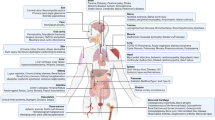
Abstract
Mesenchymal stem cells (MSCs) can be widely isolated from various tissues including bone marrow, umbilical cord, and adipose tissue, with the potential for self-renewal and multipotent differentiation. There is compelling evidence that the therapeutic effect of MSCs mainly depends on their paracrine action. Extracellular vesicles (EVs) are fundamental paracrine effectors of MSCs and play a crucial role in intercellular communication, existing in various body fluids and cell supernatants. Since MSC-derived EVs retain the function of protocells and have lower immunogenicity, they have a wide range of prospective therapeutic applications with advantages over cell therapy. We describe some characteristics of MSC-EVs, and discuss their role in immune regulation and regeneration, with emphasis on the molecular mechanism and application of MSC-EVs in the treatment of fibrosis and support tissue repair. We also highlight current challenges in the clinical application of MSC-EVs and potential ways to overcome the problem of quality heterogeneity.
Similar content being viewed by others
Facts
-
MSC-derived EVs have low-immunogenicity and strong potential for therapeutic applications.
-
MSC-derived EVs were used to treat tissue fibrosis and promote tissue regeneration.
-
MSC-derived EVs are proposed as a novel therapeutic agent to mediate immunomodulation and promote regeneration.
Open questions
-
How can MSC-derived EVs mediate immunomodulation and regeneration?
-
How can MSC-derived EVs be used to aid regeneration of fibrotic tissue?
-
How can mass manufacturing of MSC-derived EVs be achieved and the problem of quality heterogeneity overcome?
-
What are the challenges of MSC-derived EV-based immunomodulation and regeneration in clinical practice?
Introduction
Mesenchymal stem cells (MSCs) exist in various tissues such as bone marrow (BMSCs), umbilical cord blood (UC-MSCs) and umbilical cord tissue, placental tissue (hPMSCs), adipose tissue (ADSCs), and menstrual blood (MenSCs). These cells have multidirectional differentiation potential [1] to become osteoblasts, chondrocytes or adipocytes in vitro [2], and have a unique function of cytokine secretion [3]. Cell models have been applied in proliferation, transplantation, and differentiation studies, and in identification of immune responses in vitro [4]. Numerous studies have shown that MSCs have great potential in immune regulation and regeneration [5]. The U.S. FDA has approved nearly 60 clinical trials [6], mainly focused on Hematopoietic Stem Cell Transplantation (HSCT) [7], tissue healing, Autoimmune Disease (AID), and genetic therapy vectors [8]. Recently, MSCs have been widely used in clinical studies as a regenerative agent and to treat a variety of conditions including osteoarthritis [9], pulmonary fibrosis, spinal cord injury, myocardial damage, knee cartilage injury, dental pulp regeneration, and organ transplantation [10]. An increasing number of studies has revealed that the powerful therapeutic effects of MSCs are due to paracrine-like secretion of cytokines (growth factors and chemokines) [11, 12] and extracellular vesicles (EVs) as well as their involvement in cellular communication [13,14,15,16].
Application of MSCs as cell therapy is based on regulating the inflammatory response and participating in tissue repair and regeneration [17]. The therapeutic effect of MSCs is mainly attributed to their immunomodulatory function regulated by the inflammatory environment [18]. When stimulated by inflammatory factors, MSCs produce a large number of immunomodulatory factors, cell chemokines, and growth factors, thereby regulating the tissue immune microenvironment and promoting tissue regeneration [19]. There is accumulating evidence that EVs derived from MSCs preserve the therapeutic action of the parent MSCs and their use avoids the safety concerns associated with live cell therapy [20, 21]. Therefore, use of MSC-EVs to replace MSCs as cell-free therapy may be the focus of future clinical treatments [20]. We review recent studies of the role of MSC-EVs in immunomodulation and regeneration, focusing on their molecular mechanisms in the treatment of osteoarthritis, spinal cord injury, skin injury, and liver, kidney, and lung fibrosis.
Extracellular vesicles composition
Extracellular vesicles (EVs) exist in body fluids, are released by cells, and have a membrane structure [22]. They can be divided into four subgroups according to their diameter: exosomes (30–150 nm), microvesicles (100–1000 nm), apoptotic bodies (50–5000 nm, generated during cell apoptosis) [23, 24], and oncosomes (1–10 μm), newly discovered and observed in cancer cells [25]. EVs encapsulate many bioactive molecules (proteins, lipids, nucleic acids, and organelles) [26,27,28] that can be delivered to target cells. Large amounts of data suggest that exosomes and microvesicles are vital mediators of EVs in numerous physiological (pathological) processes [29] (Fig. 1).
Exosomes
Exosomes are microscopic vesicles with a density of 1.11–1.19 g/mL. They have a typical “disk-like” structure and flat spherical shape when seen under an electron microscope [24]. Many kinds of cellsin various body fluids and cell supernatants can secrete exosomes under normal and pathological conditions. Exosomes were first discovered in 1983 in sheep reticulocytes and were named “Exosomes” by Johnstone in 1987 [30]. These tiny vesicles contain specific proteins, lipids, and nucleic acids that can be transmitted and serve as signaling molecules to alter the function of other cells [31, 32].
During the formation of exosomes, the extracellular components and cell membrane proteins are wrapped by the invaginated plasma membrane to form early endosomes. These can exchange materials with intracellular organelles and develop into late endosomes, eventually forming intracellular multivesicular bodies (MVBs) [33, 34]. MVBs contain many intraluminal vesicles (ILVs) [35]. They may be degraded and released into the cytoplasm by fusion with autophagosomes or lysosomes, or released into extracellular vesicles by fusion with plasma membrane, including ILVs, resulting in exosome formation [34]. Exosome-mediated intercellular communication is achieved by direct membrane fusion, receptor-mediated endocytosis, phagocytosis, caveolae, and micropinocytosis [36,37,38].
Proteins involved in exosome biogenesis (such as transport and fusion) include Rab GTPases [39,40,41], ESCRT (endosomal sorting complex required for transport) [42], annexin, lipid raft proteins, and four transmembrane proteins (CD63, CD81, and CD9) [43, 44]. In addition, they also contain biosynthetic antibodies (Alix and TSG101) involved in MVBs [45, 46], cholesterol, ceramide, phosphoglyceride that provides structural stability, and immune-related molecule MHC-II that is involved in antigen binding and presentation. Exosomes also carry functional mRNAs and miRNAs that can be transferred between cells [47]. Exosomes released by tumors contain single-stranded DNA, genomic DNA, cDNA, and a transposable element [48, 49]. It is clear that exosomes have many functions as biomarkers of disease.
Microvesicles
Microvesicles are also known as microparticles. Biogenesis of MVs differs to that of exosomes since they are released from outward budding and fission of plasma membrane when the cell is stimulated or apoptotic [50]. Nonetheless, they share characteristics of high biocompatibility, and low immunogenicity and targeting and can be used as drug carriers [51]. Studies have shown that the use of tumor cell-derived MVs to deliver chemotherapy drugs produces in better cancer treatment results with few side effects or adverse reactions [52, 53].
MSC-derived extracellular vesicles
Although MSCs derive from a variety of sources, they can all be adherent in culture and differentiated into a variety of cell types with specific surface markers [54]. With the need for clinical treatment with MSCs, the Mesenchymal and Tissue Stem Cell Committee of the International Society for Cellular Therapy (ISCT) has proposed minimum criteria for identification of human MSCs: (1) Cultured under standard conditions they must adhere to plastic substrates; (2) On flow cytometry, the positive rate of CD105, CD73 and CD90 expression in MSC surface markers should reach 95%, and negative expression rate CD45, CD34, CD14 or CD11b, CD79a or CD19 or HLA-DR (human leukocyte antigen -DR) (≤2% positive); (3) After induction by standard methods in vitro, MSCs must be able to induce differentiation into osteoblasts, chondrocytes and adipocytes [55]. Nonetheless, further research has revealed that these standards do not fully define MSCs [56]. There is accumulating evidence that heterogeneous MSCs have multiple cell subpopulations with characteristic surface markers [57, 58], but the definition of surface markers and biological functions of these subpopulations requires ongoing exploration.
MSCs are easy to resuscitate and proliferate in vitro, enabling them to be mass-produced for clinical application [18]. In recent years, they have been the most studied stem cell type for clinical application, and have played an effective therapeutic role in graft-versus-host disease (GVHD) [7], kidney injury [59], tissue and organ transplantation, immune tolerance [60], nerve injury, rheumatic disease, and liver disease. At present, MSCs have attracted much attention in the context of the COVID-19 pandemic [61]. Leng et al. demonstrated that in an MSC treatment group, patients with COVID-19 infection were cured or their condition significantly improved as a result of regulation of increased interleukin 10 (IL 10) expression, inhibition of overactivated immune T cells and NK cells, and a significantly reduced TNF-α level [62].
Despite their advantages, there are aspects of MSC therapy that warrant consideration. First, the proliferation ability of MSCs is gradually weakened and accompanied by a certain degree of differentiation and even aging with increasing passages during in vitro culture. This impacts their regulatory and therapeutic ability [56, 63]. Second, in the in vivo environment, heredity factors and the self-renewal ability of MSCs cannot be controlled with consequent potential for tumorigenicity [64]. In addition, although MSCs have a strong regenerative regulatory potential, it is uncertain whether they can target or remain at the damaged site following intravenous injection [65]. There is some evidence that only a small number of MSCs reach the target site due to the host body’s scavenging capacity [66, 67]. Although in-situ injection can partially solve these problems, there remain problems with cell differentiation and aging, and the clinical effects are not optimistic [68]. MSCs have also been found to cause and promote the growth of various types of cancer [69]. In addition, there are the usual associated risks of cell therapy such as viral infection and immune rejection as well as problems with storage and transportation [70].
The discovery that most therapeutic effects of MSCs depend on their paracrine action and that EVs can replace their parent cells offers exciting prospects for researchers [21]. EVs offer great advantages [71]: they are not self-replicating and largely avoid the risk of tumorigenicity [72]; compared with cell therapy, EVs are safer; as nanoparticles they have both biocompatibility and low immunogenicity, enabling them to cross-protective barriers such as the blood-brain barrier [73]; they can be continuously secreted by immortalized cells to obtain a sufficient number [74]; EVs protect their internal biomolecular activity via their lipid membrane structure, can be preserved for a prolonged period at -80°C, and are not subject to deactivation, even after repeated freezing and thawing [75, 76]; and they have an encapsulation capability, can load specific drugs and transport them to target cells [77].
