov/ct2/show/NCT02310906.
Watanabe N, Nagata T, Satou Y, Masuda S, Saito T, Kitagawa H, et al. NS-065/NCNP-01: an antisense oligonucleotide for potential treatment of exon 53 skipping in Duchenne muscular dystrophy. Mol Ther Nucleic Acids. 2018;13:442–9.
Echigoya Y, Mouly V, Garcia L, Yokota T, Duddy W. In silico screening based on predictive algorithms as a design tool for exon skipping oligonucleotides in duchenne muscular dystrophy. PLoS ONE. 2015;10:1–24.
Komaki H, Nagata T, Saito T, Masuda S, Takeshita E, Sasaki M, et al. Systemic administration of the antisense oligonucleotide NS-065/NCNP-01 for skipping of exon 53 in patients with Duchenne muscular dystrophy. Sci Transl Med. 2018;10:1–11.
Clemens P, Rao V, Connolly A, Harper A, Mah J, Smith E, et al. A phase II, dose finding study to assess the safety, tolerability, pharmacokinetics, and pharmacodynamics of NS-065/NCNP-01 (Viltolarsen) in boys with Duchenne muscular dystrophy (DMD). Mendoza: Poster presented at the 23rd International Annual Congress of the World Muscle Society; 2018.
Fall AM, Johnsen R, Honeyman K, Iversen P, Fletcher S, Wilton SD. Induction of revertant fibres in the mdx mouse using antisense oligonucleotides. Genetic Vaccines Ther. 2006;4:1–12.
Nichols BM, Aoki Y, Kuraoka M, Lee JJA, Takeda S, Yokota T. Multi-exon skipping using cocktail antisense oligonucleotides in the Canine X-linked muscular dystrophy. J Vis Exp. 2016;111:1–15.
Echigoya Y, Lim KRQ, Melo D, Bao B, Trieu N, Mizobe Y, et al. Exons 45–55 skipping using mutation-tailored cocktails of antisense morpholinos in the DMD gene. Mol Ther. 2019;27:2005–17.
Yazaki M, Yoshida K, Nakamura A, Koyama J, Nanba T, Ohori N, et al. Clinical characteristics of aged Becker muscular dystrophy patients with onset after 30 years. Eur Neurol. 1999;42:145–9.
Echigoya Y, Aoki Y, Miskew B, Panesar D, Touznik A, Nagata T, et al. Long-term efficacy of systemic multiexon skipping targeting Dystrophin exons 45–55 with a cocktail of vivo-morpholinos in Mdx52 Mice. Mol Ther Nucleic Acids. 2015;4:1–10.
Aoki Y, Yokota T, Nagata T, Nakamura A, Tanihata J, Saito T, et al. Bodywide skipping of exons 45–55 in dystrophic mdx52 mice by systemic antisense delivery. PNAS. 2012;109:13763–8.
Yokota T, Takeda S, Lu QL, Partridge TA, Nakamura A, Hoffman EP. A renaissance for antisense oligonucleotide drugs in neurology: exon skipping breaks new ground. Arch Neurol. 2009;66:32–38.
Aartsma-Rus A, Van Vliet L, Hirschi M, Janson AAM, Heemskerk H, Winter CLD, et al. Guidelines for antisense oligonucleotide design and insight into splice-modulating mechanisms. Mol Ther. 2009;17:548–53.
Popplewell LJ, Trollet C, Dickson G, Graham IR. Design of phosphorodiamidate morpholino oligomers (PMOs) for the induction of exon skipping of the human DMD gene. Mol Ther. 2009;17:554–61.
Harding PL, Fall AM, Honeyman K, Fletcher S, Wilton SD. The influence of antisense oligonucleotide length on dystrophin exon skipping. Mol Ther. 2017;15:157–66.
Nguyen Q, Yokota T. Immortalized muscle cell model to test the exon skipping efficacy for Duchenne muscular dystrophy. J Pers Med. 2017;7:1–9.
Shimo T, Tachibana K, Saito K, Yoshida T, Tomita E, Waki R, et al. Design and evaluation of locked nucleic acid-based splice-switching oligonucleotides in vitro. Nucleic Acid Res. 2014;42:8174–87.
Mamchaoui K, Trollet C, Bigot A, Negroni E, Chaouch S, Wolff A, et al. Immortalized pathological human myoblasts: towards a universal tool for the study of neuromuscular disorders. Skeletal Muscle. 2011;1:1–10.
Acknowledgements
This work was supported by Muscular Dystrophy Canada, the Friends of Garrett Cumming Research Fund, the HM Toupin Neurological Science Research Fund, Canadian Institutes of Health Research (CIHR), Alberta Innovates: Health Solutions (AIHS), Jesse’s Journey, Canada Foundation for Innovation (CFI), Alberta Advanced Education and Technology, and the Women and Children’s Health Research Institute (WCHRI).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Dzierlega, K., Yokota, T. Optimization of antisense-mediated exon skipping for Duchenne muscular dystrophy. Gene Ther 27, 407–416 (2020). https://doi.org/10.1038/s41434-020-0156-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41434-020-0156-6
This article is cited by
-
Possibilities and limitations of antisense oligonucleotide therapies for the treatment of monogenic disorders
Communications Medicine (2024)
-
Electrical impedance myography detects dystrophin-related muscle changes in mdx mice
Skeletal Muscle (2023)
-
In vivo restoration of dystrophin expression in mdx mice using intra-muscular and intra-arterial injections of hydrogel microsphere carriers of exon skipping antisense oligonucleotides
Cell Death & Disease (2022)
-
Hydrogen sulfide as a therapeutic option for the treatment of Duchenne muscular dystrophy and other muscle-related diseases
Cellular and Molecular Life Sciences (2022)
-
Pharmacology and toxicology of eteplirsen and SRP-5051 for DMD exon 51 skipping: an update
Archives of Toxicology (2022)