Notably, MSC-EVs express EV surface markers CD63, CD9 and CD81, as well as mesenchymal stem cell surface markers CD44, CD73, and CD90 [78]. In addition, proteins contained in the extracellular vesicles secreted by MSCs are a specific protein subclass that determines their unique biological functions [36]. At the same time, the encapsulated mRNA and miRNA in MSC-EVs form the molecular basis for their function [79]. Accordingly, MSC-EVs transmit information and communicate with target cells through internal substances, thus changing the activity and function of target cells [80].
With their unique advantages, MSC-EVs play an important role in immune regulation and regeneration. Studies of the promotion of regeneration through immune regulation are described in detail below. Meanwhile, in the treatment of autoimmune diseases, Wu et al. found that BM-MSC-derived EVs targeted inhibition of the cyclin I-activated ATM/ATR/p53 signaling pathway by upregulation of miR- 34a, thereby inhibiting RA fibroblast-like synoviocytes (RA-FLSs) and significantly ameliorating RA inflammation in vivo [81]. Another study on the regulation of type-I autoimmune diabetes mellitus (T1DM) showed that AD-MSC-derived exosomes ameliorated T1DM symptoms by upregulating the expression of regulatory T cells, interleukin 4 (IL 4), IL 10 and transforming growth factor-beta (TGF-β) and down-regulating IL 17 and interferon-gamma (IFN-γ) [82]. Additional studies of autoimmune disease regulation have been summarized elsewhere [83]. Recently MSC-EVs have also been applied in clinical practice. Nassar et al. are in the process of evaluating the effect of human UC-MSC-derived EVs on islet β cells in patients with T1DM (trial NCT02138331). Recent clinical trials have been conducted to evaluate the safety and efficacy of MSC-EVs in patients with a variety of diseases based on their potential for immune regulation and regeneration (Table 1).
Application of MSC-EVs in immune regulation and regeneration
The therapeutic potential of MSC-EVs has been reported in immune regulation and tissue regeneration based on EV-mediated cellular communication between MSCs and several target cells, including macrophages, microglia, chondrocytes, articular chondrocytes, endothelial cells, fibroblasts, pericytes, neural stem cells (NSC), neurons, hepatic stellate cells, and podocytes. In this paper, we discuss the molecular mechanisms of MSC-EVs in tissue repair and anti-fibrosis, in which several clusters of miRNA and their downstream pathways have been revealed to play important roles in osteoarthritis, spinal cord injury, skin injury, liver fibrosis, kidney fibrosis, and lung fibrosis (Tables 2–7).
Support tissue repair
Osteoarthritis
Osteoarthritis (OA) is the principal form of joint disease with unclear pathogenesis, presenting with pain and stiffness, and in some cases, disability [84]. Recently, MSC-EVs have been proven to have both regenerative and immunoregulatory benefits in OA (Table 2).
Several studies have reported that hBMSC-EVs play a significant role in the treatment of OA by inhibiting some pro-inflammatory pathways and factors, and enhancing the proliferation and migration of chondrocytes. Vonk et al. determined that MSC-EVs blocked NFκB signaling by inhibiting phosphorylation of IκBα, thereby down-regulating TNF-α-induced COX2 expression, and interleukins and collagenase activity. Additionally, MSC-EVs up-regulated the expression of SOX9 and WNT7A, and promoted the production of proteoglycan and type II collagen in in vitro studies [85]. Li et al. concluded that hBMSC-EVs promoted OA-chondrocyte (OA-CH) proliferation and migration and reduced apoptosis via downregulation of MMP13, ALPL, IL-1β-activated pro-inflammatory Erk1/2, PI3K/Akt, p38, TAK1, and NF-κB signaling pathways and increased gene expression of PRG4, BCL2, and ACAN (aggrecan) [86]. In addition, in OA-like chondrocytes, MSC-EVs induced the expression of type II collagen and aggrecan (chondrocyte markers), while inhibiting MMP-13 and ADAMTS5 (catabolic) and iNOS (inflammatory markers). In a CIOA model, treated mice also exhibited reduced cartilage and bone degeneration [87]. In an OA model, Ruiz showed that the effect of MSC-EVs was due to the presence of TGFBI mRNA and protein [88]. Analogously, in the same model, BMSC-EVs promoted the conversion of RAW264.7 from M1 to M2, reduced the expression of proinflammatory cytokines IL-1β, TNF-α, and IL-6, and enhanced the expression of IL-10, chondrogenic genes, collagen II and SOX9 [89]. Interestingly, Woo et al. revealed in their monosodium iodoacetate (MIA) rat and the surgical destabilization of the medial meniscus (DMM) mouse model that MSC-EVs could ameliorate cartilage degeneration by increasing type II collagen synthesis and decreasing MMP-1, MMP-3, MMP-13 and ADAMTS-5 expression in the presence of IL-1β [90].
Recent studies have also examined the effect of miRNAs in MSC-EVs. In synovial-derived MSC-EVs (SMSC-EVs), Tao et al. overexpressed miR-140-5p to block Wnt5a and Wnt5b to activate YAP via the Wnt signaling pathway and significantly reduce extracellular matrix (ECM) secretion [91]. Wang et al. found that exosomes derived from miR 155-5p–overexpressing SMSCs (SMSC-155-5p-Exos) promoted ECM secretion by targeting Runx2, which enhanced cartilage regeneration and ameliorated OA [92]. Likewise, SMSC-EVs highly expressed miR-31 and relieved OA via the KDM2A/E2F1/PTTG1 axis [93]. Of interest, hypoxia increased the expression of miR-216a-3p in HIF-1α-induced BMSC-EVs and promoted down-regulation of JAK2, promoting proliferation, migration, and reduced apoptosis of chondrocytes via inhibition of the JAK2/STAT3 signaling pathway [94]. A combination of these miRNAs and MSC-EVs may serve as a potential therapy for OA. In contrast, several studies have shown that miRNAs cause side effects in OA. Intra-articular injection of antagomir-miR-100-5p dramatically attenuated the infrapatellar fat pad (IPFP) MSC-EV (MSCIPFP-EVs)-mediated protective effect on articular cartilage in vivo [95]. MiR-29b-3p targets FoxO3 gene and enhances chondrocyte destruction. lncRNA H19 from umbilical cord MSC-EVs could competitively bind to miR-29b-3p to attenuate its inhibition of the target gene FoxO3 [96].
Spinal cord injury
Spinal cord injury (SCI) arises following damage to its structure and function by various pathogenic factors, with consequent spinal cord dysfunction including that of movement, sensation, and reflexes [97]. Due to the limited regenerative ability of nerve components, MSC-EVs have been recently viewed as a promising clinical treatment for SCI (Table 3).
A rat model of SCI has commonly been applied to evaluate treatment with MSC-EVs. They have been found to be able to regulate immunity and restore function through a variety of pathways. First, Huang et al. studied the administration of hBMSC-Exos in an animal model, and demonstrated that inhibition of apoptosis protein (Bax) and pro-inflammatory factors (TNFα and IL 1β), and promotion of anti-apoptotic protein (Bcl-2), anti-inflammatory protein (IL 10) and angiogenesis, could improve motor function [98]. Interestingly, the reduced pericyte migration mediated by BMSC-EVs correlated with inhibition of the NF-KB P65 signaling pathway with consequent weakening of the blood-spinal cord barrier (BSCB) [99]. In addition, Zhou et al. showed that treatment with BMSC-Exos suppressed the expression of caspase 1 and IL 1β by reducing pyroptosis, and enhanced neuronal regeneration to ameliorate motor ability in rats with spinal cord injury [100]. Han et al. found that TGF-β in BMSC-EVs enhanced the expression of Smad6, inhibited the excessive differentiation of neural stem cells (NSCs) into astrocytes, and promoted regeneration of neurons [101]. Consecutively, Nakazaki et al. proposed that BMSC-EVs should be administered over 3 days to up-regulate transforming growth factor -β (TGF-β), TGF-β receptor, and relative proteins of tight junction [102]. More intriguingly, Zhou et al. provided evidence that exosomes secreted by hPMSCs increased the activation of proliferating endogenous nerve stem/progenitor cells in vivo, while promoting NSC proliferation and upregulating MEK, ERK, and CREB phosphorylation levels in vitro, resulting in functional recovery [103].
MiRNAs have always been potent biological effectors of MSC-EVs, and without exception, they play a strong role in immune regulation and regeneration in spinal cord injury. Jia et al. confirmed that overexpression of miR-381 in MSC-EVs could promote SCI repair by up-regulating Ras homologous A (RhoA)/ RHO kinase activity and down-regulating BRD4 expression and DRG cell apoptosis by WNT5A [104]. Li et al. observed that miR-133 carried by MSC-Exos could directly target and down-regulate the expression of RhoA, and also promote expression of ERK1/2 STAT3 and CREB signaling pathway proteins related to neuronal survival and axon regeneration, thus rescuing neuron apoptosis and promoting axon regeneration [105]. Of interest, when miR-17-92, miR-26a, and miR-216a-5p were enriched in BMSC-Exos, they respectively induced activation of mTOR/PI3K/Akt, PTEN/ Akt /mTOR, and the TLR4/NF-κB/PI3K/ Akt signaling pathway cascade, with consequent promotion of axonal regeneration and nerve function repair after SCI [106,107,108]. In addition, miRNA-22 encapsulated in BMSC-EVs promotes neurogenesis and inflammation suppression by downregulating the expression of inflammatory cytokines and GSDMD, and blocking the pyroptosis of microglia after SCI [109]. Overexpression of miR-199a-3p/145-5p in exosomes secreted by human umbilical cord-derived MSCs has been shown to activate the NGF/TrkA signaling pathway affecting TrkA ubiquitination, and improve locomotor function in rats with SCI [110].
Skin injury
Skin injury is quite common. Skin regeneration is typically accompanied by four overlapping processes: inflammation, angiogenesis, new tissue formation, and remodeling [111,112,113] (Table 4).
There is recent evidence that human-derived MSC-Exos effectively benefit skin damage and accelerate wound healing by modulating related signaling pathways. Intriguingly, Zhou et al. adopted a combination therapy, applying hADSC-Exos both locally and intravenously to accelerate skin wound healing. Mechanistically, hADSC-Exos achieved this effect by down-regulating TNF-α, IL-6, CD14, CD19, CD68, and C-caspase 3, and up-regulating VEGF, CD31, Ki67, PCNA, filaggrin, loricrin and AQP3 [114]. Jiang et al. demonstrated that hBMSC-Exos suppressed TGF-β1, Smad2, Smad3, and Smad4 by targeting the TGF-β/Smad signaling pathway, but increased the expression of TGF-β3 and Smad7, thus improving scar formation and promoting wound healing [115]. Remarkably, fetal dermal mesenchymal stem cell-derived exosomes (FDMSC-Exos) have been shown to activate adult dermal fibroblast (ADFs) to promote cell proliferation, migration and secretion by targeting Jagged 1 ligand in the Notch signaling pathway, and ultimately accelerate wound healing [116].
Similar effects have also been observed for human-derived MSC-Exos carrying miRNAs. Of interest, He et al. showed that hBMMSCs and jaw bone marrow MSCs (JMMSCs) could induce macrophages toward M2 polarization and promote wound healing. The mechanism suggested that exosomes secreted by donors may regulate the polarization of macrophages by carrying miR-223 targeting Pknox1. Nonetheless, researchers cannot confirm whether other miRNAs or factors carried by these exosomes are involved in the induction of M2 polarization, and further studies are needed [117]. Likewise, Wu et al. utilized BMSC-Exos treated with 50 µg/mL Fe3O4 nanoparticles and 100 mT SMF to form a functional exosome (mag-BMSC-Exos). Notably, miR-21-5p was overexpressed in mag-BMSC-Exos and promoted angiogenesis in vivo and in vitro to accelerate skin wound healing by targeting SPRY2 to activate the PI3K/AKT and ERK1/2 signaling pathways [118]. Additionally, Cheng et al. found that hUCMSCs-EVs are highly enriched with miR-27b and promote the expression of JUNB and IRE1α by targeting the Itchy E3 ubiquitin-protein ligase (ITCH), thereby accelerating cutaneous wound healing [119]. In addition, hUMSC-Exos can be enriched with a set of microRNAs (miR-21, -23A, -125b, and -145) to attenuate excess myofibroblast formation and scarring via repression of the TGF-β2 /SMAD2 pathways [120]. Another study showed that hADSC-Exos derived miR-19b regulate the TGF-β pathway by targeting CCL1 [121]. Li et al. verified that hADSC-Exos down-regulated the expression of Col1, Col3, α-SMA, IL-17RA, and P-SMad2/P-SMad3, and up-regulated the level of SIP1 by suppressing multiplication and migration of hypertrophic scar-derived fibroblasts (HSFs). In addition, miR-192-5p was highly enriched in ADSC-EXO and reduced the level of pro-fibrosis protein, improved hypertrophic scar fibrosis, and accelerated wound healing via targeted inhibition of IL-17RA expression [122]. Alongside this, overexpression of miR-486-5P in hADSC-EVs enhanced the migration of human skin fibroblasts (HSFs) and the angiogenic activity of human microvascular endothelial cells (HMECs) by targeting Sp5 and motivating CCND2 expression, thereby promoting wound healing [123]. Interestingly, Gao et al. found that overexpression of Mir-135a in hAMSC-Exos significantly down-regulated LATS2, thereby increasing cell migration and promoting wound healing [124].
Anti-fibrosis
Liver fibrosis
Liver fibrosis is a pathophysiological process and refers to the abnormal proliferation of intrahepatic connective tissue due to various pathogenic factors [125]. Recently, use of MSC-EVs has been considered a new therapeutic approach to repair liver fibrosis (Table 5). Rong et al. showed that human bone MSC-EVs inhibited expression of Wnt/β-catenin pathway components, α-SMA, and type I collagen, thereby preventing stellate cell activation and increasing hepatocyte regeneration. In vivo injection of hBMSC-Exos has been shown to effectively alleviate CCL4-induced liver fibrosis in rats and restore liver function [126]. Likewise, using a CCL4-induced liver fibrosis animal model, Ohara et al. proved that EVs from amnion-derived MSCs (AMSC-EVs) could significantly reduce the number of Kupffer cells (KCs), mRNA expression of inflammatory factors, activation of hepatic stellate cells (HSC), and the lipopolysaccharide (LPS)/toll-like receptor 4 (TLR4) signaling pathway, thereby reducing inflammation and fibrosis [127].
The anti-fibrotic effect of miRNAs in MSC-EVs has become a focus of research into CCL4-induced liver fibrosis in rats. MiRNA-181-5p overexpression in ADSC-EVs has been shown to down-regulate transcription 3 (STAT3) and Bcl-2 and activated autophagy in HST-T6 cells, alongside a significant decrease in collagen I, vimentin, a-SMA, and fibronectin in liver [128]. Similarly, high expression of miR-122 in ADSC-EVs modulated the expression of target genes such as insulin-like growth factor receptor 1 (IGF1R) cyclin G(CCNG1), and proline-4-hydroxylase A1(P4HA1), thereby more effectively blocking the proliferation of HSCs and collagen maturation [129]. Interestingly, Kim et al. reported that miR-486-5p was highly expressed in T-MSC-EVs that could target the hedgehog receptor, smoothened (Smo), and inhibit hedgehog signaling, thereby attenuate the activation of HSCs and liver fibrosis [130].
Kidney fibrosis
Renal fibrosis is a gradual pathophysiological process during which kidney function progresses from healthy to injured, then to damage with an ultimate loss of function [131]. Increasingly, MSC-EVs have been studied in the treatment of renal fibrosis using various models (Table 6).
Ji et al. determined that hUC-MSC-Exos repressed Yes-associated protein (YAP) through casein kinase 1δ (CK1δ) and E3 ubiquitin ligase β-TRCP in a rat model of unilateral ureteral obstruction (UUO), thus ameliorating renal fibrosis [132]. Similar effects in a UUO model were confirmed in Liu’s study. They revealed that hUC-MSC-Exos attenuated renal fibrosis by inhibiting the ROS-mediated p38MAPK/ERK signaling pathway [133]. Likewise, Shi et al. showed that milk fat globule–epidermal growth factor–factor 8 (MFG-E8) was included in BMSC-EVs, and ameliorated renal fibrosis by blocking the RhoA/ROCK pathway in a UUO model [134]. Of interest, in a UUO mouse model, BMSC-Exos loaded miR-34c-5p inhibited core fucosylation (CF) by cd81-EGFR complex, thereby improving renal interstitial fibrosis (RIF) [135]. Correspondingly, recent studies also suggest that exosomes from ADSCs ameliorate the development of DN via miRNAs. Jin et al. used miRNA-215-5p to inhibit ZEB2 and improved diabetic nephropathy (DN) symptoms. They also revealed that upregulated expression of miR-486 could suppress the Smad1/mTOR signaling pathway in podocytes [136, 137]. MV-miR-451a from hUMSCs repressed cell cycle inhibitor P15 and P19 expression by targeting their 3′-UTR sites, thereby decreasing α-SMA and increasing e-cadherin expression. This resulted in epithelial-mesenchymal transformation (EMT) reversal and improved DN symptoms [138]. In another study of amelioration of DN, BMSC-Exos significantly enhanced the expression of LC3 and Beclin-1, and decreased the level of mTOR and fibrotic markers in a streptozotocin-induced rat model of diabetes mellitus [139]. Interestingly, Grange et al. reported that renal fibrosis and the expression of collagen I were significantly ameliorated via multiple injections of HLSCs (human liver stem-like cells) and MSC-EVs in NOD/SCID/IL2Rγ KO (NSG) mice. Additionally, related genes (Serpina1a, FAS ligand, CCL3, TIMP1, MMP3, collagen I, and SNAI1) were significantly downregulated, thereby attenuating DN symptoms [140].
Lung fibrosis
Pulmonary fibrosis is a terminal change in lung disease characterized by fibroblast proliferation and accumulation of a large amount of extracellular matrix accompanied by inflammatory injury and destruction of tissue. Normal alveolar tissue is damaged and abnormal repair leads to structural abnormalities [141, 142]. The etiology in the vast majority of patients with pulmonary fibrosis is unknown [143]. Idiopathic pulmonary fibrosis (IPF) manifests mainly with pulmonary fibrotic lesions and is a serious interstitial lung disease that can lead to progressive loss of lung function. IPF has a higher mortality than most tumors and is considered a tumor-like disease [142]. Recently, MSC-EVs have become an effective treatment for pulmonary fibrosis (Table 7).
BMSC-Exos exert their therapeutic effect through immunomodulation. In a mouse model, BMSC-Exos have been shown to significantly ameliorate hyperoxia (HYRX)-induced bronchopulmonary dysplasia (BPD), alveolar fibrosis, and pulmonary vascular remodeling by suppressing M1 macrophage production and enhancing M2 macrophage generation [144]. Likewise, BMSC-Exos have been shown to significantly reverse fibrosis in a bleomycin-induced pulmonary fibrosis model by regulating total lung imbalance of MΦ phenotype [145]. In addition, the Wnt5a/BMP signaling pathway regulated by UC-MSC-Exos can enhance Wnt5a, Wnt11, BMPR2, BMP4, and BMP9 expression, and down-regulate that of β-catenin, Cyclin D1 and TGF-β1. In a monocrotaline (MCT)-induced rat model of pulmonary hypertension (PH), MSC-Exos were shown to significantly ameliorate pulmonary vascular remodeling and pulmonary fibrosis [146]. Of interest, Chaubey et al. showed that UC-MSC-Exos played a therapeutic role in improving pulmonary inflammation, pulmonary simplification, pulmonary hypertension, and right ventricular hypertrophy through immunomodulatory glycoprotein TSG-6 in a neonatal BPD mouse model [147].
Additionally, MSC-EVs can reverse lung injury and pulmonary fibrosis by expressing influential miRNAs. Wan et al. determined that high expression of miR-29b-3p by BMSC-EVs ameliorated IPF by FZD6 [148]. Zhou et al. found that miR-186 enriched by BMSC-EVs repressed the expression of SOX4 and Dickkopf-1 (Dkk1), thereby effectively inhibiting fibroblast development and attenuating IPF [149]. In addition, Lei’s study revealed that hPMSC -EVs could carry miR-214-3p and downregulate ATM/P53/P21 signaling, thus relieving radiation-induced lung inflammation and fibrosis [150]. In BLM-induced lung fibrosis and a mouse model of alveolar epithelial cell damage, exosomes secreted from MenSCs (MenSCs-Exos) have been shown to ameliorate pulmonary fibrosis by transferring miRNA Let-7 to suppress reactive oxygen species (ROS), mitochondrial DNA (mtDNA) damage, and activation of NLRP3 inflammasome [151]. Similarly, Xiao et al. used another LPS-induced Acute Lung Injury (ALI) mouse model and demonstrated that MSC-Exos repressed NF-κB and hedgehog pathways by transporting miR-23a-3p and miR-182-5p, thereby improving lung injury and fibrosis [152].
Challenges and application of MSC-EVS as an advanced therapy
Although MSC-EV-based therapy holds great promise as a novel “cell-free” therapeutic product, there remain many challenges to overcome prior to their clinical application. At present, several limitations restrict the clinical translation of MSC-EVs including the discrepancies in the components of EVs from various sources and the lack of standard operation processes for largescale production, both of which largely depend on quality control of the sources of EVs. It is plausible to overcome these hurdles by introducing a strategy to control the quality of MSCs from the original source of EVs.
The quality of MSC-derived EVs from different groups and batches is heterogeneous
MSCs are most commonly derived from bone marrow, fat, umbilical cord and other tissues, but maintaining consistent quality of MSCs and their EVs from different sources and across batches is difficult. This severely restricts the quality control and management of MSCs and their EVs as drugs, and increases the problem of drug resistance [153]. This results in limited reproducibility of functional measurements in vitro and in vivo [154].
In the angiogenesis study, BMSC-, ADSC-, and UCBMSC-derived EVs were compared and found to reduce myocardial apoptosis, facilitate angiogenesis, and improve cardiovascular function. Notably, EVs from ADSCs stimulated cardioprotection factors VEGF, bFGF, and HGF [155]. In addition, BMSC-derived EVs appeared to have a greater angiogenic potential than ADSC-derived EVs when compared in two independent ischemic model studies, with an approximately 4-fold increase in endothelial cell numbers compared with controls, and a 1.5-fold change in the latter [156, 157]. Nonetheless, another study showed that EVs from endometrial mesenchymal stem cells resulted in a greater level of angiogenesis than EVs from BMSCS or ADMSCs [158].
In studies of osteogenesis studies, in two separate rat skull defect studies, BMSC-EV treatment increased bone volume four-fold relative to the control group [159], while ADSC-EV increased bone volume by about 1.33 times [160]. In other studies, BMSC- and ADSC-derived EVs accelerated chondrocyte proliferation, migration, and osteogenic differentiation [161, 162].
Comparison of the immunomodulatory differences of MSC-derived EVs from different sources revealed that BMSC-EVs and ADSC-EVs could induce M2 polarization of macrophages in vivo and in vitro [163, 164]. Interestingly, in a separate experiment, Wang et al. showed that BMSC-EVs prompted a significant (3.2-fold) increase in the expression of CD206 of M2-polarization marker in an acute lung injury mouse model [163]. Nonetheless Liu et al. reported that the M2 polarization ability of ADSC-EVs increased only by a factor of 1.5 in a mouse model [165].
The proliferation capacity of MSCs extracted from adult tissues was limited, and affected the largescale production of EVs
To develop MSC-EVs into commercially advanced therapeutic products (ATPs), quality assurance (QA) is required of the original material, including parental groups or cells used in the manufacture of MSCs. There remain many difficulties in mass production of EVs from adult tissues for clinical trials since proprietary MSCs have a limited number of passage times, age easily, and come at a high financial cost. In addition, their heterogenicity makes traditional cell culture inefficient in terms of time and cost.
MSCs derived from pluripotent stem cells overcome the problems of mass production of MSC-EVs and quality heterogeneity
The original source MSCs requires good, consistent, and controllable quality, with a strong ability to proliferate and to secrete large numbers of EVs. To achieve this, we established an induction system of MSCs using pluripotent stem cells to overcome the problems of mass production of MSC-EVs and variation in quality. We successfully induced MSCs from pluripotent stem cells (PSC) [166,167,168,169,170]. Compared with MSCs extracted from traditional sources, our MSCs were derived from the same parent PSCs, consequently overcoming the problem of EV heterogeneity when MSCs from a variety of sources are used. Recently, GMP-grade MSCs derived from human PSCs (hPSC) have been used in clinical trials for refractory graft-versus-host disease (GVHD) [171]. The therapeutic potential of MSC-EVs has been shown in preclinical studies of both acute GVHD (aGVHD)[172,173,174] and chronic GVHD (cGVHD) [175] models. The preliminary benefits of hPMSC-EVs have been reported in a patient with cutaneous cGVHD. The stiffening and dryness of skin were improved significantly after intravenous injection of hPMSC-EVs [176]. Based on the preliminary efficacy and safety profiles, a phase 1 study has been launched to evaluate the safety and efficacy of BM-MSC-derived EVs in patients with acute or chronic rejection following abdominal solid organ transplantation (NCT05215288, Table 1). It is plausible that hPSC-MSC-derived EVs will promote the clinical translation of MSC-EVs owing to the quality control and largescale productive advantages of hPSC-MSCs compared with traditional MSC. hPSC-MSCs have more passages (more than 30 generations), strong amplification ability, can withstand senescence [166, 167, 170], and have strong secretion ability (including cytokines and exosomes) [168] compared with the traditional MSCs. Nonetheless, the passage times of traditional MSCs are generally less than 10 generations, and the proliferation and differentiation abilities of MSCs are reduced after numerous passages in culture, and affects the secretion of extracellular vesicles. Therefore, our hPSC-MSCs have great advantages for large-scale production and cost control of EVs. Mass production of MSCs and their EVs is now possible using bioreactors and microcarriers to maximize MSC growth and EV release per unit surface area. We evaluated mesenchymal stem cells from different sources and found that PSC-MSCs had the highest EV production. To optimize EV production, we acquired hPSC-MSCs in a scalable cell factory-based culture and were able to overcome the major obstacles during transformation of MSC-EVs into ATPs.
Conclusions and future perspective
Extracellular vesicles derived from mesenchymal stem cells play a critical role in the development of immune regulation and regeneration. These EVs mimic the effects of stem cells and perform powerful functions by modulating immune pathways, promoting effector cell migration and proliferation, and reducing apoptosis. To date, 15 clinical trials have been registered in ClinicalTrial.gov, but none has been completed. Although EVs compared with MSC cell therapy incite a lower immune response and have a higher safety profile, there remain challenges to their clinical application [56]. In addition, the successful application of EVs depends on low cost for mass production, as well as improved separation efficiency and more accurate characterization methods. This review has discussed the therapeutic effects of EVs based on the function of MSCs or the introduction of specific molecules (such as miRNAs and lncRNAs). As work continues, researchers are actively developing engineered EVs that are more effective and capable of targeting, through loading of bioactive molecules and surface modification. Of interest, Feng et al. developed ε-polylysine-polyethylene-distearyl phosphatidylethanolamine (PPD) to modify MSC-EVs and invert their surface charge. As a result, the steric and electrostatic hindrance of cartilage matrix were alleviated, and the efficiency of MSC-EVs in the treatment of OA was improved [177]. These treatment strategies have achieved promising results at the initial stage and provide exciting new avenues for regenerative medicine therapy.
Data availability
All relevant data are included in this manuscript.
References
Maqsood M, Kang M, Wu X, Chen J, Teng L, Qiu L. Adult mesenchymal stem cells and their exosomes: Sources, characteristics, and application in regenerative medicine. Life Sci. 2020;256:118002.
Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–7.
Santamaria G, Brandi E, Vitola P, Grandi F, Ferrara G, Pischiutta F, et al. Intranasal delivery of mesenchymal stem cell secretome repairs the brain of Alzheimer’s mice. Cell Death Differ. 2021;28:203–18.
Reagan MR, Kaplan DL. Concise review: Mesenchymal stem cell tumor-homing: detection methods in disease model systems. Stem Cells. 2011;29:920–7.
Uccelli A, Moretta L, Pistoia V. Mesenchymal stem cells in health and disease. Nat Rev Immunol. 2008;8:726–36.
Wiest EF, Zubair AC. Challenges of manufacturing mesenchymal stromal cell-derived extracellular vesicles in regenerative medicine. Cytotherapy. 2020;22:606–12.
Martínez-Carrasco R, Sánchez-Abarca LI, Nieto-Gómez C, Martín García E, Sánchez-Guijo F, Argüeso P, et al. Subconjunctival injection of mesenchymal stromal cells protects the cornea in an experimental model of GVHD. Ocul Surf. 2019;17:285–94.
Levy O, Kuai R, Siren EMJ, Bhere D, Milton Y, Nissar N, et al. Shattering barriers toward clinically meaningful MSC therapies. Sci Adv. 2020;6:eaba6884.
Park YB, Ha CW, Lee CH, Yoon YC, Park YG. Cartilage regeneration in osteoarthritic patients by a composite of allogeneic umbilical cord blood-derived mesenchymal stem cells and hyaluronate hydrogel: results from a clinical trial for safety and proof-of-concept with 7 years of extended follow-up. Stem Cells Transl Med. 2017;6:613–21.
Han Y, Li X, Zhang Y, Han Y, Chang F, Ding J. Mesenchymal Stem Cells for Regenerative Medicine. Cells. 2019;8:886.
van den Bos C, Mosca JD, Winkles J, Kerrigan L, Burgess WH, Marshak DR. Human mesenchymal stem cells respond to fibroblast growth factors. Hum Cell. 1997;10:45–50.
Kinnaird T, Stabile E, Burnett MS, Lee CW, Barr S, Fuchs S, et al. Marrow-derived stromal cells express genes encoding a broad spectrum of arteriogenic cytokines and promote in vitro and in vivo arteriogenesis through paracrine mechanisms. Circ Res. 2004;94:678–85.
Asai A, Aihara E, Watson C, Mourya R, Mizuochi T, Shivakumar P, et al. Paracrine signals regulate human liver organoid maturation from induced pluripotent stem cells. Development. 2017;144:1056–64.
Quaglia M, Dellepiane S, Guglielmetti G, Merlotti G, Castellano G, Cantaluppi V. Extracellular vesicles as mediators of cellular crosstalk between immune system and kidney graft. Front Immunol. 2020;11:74.
Rahmani A, Saleki K, Javanmehr N, Khodaparast J, Saadat P, Nouri HR. Mesenchymal stem cell-derived extracellular vesicle-based therapies protect against coupled degeneration of the central nervous and vascular systems in stroke. Ageing Res Rev. 2020;62:101106.
Kourembanas S. Exosomes: vehicles of intercellular signaling, biomarkers, and vectors of cell therapy. Annu Rev Physiol. 2015;77:13–27.
Hosseini S, Shamekhi MA, Jahangir S, Bagheri F, Eslaminejad MB. The robust potential of mesenchymal stem cell-loaded constructs for hard tissue regeneration after cancer removal. Adv Exp Med Biol. 2019;1084:17–43.
Castro-Manrreza ME, Montesinos JJ. Immunoregulation by mesenchymal stem cells: biological aspects and clinical applications. J Immunol Res. 2015;2015:394917.
Li N, Hua J. Interactions between mesenchymal stem cells and the immune system. Cell Mol Life Sci. 2017;74:2345–60.
Liang X, Ding Y, Zhang Y, Tse HF, Lian Q. Paracrine mechanisms of mesenchymal stem cell-based therapy: current status and perspectives. Cell Transpl. 2014;23:1045–59.
Jafarinia M, Alsahebfosoul F, Salehi H, Eskandari N, Ganjalikhani-Hakemi M. Mesenchymal stem cell-derived extracellular vesicles: a novel cell-free therapy. Immunol Invest. 2020;49:758–80.
Lötvall J, Hill AF, Hochberg F, Buzás EI, Di Vizio D, Gardiner C, et al. Minimal experimental requirements for definition of extracellular vesicles and their functions: a position statement from the International Society for Extracellular Vesicles. J Extracell Vesicles. 2014;3:26913.
Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol. 2013;200:373–83.
Colombo M, Raposo G, Théry C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol. 2014;30:255–89.
Di Vizio D, Kim J, Hager MH, Morello M, Yang W, Lafargue CJ, et al. Oncosome formation in prostate cancer: association with a region of frequent chromosomal deletion in metastatic disease. Cancer Res. 2009;69:5601–9.
Graner MW, Alzate O, Dechkovskaia AM, Keene JD, Sampson JH, Mitchell DA, et al. Proteomic and immunologic analyses of brain tumor exosomes. FASEB J. 2009;23:1541–57.
Pathan M, Fonseka P, Chitti SV, Kang T, Sanwlani R, Van Deun J, et al. Vesiclepedia 2019: a compendium of RNA, proteins, lipids and metabolites in extracellular vesicles. Nucleic Acids Res. 2019;47:D516–d519.
Nguyen HP, Simpson RJ, Salamonsen LA, Greening DW. Extracellular vesicles in the intrauterine environment: challenges and potential functions. Biol Reprod. 2016;95:109.
Jeppesen DK, Fenix AM, Franklin JL, Higginbotham JN, Zhang Q, Zimmerman LJ, et al. Reassessment of exosome composition. Cell. 2019;177:428–45.
Johnstone RM, Adam M, Hammond JR, Orr L, Turbide C. Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes). J Biol Chem. 1987;262:9412–20.
Wortzel I, Dror S, Kenific CM, Lyden D. Exosome-mediated metastasis: communication from a distance. Dev Cell. 2019;49:347–60.
Shao H, Im H, Castro CM, Breakefield X, Weissleder R, Lee H. New technologies for analysis of extracellular vesicles. Chem Rev. 2018;118:1917–50.
van Niel G, D'Angelo G, Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol. 2018;19:213–28.
Piper RC, Katzmann DJ. Biogenesis and function of multivesicular bodies. Annu Rev Cell Dev Biol. 2007;23:519–47.
van Niel G, Porto-Carreiro I, Simoes S, Raposo G. Exosomes: a common pathway for a specialized function. J Biochem. 2006;140:13–21.
Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science. 2020;367:6478.
Costa Verdera H, Gitz-Francois JJ, Schiffelers RM, Vader P. Cellular uptake of extracellular vesicles is mediated by clathrin-independent endocytosis and macropinocytosis. J Control Rel. 2017;266:100–8.
Rai AK, Johnson PJ. Trichomonas vaginalis extracellular vesicles are internalized by host cells using proteoglycans and caveolin-dependent endocytosis. Proc Natl Acad Sci USA. 2019;116:21354–60.
Vanlandingham PA, Ceresa BP. Rab7 regulates late endocytic trafficking downstream of multivesicular body biogenesis and cargo sequestration. J Biol Chem. 2009;284:12110–24.
Ostrowski M, Carmo NB, Krumeich S, Fanget I, Raposo G, Savina A, et al. Rab27a and Rab27b control different steps of the exosome secretion pathway. Nat Cell Biol. 2010;12:19–30.
Zeigerer A, Gilleron J, Bogorad RL, Marsico G, Nonaka H, Seifert S, et al. Rab5 is necessary for the biogenesis of the endolysosomal system in vivo. Nature. 2012;485:465–70.
Henne WM, Buchkovich NJ, Emr SD. The ESCRT pathway. Dev Cell. 2011;21:77–91.
van Niel G, Charrin S, Simoes S, Romao M, Rochin L, Saftig P, et al. The tetraspanin CD63 regulates ESCRT-independent and -dependent endosomal sorting during melanogenesis. Dev Cell. 2011;21:708–21.
Verweij FJ, van Eijndhoven MA, Hopmans ES, Vendrig T, Wurdinger T, Cahir-McFarland E, et al. LMP1 association with CD63 in endosomes and secretion via exosomes limits constitutive NF-κB activation. EMBO J. 2011;30:2115–29.
Nabhan JF, Hu R, Oh RS, Cohen SN, Lu Q. Formation and release of arrestin domain-containing protein 1-mediated microvesicles (ARMMs) at plasma membrane by recruitment of TSG101 protein. Proc Natl Acad Sci USA. 2012;109:4146–51.
Kowal J, Tkach M, Théry C. Biogenesis and secretion of exosomes. Curr Opin Cell Biol. 2014;29:116–25.
Valadi H, Ekström K, Bossios A, Sjöstrand M, Lee JJ, Lötvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9:654–9.
Balaj L, Lessard R, Dai L, Cho YJ, Pomeroy SL, Breakefield XO, et al. Tumour microvesicles contain retrotransposon elements and amplified oncogene sequences. Nat Commun. 2011;2:180.
Thakur BK, Zhang H, Becker A, Matei I, Huang Y, Costa-Silva B, et al. Double-stranded DNA in exosomes: a novel biomarker in cancer detection. Cell Res. 2014;24:766–9.
Willms E, Cabañas C, Mäger I, Wood MJA, Vader P. Extracellular vesicle heterogeneity: subpopulations, isolation techniques, and diverse functions in cancer progression. Front Immunol. 2018;9:738.
Skog J, Würdinger T, van Rijn S, Meijer DH, Gainche L, Sena-Esteves M, et al. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat Cell Biol. 2008;10:1470–6.
Tang K, Zhang Y, Zhang H, Xu P, Liu J, Ma J, et al. Delivery of chemotherapeutic drugs in tumour cell-derived microparticles. Nat Commun. 2012;3:1282.
Ma J, Zhang Y, Tang K, Zhang H, Yin X, Li Y, et al. Reversing drug resistance of soft tumor-repopulating cells by tumor cell-derived chemotherapeutic microparticles. Cell Res. 2016;26:713–27.
Viswanathan S, Shi Y, Galipeau J, Krampera M, Leblanc K, Martin I, et al. Mesenchymal stem versus stromal cells: International Society for Cell & Gene Therapy (ISCT®) Mesenchymal Stromal Cell committee position statement on nomenclature. Cytotherapy. 2019;21:1019–24.
Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–7.
Zhou T, Yuan Z, Weng J, Pei D, Du X, He C, et al. Challenges and advances in clinical applications of mesenchymal stromal cells. J Hematol Oncol. 2021;14:24.
Holan V, Trosan P, Cejka C, Javorkova E, Zajicova A, Hermankova B, et al. A comparative study of the therapeutic potential of mesenchymal stem cells and limbal epithelial stem cells for ocular surface reconstruction. Stem Cells Transl Med. 2015;4:1052–63.
Pogozhykh O, Pogozhykh D, Neehus AL, Hoffmann A, Blasczyk R, Müller T. Molecular and cellular characteristics of human and non-human primate multipotent stromal cells from the amnion and bone marrow during long term culture. Stem Cell Res Ther. 2015;6:150.
Yun CW, Lee SH. Potential and therapeutic efficacy of cell-based therapy using mesenchymal stem cells for acute/chronic kidney disease. Int J Mol Sci. 2019;20:1619.
Ankrum JA, Ong JF, Karp JM. Mesenchymal stem cells: immune evasive, not immune privileged. Nat Biotechnol. 2014;32:252–60.
Al-Khawaga S, Abdelalim EM. Potential application of mesenchymal stem cells and their exosomes in lung injury: an emerging therapeutic option for COVID-19 patients. Stem Cell Res Ther. 2020;11:437.
Leng Z, Zhu R, Hou W, Feng Y, Yang Y, Han Q, et al. Transplantation of ACE2(-) mesenchymal stem cells improves the outcome of patients with COVID-19 Pneumonia. Aging Dis. 2020;11:216–28.
Neuhuber B, Swanger SA, Howard L, Mackay A, Fischer I. Effects of plating density and culture time on bone marrow stromal cell characteristics. Exp Hematol. 2008;36:1176–85.
Jeong JO, Han JW, Kim JM, Cho HJ, Park C, Lee N, et al. Malignant tumor formation after transplantation of short-term cultured bone marrow mesenchymal stem cells in experimental myocardial infarction and diabetic neuropathy. Circ Res. 2011;108:1340–7.
Karp JM, Leng Teo GS. Mesenchymal stem cell homing: the devil is in the details. Cell Stem Cell. 2009;4:206–16.
van Hennik PB, de Koning AE, Ploemacher RE. Seeding efficiency of primitive human hematopoietic cells in nonobese diabetic/severe combined immune deficiency mice: implications for stem cell frequency assessment. Blood. 1999;94:3055–61.
Cui J, Wahl RL, Shen T, Fisher SJ, Recker E, Ginsburg D, et al. Bone marrow cell trafficking following intravenous administration. Br J Haematol. 1999;107:895–902.
Meyer GP, Wollert KC, Lotz J, Steffens J, Lippolt P, Fichtner S, et al. Intracoronary bone marrow cell transfer after myocardial infarction: eighteen months' follow-up data from the randomized, controlled BOOST (BOne marrOw transfer to enhance ST-elevation infarct regeneration) trial. Circulation. 2006;113:1287–94.
Adamo A, Dal Collo G, Bazzoni R, Krampera M. Role of mesenchymal stromal cell-derived extracellular vesicles in tumour microenvironment. Biochim Biophys Acta Rev Cancer. 2019;1871:192–8.
Ankrum J, Karp JM. Mesenchymal stem cell therapy: Two steps forward, one step back. Trends Mol Med. 2010;16:203–9.
Zhang B, Yin Y, Lai RC, Tan SS, Choo AB, Lim SK. Mesenchymal stem cells secrete immunologically active exosomes. Stem Cells Dev. 2014;23:1233–44.
Phinney DG, Pittenger MF. Concise review: MSC-derived exosomes for cell-free therapy. Stem Cells. 2017;35:851–8.
Milbank E, Dragano NRV, González-García I, Garcia MR, Rivas-Limeres V, Perdomo L, et al. Small extracellular vesicle-mediated targeting of hypothalamic AMPKα1 corrects obesity through BAT activation. Nat Metab. 2021;3:1415–31.
Xunian Z, Kalluri R. Biology and therapeutic potential of mesenchymal stem cell-derived exosomes. Cancer Sci. 2020;111:3100–10.
Zhuang WZ, Lin YH, Su LJ, Wu MS, Jeng HY, Chang HC, et al. Mesenchymal stem/stromal cell-based therapy: mechanism, systemic safety and biodistribution for precision clinical applications. J Biomed Sci. 2021;28:28.
Watanabe Y, Tsuchiya A, Terai S. The development of mesenchymal stem cell therapy in the present, and the perspective of cell-free therapy in the future. Clin Mol Hepatol. 2021;27:70–80.
Herrmann IK, Wood MJA, Fuhrmann G. Extracellular vesicles as a next-generation drug delivery platform. Nat Nanotechnol. 2021;16:748–59.
Racchetti G, Meldolesi J. Extracellular vesicles of mesenchymal stem cells: therapeutic properties discovered with extraordinary success. Biomedicines. 2021;9:667.
Qiu G, Zheng G, Ge M, Wang J, Huang R, Shu Q, et al. Mesenchymal stem cell-derived extracellular vesicles affect disease outcomes via transfer of microRNAs. Stem Cell Res Ther. 2018;9:320.
Varderidou-Minasian S, Lorenowicz MJ. Mesenchymal stromal/stem cell-derived extracellular vesicles in tissue repair: challenges and opportunities. Theranostics. 2020;10:5979–97.
Wu H, Zhou X, Wang X, Cheng W, Hu X, Wang Y, et al. miR-34a in extracellular vesicles from bone marrow mesenchymal stem cells reduces rheumatoid arthritis inflammation via the cyclin I/ATM/ATR/p53 axis. J Cell Mol Med. 2021;25:1896–910.
Nojehdehi S, Soudi S, Hesampour A, Rasouli S, Soleimani M, Hashemi SM. Immunomodulatory effects of mesenchymal stem cell-derived exosomes on experimental type-1 autoimmune diabetes. J Cell Biochem. 2018;119:9433–43.
Shen Z, Huang W, Liu J, Tian J, Wang S, Rui K. Effects of mesenchymal stem cell-derived exosomes on autoimmune diseases. Front Immunol. 2021;12:749192.
Burr DB, Gallant MA. Bone remodelling in osteoarthritis. Nat Rev Rheumatol. 2012;8:665–73.
Vonk LA, van Dooremalen SFJ, Liv N, Klumperman J, Coffer PJ, Saris DBF, et al. Mesenchymal stromal/stem cell-derived extracellular vesicles promote human cartilage regeneration in vitro. Theranostics. 2018;8:906–20.
Li S, Stöckl S, Lukas C, Götz J, Herrmann M, Federlin M, et al. hBMSC-derived extracellular vesicles attenuate IL-1β-induced catabolic effects on OA-chondrocytes by regulating pro-inflammatory signaling pathways. Front Bioeng Biotechnol. 2020;8:603598.
Cosenza S, Ruiz M, Toupet K, Jorgensen C, Noël D. Mesenchymal stem cells derived exosomes and microparticles protect cartilage and bone from degradation in osteoarthritis. Sci Rep. 2017;7:16214.
Ruiz M, Toupet K, Maumus M, Rozier P, Jorgensen C, Noël D. TGFBI secreted by mesenchymal stromal cells ameliorates osteoarthritis and is detected in extracellular vesicles. Biomaterials. 2020;226:119544.
Zhang J, Rong Y, Luo C, Cui W. Bone marrow mesenchymal stem cell-derived exosomes prevent osteoarthritis by regulating synovial macrophage polarization. Aging. 2020;12:25138–52.
Woo CH, Kim HK, Jung GY, Jung YJ, Lee KS, Yun YE, et al. Small extracellular vesicles from human adipose-derived stem cells attenuate cartilage degeneration. J Extracell Vesicles. 2020;9:1735249.
Tao SC, Yuan T, Zhang YL, Yin WJ, Guo SC, Zhang CQ. Exosomes derived from miR-140-5p-overexpressing human synovial mesenchymal stem cells enhance cartilage tissue regeneration and prevent osteoarthritis of the knee in a rat model. Theranostics. 2017;7:180–95.
Wang Z, Yan K, Ge G, Zhang D, Bai J, Guo X, et al. Exosomes derived from miR-155-5p-overexpressing synovial mesenchymal stem cells prevent osteoarthritis via enhancing proliferation and migration, attenuating apoptosis, and modulating extracellular matrix secretion in chondrocytes. Cell Biol Toxicol. 2021;37:85–96.
Wang K, Li F, Yuan Y, Shan L, Cui Y, Qu J, et al. Synovial mesenchymal stem cell-derived EV-packaged miR-31 downregulates Histone Demethylase KDM2A to prevent knee osteoarthritis. Mol Ther Nucleic Acids. 2020;22:1078–91.
Rong Y, Zhang J, Jiang D, Ji C, Liu W, Wang J, et al. Hypoxic pretreatment of small extracellular vesicles mediates cartilage repair in osteoarthritis by delivering miR-216a-5p. Acta Biomater. 2021;122:325–42.
Wu J, Kuang L, Chen C, Yang J, Zeng WN, Li T, et al. miR-100-5p-abundant exosomes derived from infrapatellar fat pad MSCs protect articular cartilage and ameliorate gait abnormalities via inhibition of mTOR in osteoarthritis. Biomaterials. 2019;206:87–100.
Yan L, Liu G, Wu X. The umbilical cord mesenchymal stem cell-derived exosomal lncRNA H19 improves osteochondral activity through miR-29b-3p/FoxO3 axis. Clin Transl Med. 2021;11:e255.
McDonald JW, Sadowsky C. Spinal-cord injury. Lancet. 2002;359:417–25.
Huang JH, Yin XM, Xu Y, Xu CC, Lin X, Ye FB, et al. Systemic administration of exosomes released from mesenchymal stromal cells attenuates apoptosis, inflammation, and promotes angiogenesis after spinal cord injury in rats. J Neurotrauma. 2017;34:3388–96.
Lu Y, Zhou Y, Zhang R, Wen L, Wu K, Li Y, et al. Bone mesenchymal stem cell-derived extracellular vesicles promote recovery following spinal cord injury via improvement of the integrity of the blood-spinal cord barrier. Front Neurosci. 2019;13:209.
Zhou Y, Wen LL, Li YF, Wu KM, Duan RR, Yao YB, et al. Exosomes derived from bone marrow mesenchymal stem cells protect the injured spinal cord by inhibiting pericyte pyroptosis. Neural Regen Res. 2022;17:194–202.
Han T, Song P, Wu Z, Xiang X, Liu Y, Wang Y, et al. MSC secreted extracellular vesicles carrying TGF-beta upregulate Smad 6 expression and promote the regrowth of neurons in spinal cord injured rats. Stem Cell Rev Rep. 2021;18:1078–96.
Nakazaki M, Morita T, Lankford KL, Askenase PW, Kocsis JD. Small extracellular vesicles released by infused mesenchymal stromal cells target M2 macrophages and promote TGF-β upregulation, microvascular stabilization, and functional recovery in a rodent model of severe spinal cord injury. J Extracell Vesicles. 2021;10:e12137.
Zhou W, Silva M, Feng C, Zhao S, Liu L, Li S, et al. Exosomes derived from human placental mesenchymal stem cells enhanced the recovery of spinal cord injury by activating endogenous neurogenesis. Stem Cell Res Ther. 2021;12:174.
Jia X, Huang G, Wang S, Long M, Tang X, Feng D, et al. Extracellular vesicles derived from mesenchymal stem cells containing microRNA-381 protect against spinal cord injury in a rat model via the BRD4/WNT5A axis. Bone Jt Res. 2021;10:328–39.
Li D, Zhang P, Yao X, Li H, Shen H, Li X, et al. Exosomes derived from miR-133b-modified mesenchymal stem cells promote recovery after spinal cord injury. Front Neurosci. 2018;12:845.
Xin H, Liu Z, Buller B, Li Y, Golembieski W, Gan X, et al. MiR-17-92 enriched exosomes derived from multipotent mesenchymal stromal cells enhance axon-myelin remodeling and motor electrophysiological recovery after stroke. J Cereb Blood Flow Metab. 2021;41:1131–44.
Chen Y, Tian Z, He L, Liu C, Wang N, Rong L, et al. Exosomes derived from miR-26a-modified MSCs promote axonal regeneration via the PTEN/AKT/mTOR pathway following spinal cord injury. Stem Cell Res Ther. 2021;12:224.
Liu W, Rong Y, Wang J, Zhou Z, Ge X, Ji C, et al. Exosome-shuttled miR-216a-5p from hypoxic preconditioned mesenchymal stem cells repair traumatic spinal cord injury by shifting microglial M1/M2 polarization. J Neuroinflammation. 2020;17:47.
Sheng Y, Zhou X, Wang J, Shen H, Wu S, Guo W, et al. MSC derived EV loaded with miRNA-22 inhibits the inflammatory response and nerve function recovery after spinal cord injury in rats. J Cell Mol Med. 2021;25:10268–78.
Wang Y, Lai X, Wu D, Liu B, Wang N, Rong L. Umbilical mesenchymal stem cell-derived exosomes facilitate spinal cord functional recovery through the miR-199a-3p/145-5p-mediated NGF/TrkA signaling pathway in rats. Stem Cell Res Ther. 2021;12:117.
Driskell RR, Lichtenberger BM, Hoste E, Kretzschmar K, Simons BD, Charalambous M, et al. Distinct fibroblast lineages determine dermal architecture in skin development and repair. Nature. 2013;504:277–81.
van Zanten MC, Mistry RM, Suami H, Campbell-Lloyd A, Finkemeyer JP, Piller NB, et al. The lymphatic response to injury with soft-tissue reconstruction in high-energy open tibial fractures of the lower extremity. Plast Reconstr Surg. 2017;139:483–91.
Falanga V. Wound healing and its impairment in the diabetic foot. Lancet. 2005;366:1736–43.
Zhou Y, Zhao B, Zhang XL, Lu YJ, Lu ST, Cheng J, et al. Combined topical and systemic administration with human adipose-derived mesenchymal stem cells (hADSC) and hADSC-derived exosomes markedly promoted cutaneous wound healing and regeneration. Stem Cell Res Ther. 2021;12:257.
Jiang T, Wang Z, Sun J. Human bone marrow mesenchymal stem cell-derived exosomes stimulate cutaneous wound healing mediates through TGF-β/Smad signaling pathway. Stem Cell Res Ther. 2020;11:198.
Wang X, Jiao Y, Pan Y, Zhang L, Gong H, Qi Y, et al. Fetal dermal mesenchymal stem cell-derived exosomes accelerate cutaneous wound healing by activating notch signaling. Stem Cells Int. 2019;2019:2402916.
He X, Dong Z, Cao Y, Wang H, Liu S, Liao L, et al. MSC-derived exosome promotes M2 polarization and enhances cutaneous wound healing. Stem Cells Int. 2019;2019:7132708.
Wu D, Kang L, Tian J, Wu Y, Liu J, Li Z, et al. Exosomes derived from bone mesenchymal stem cells with the stimulation of Fe(3)O(4) nanoparticles and static magnetic field enhance wound healing through upregulated miR-21-5p. Int J Nanomed. 2020;15:7979–93.
Cheng S, Xi Z, Chen G, Liu K, Ma R, Zhou C. Extracellular vesicle-carried microRNA-27b derived from mesenchymal stem cells accelerates cutaneous wound healing via E3 ubiquitin ligase ITCH. J Cell Mol Med. 2020;24:11254–71.
Fang S, Xu C, Zhang Y, Xue C, Yang C, Bi H, et al. Umbilical cord-derived mesenchymal stem cell-derived exosomal MicroRNAs suppress myofibroblast differentiation by inhibiting the transforming growth Factor-β/SMAD2 pathway during wound healing. Stem Cells Transl Med. 2016;5:1425–39.
Cao G, Chen B, Zhang X, Chen H. Human adipose-derived mesenchymal stem cells-derived exosomal microRNA-19b promotes the healing of skin wounds through modulation of the CCL1/TGF-β signaling axis. Clin Cosmet Investig Dermatol. 2020;13:957–71.
Li Y, Zhang J, Shi J, Liu K, Wang X, Jia Y, et al. Exosomes derived from human adipose mesenchymal stem cells attenuate hypertrophic scar fibrosis by miR-192-5p/IL-17RA/Smad axis. Stem Cell Res Ther. 2021;12:221.
Lu Y, Wen H, Huang J, Liao P, Liao H, Tu J, et al. Extracellular vesicle-enclosed miR-486-5p mediates wound healing with adipose-derived stem cells by promoting angiogenesis. J Cell Mol Med. 2020;24:9590–604.
Gao S, Chen T, Hao Y, Zhang F, Tang X, Wang D, et al. Exosomal miR-135a derived from human amnion mesenchymal stem cells promotes cutaneous wound healing in rats and fibroblast migration by directly inhibiting LATS2 expression. Stem Cell Res Ther. 2020;11:56.
Hernandez-Gea V, Friedman SL. Pathogenesis of liver fibrosis. Annu Rev Pathol. 2011;6:425–56.
Rong X, Liu J, Yao X, Jiang T, Wang Y, Xie F. Human bone marrow mesenchymal stem cells-derived exosomes alleviate liver fibrosis through the Wnt/β-catenin pathway. Stem Cell Res Ther. 2019;10:98.
Ohara M, Ohnishi S, Hosono H, Yamamoto K, Yuyama K, Nakamura H, et al. Extracellular vesicles from amnion-derived mesenchymal stem cells ameliorate hepatic inflammation and fibrosis in rats. Stem Cells Int. 2018;2018:3212643.
Qu Y, Zhang Q, Cai X, Li F, Ma Z, Xu M, et al. Exosomes derived from miR-181-5p-modified adipose-derived mesenchymal stem cells prevent liver fibrosis via autophagy activation. J Cell Mol Med. 2017;21:2491–502.
Lou G, Yang Y, Liu F, Ye B, Chen Z, Zheng M, et al. MiR-122 modification enhances the therapeutic efficacy of adipose tissue-derived mesenchymal stem cells against liver fibrosis. J Cell Mol Med. 2017;21:2963–73.
Kim J, Lee C, Shin Y, Wang S, Han J, Kim M, et al. sEVs from tonsil-derived mesenchymal stromal cells alleviate activation of hepatic stellate cells and liver fibrosis through miR-486-5p. Mol Ther. 2021;29:1471–86.
Romagnani P, Remuzzi G, Glassock R, Levin A, Jager KJ, Tonelli M, et al. Chronic kidney disease. Nat Rev Dis Prim. 2017;3:17088.
Ji C, Zhang J, Zhu Y, Shi H, Yin S, Sun F, et al. Exosomes derived from hucMSC attenuate renal fibrosis through CK1δ/β-TRCP-mediated YAP degradation. Cell Death Dis. 2020;11:327.
Liu B, Hu D, Zhou Y, Yu Y, Shen L, Long C, et al. Exosomes released by human umbilical cord mesenchymal stem cells protect against renal interstitial fibrosis through ROS-mediated P38MAPK/ERK signaling pathway. Am J Transl Res. 2020;12:4998–5014.
Shi Z, Wang Q, Zhang Y, Jiang D. Extracellular vesicles produced by bone marrow mesenchymal stem cells attenuate renal fibrosis, in part by inhibiting the RhoA/ROCK pathway, in a UUO rat model. Stem Cell Res Ther. 2020;11:253.
Hu X, Shen N, Liu A, Wang W, Zhang L, Sui Z, et al. Bone marrow mesenchymal stem cell-derived exosomal miR-34c-5p ameliorates RIF by inhibitingthe core fucosylation of multiple proteins. Mol Ther. 2021;30:763–81.
Jin J, Wang Y, Zhao L, Zou W, Tan M, He Q. Exosomal miRNA-215-5p derived from adipose-derived stem cells attenuates epithelial-mesenchymal transition of podocytes by inhibiting ZEB2. Biomed Res Int. 2020;2020:2685305.
Jin J, Shi Y, Gong J, Zhao L, Li Y, He Q, et al. Exosome secreted from adipose-derived stem cells attenuates diabetic nephropathy by promoting autophagy flux and inhibiting apoptosis in podocyte. Stem Cell Res Ther. 2019;10:95.
Zhong L, Liao G, Wang X, Li L, Zhang J, Chen Y, et al. Mesenchymal stem cells-microvesicle-miR-451a ameliorate early diabetic kidney injury by negative regulation of P15 and P19. Exp Biol Med. 2018;243:1233–42.
Ebrahim N, Ahmed IA, Hussien NI, Dessouky AA, Farid AS, Elshazly AM, et al. Mesenchymal stem cell-derived exosomes ameliorated diabetic nephropathy by autophagy induction through the mTOR signaling pathway. Cells. 2018;7:226.
Grange C, Tritta S, Tapparo M, Cedrino M, Tetta C, Camussi G, et al. Stem cell-derived extracellular vesicles inhibit and revert fibrosis progression in a mouse model of diabetic nephropathy. Sci Rep. 2019;9:4468.
Spagnolo P, Distler O, Ryerson CJ, Tzouvelekis A, Lee JS, Bonella F, et al. Mechanisms of progressive fibrosis in connective tissue disease (CTD)-associated interstitial lung diseases (ILDs). Ann Rheum Dis. 2021;80:143–50.
Richeldi L, Collard HR, Jones MG. Idiopathic pulmonary fibrosis. Lancet. 2017;389:1941–52.
Shenderov K, Collins SL, Powell JD, Horton MR. Immune dysregulation as a driver of idiopathic pulmonary fibrosis. J Clin Invest. 2021;131:e143226.
Willis GR, Fernandez-Gonzalez A, Anastas J, Vitali SH, Liu X, Ericsson M, et al. Mesenchymal stromal cell exosomes ameliorate experimental bronchopulmonary dysplasia and restore lung function through macrophage immunomodulation. Am J Respir Crit Care Med. 2018;197:104–16.
Mansouri N, Willis GR, Fernandez-Gonzalez A, Reis M, Nassiri S, Mitsialis SA, et al. Mesenchymal stromal cell exosomes prevent and revert experimental pulmonary fibrosis through modulation of monocyte phenotypes. JCI Insight. 2019;4:e128060.
Zhang Z, Ge L, Zhang S, Wang J, Jiang W, Xin Q, et al. The protective effects of MSC-EXO against pulmonary hypertension through regulating Wnt5a/BMP signalling pathway. J Cell Mol Med. 2020;24:13938–48.
Chaubey S, Thueson S, Ponnalagu D, Alam MA, Gheorghe CP, Aghai Z, et al. Early gestational mesenchymal stem cell secretome attenuates experimental bronchopulmonary dysplasia in part via exosome-associated factor TSG-6. Stem Cell Res Ther. 2018;9:173.
Wan X, Chen S, Fang Y, Zuo W, Cui J, Xie S. Mesenchymal stem cell-derived extracellular vesicles suppress the fibroblast proliferation by downregulating FZD6 expression in fibroblasts via micrRNA-29b-3p in idiopathic pulmonary fibrosis. J Cell Physiol. 2020;235:8613–25.
Zhou J, Lin Y, Kang X, Liu Z, Zhang W, Xu F. microRNA-186 in extracellular vesicles from bone marrow mesenchymal stem cells alleviates idiopathic pulmonary fibrosis via interaction with SOX4 and DKK1. Stem Cell Res Ther. 2021;12:96.
Lei X, He N, Zhu L, Zhou M, Zhang K, Wang C, et al. Mesenchymal stem cell-derived extracellular vesicles attenuate radiation-induced lung injury via miRNA-214-3p. Antioxid Redox Signal. 2021;35:849–62.
Sun L, Zhu M, Feng W, Lin Y, Yin J, Jin J, et al. Exosomal miRNA Let-7 from menstrual blood-derived endometrial stem cells alleviates pulmonary fibrosis through regulating mitochondrial DNA damage. Oxid Med Cell Longev. 2019;2019:4506303.
Xiao K, He W, Guan W, Hou F, Yan P, Xu J, et al. Mesenchymal stem cells reverse EMT process through blocking the activation of NF-κB and Hedgehog pathways in LPS-induced acute lung injury. Cell Death Dis. 2020;11:863.
Yin JQ, Zhu J, Ankrum JA. Manufacturing of primed mesenchymal stromal cells for therapy. Nat Biomed Eng. 2019;3:90–104.
Witwer KW, Van Balkom BWM, Bruno S, Choo A, Dominici M, Gimona M, et al. Defining mesenchymal stromal cell (MSC)-derived small extracellular vesicles for therapeutic applications. J Extracell Vesicles. 2019;8:1609206.
Xu H, Wang Z, Liu L, Zhang B, Li B. Exosomes derived from adipose tissue, bone marrow, and umbilical cord blood for cardioprotection after myocardial infarction. J Cell Biochem. 2020;121:2089–102.
Doeppner TR, Herz J, Görgens A, Schlechter J, Ludwig AK, Radtke S, et al. Extracellular Vesicles improve post-stroke neuroregeneration and prevent postischemic immunosuppression. Stem Cells Transl Med. 2015;4:1131–43.
Chen KH, Chen CH, Wallace CG, Yuen CM, Kao GS, Chen YL, et al. Intravenous administration of xenogenic adipose-derived mesenchymal stem cells (ADMSC) and ADMSC-derived exosomes markedly reduced brain infarct volume and preserved neurological function in rat after acute ischemic stroke. Oncotarget. 2016;7:74537–56.
Wang K, Jiang Z, Webster KA, Chen J, Hu H, Zhou Y, et al. Enhanced cardioprotection by human endometrium mesenchymal stem cells driven by exosomal MicroRNA-21. Stem Cells Transl Med. 2017;6:209–22.
Qin Y, Wang L, Gao Z, Chen G, Zhang C. Bone marrow stromal/stem cell-derived extracellular vesicles regulate osteoblast activity and differentiation in vitro and promote bone regeneration in vivo. Sci Rep. 2016;6:21961.
Chen S, Tang Y, Liu Y, Zhang P, Lv L, Zhang X, et al. Exosomes derived from miR-375-overexpressing human adipose mesenchymal stem cells promote bone regeneration. Cell Prolif. 2019;52:e12669.
Narayanan R, Huang CC, Ravindran S. Hijacking the cellular mail: exosome mediated differentiation of mesenchymal. Stem Cells Stem Cells Int. 2016;2016:3808674.
Takeuchi R, Katagiri W, Endo S, Kobayashi T. Exosomes from conditioned media of bone marrow-derived mesenchymal stem cells promote bone regeneration by enhancing angiogenesis. PLoS One. 2019;14:e0225472.
Wang J, Huang R, Xu Q, Zheng G, Qiu G, Ge M, et al. Mesenchymal stem cell-derived extracellular vesicles alleviate acute lung injury via transfer of miR-27a-3p. Crit Care Med. 2020;48:e599–e610.
Li R, Zhao K, Ruan Q, Meng C, Yin F. Bone marrow mesenchymal stem cell-derived exosomal microRNA-124-3p attenuates neurological damage in spinal cord ischemia-reperfusion injury by downregulating Ern1 and promoting M2 macrophage polarization. Arthritis Res Ther. 2020;22:75.
Liu W, Yu M, Xie D, Wang L, Ye C, Zhu Q, et al. Melatonin-stimulated MSC-derived exosomes improve diabetic wound healing through regulating macrophage M1 and M2 polarization by targeting the PTEN/AKT pathway. Stem Cell Res Ther. 2020;11:259.
Lian Q, Lye E, Suan Yeo K, Khia Way Tan E, Salto-Tellez M, Liu TM, et al. Derivation of clinically compliant MSCs from CD105+, CD24- differentiated human ESCs. Stem Cells. 2007;25:425–36.
Lian Q, Zhang Y, Zhang J, Zhang HK, Wu X, Zhang Y, et al. Functional mesenchymal stem cells derived from human induced pluripotent stem cells attenuate limb ischemia in mice. Circulation. 2010;121:1113–23.
Zhang Y, Liang X, Liao S, Wang W, Wang J, Li X, et al. Potent paracrine effects of human induced pluripotent stem cell-derived mesenchymal stem cells attenuate doxorubicin-induced cardiomyopathy. Sci Rep. 2015;5:11235.
Li X, Zhang Y, Yeung SC, Liang Y, Liang X, Ding Y, et al. Mitochondrial transfer of induced pluripotent stem cell-derived mesenchymal stem cells to airway epithelial cells attenuates cigarette smoke-induced damage. Am J Respir Cell Mol Biol. 2014;51:455–65.
Sze SK, de Kleijn DP, Lai RC, Khia Way Tan E, Zhao H, Yeo KS, et al. Elucidating the secretion proteome of human embryonic stem cell-derived mesenchymal stem cells. Mol Cell Proteom. 2007;6:1680–9.
Mendt M, Daher M, Basar R, Shanley M, Kumar B, Wei Inng FL, et al. Metabolic reprogramming of GMP grade cord tissue derived mesenchymal stem cells enhances their suppressive potential in GVHD. Front Immunol. 2021;12:631353.
Wang L, Gu Z, Zhao X, Yang N, Wang F, Deng A, et al. Extracellular vesicles released from human umbilical cord-derived mesenchymal stromal cells prevent life-threatening acute graft-versus-host disease in a mouse model of allogeneic hematopoietic stem cell transplantation. Stem Cells Dev. 2016;25:1874–83.
Dal Collo G, Adamo A, Gatti A, Tamellini E, Bazzoni R, Takam Kamga P, et al. Functional dosing of mesenchymal stromal cell-derived extracellular vesicles for the prevention of acute graft-versus-host-disease. Stem Cells. 2020;38:698–711.
Fujii S, Miura Y, Fujishiro A, Shindo T, Shimazu Y, Hirai H, et al. Graft-versus-host disease amelioration by human bone marrow mesenchymal stromal/stem cell-derived extracellular vesicles is associated with peripheral preservation of naive T cell populations. Stem Cells. 2018;36:434–45.
Lai P, Chen X, Guo L, Wang Y, Liu X, Liu Y, et al. A potent immunomodulatory role of exosomes derived from mesenchymal stromal cells in preventing cGVHD. J Hematol Oncol. 2018;11:135.
Norooznezhad AH, Yarani R, Payandeh M, Hoseinkhani Z, Kiani S, Taghizadeh E, et al. Human placental mesenchymal stromal cell-derived exosome-enriched extracellular vesicles for chronic cutaneous graft-versus-host disease: A case report. J Cell Mol Med. 2022;26:588–92.
Feng K, Xie X, Yuan J, Gong L, Zhu Z, Zhang J, et al. Reversing the surface charge of MSC-derived small extracellular vesicles by εPL-PEG-DSPE for enhanced osteoarthritis treatment. J Extracell Vesicles. 2021;10:e12160.
Acknowledgements
For the collaboration and general support, we would like to thank our colleagues from the cord blood bank centre, as well as all collaboration partners. Graphs were assembled using dynamic BioRender assets (icons, lines, shapes, and/or text).
Funding
This study is in part supported by Start-up Grant for Stem Cell Regenerative Medicine (Guangzhou Women and Children’s Medical Centre, Grant No: 5001-4001010), and Shenzhen Science and Technology Program (JCYJ20210324114606019).
Author information
Authors and Affiliations
Contributions
KM collected the literature and wrote the manuscript. HL contributed to the revisions of the manuscript and tables for important intellectual content. YJ, CZ, CS, LJ, GL, ZX, and ZX contributed to the literature summary. XX, YX, WY, ZJ, TH, and XA contributed to review and language editing. LQ conceptualized the manuscript and contributed to funding acquisition. All authors read and gave final approval for publication.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Edited by Dr Yufang Shi
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kou, M., Huang, L., Yang, J. et al. Mesenchymal stem cell-derived extracellular vesicles for immunomodulation and regeneration: a next generation therapeutic tool?. Cell Death Dis 13, 580 (2022). https://doi.org/10.1038/s41419-022-05034-x
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41419-022-05034-x
This article is cited by
-
ECM stiffness affects cargo sorting into MSC-EVs to regulate their secretion and uptake behaviors
Journal of Nanobiotechnology (2024)
-
Effectiveness of extracellular vesicles derived from hiPSCs in repairing hyperoxia-induced injury in a fetal murine lung explant model
Stem Cell Research & Therapy (2024)
-
Engineered extracellular vesicle-encapsulated CHIP as novel nanotherapeutics for treatment of renal fibrosis
npj Regenerative Medicine (2024)
-
Mesenchymal Stem Cell-based Scaffolds in Regenerative Medicine of Dental Diseases
Stem Cell Reviews and Reports (2024)
-
The Main Mechanisms of Mesenchymal Stem Cell-Based Treatments against COVID-19
Tissue Engineering and Regenerative Medicine (2024)