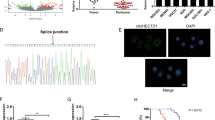
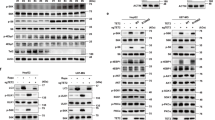
Abstract
Several recent studies have suggested that TLKs are related to tumor progression. However, the function and mechanism of action of TLK2 in gastric cancer (GC) remain elusive. In this study, TLK2 was found to be significantly upregulated in patients with GC and was identified as an independent prognostic factor for GC. Consistently, TLK2 knockdown markedly reduced the aggressiveness of GC, whereas its overexpression had the opposite effect. IP-MS revealed that the effects of TLK2 on GC were mainly associated with metabolism reprogramming. TLK2 knockdown suppressed amino acid synthesis by downregulating the mTORC1 pathway and ASNS expression in GC cells. Mechanistically, mTORC1 directly interacts with the ASNS protein and inhibits its degradation. Further experiments validated that the ASNS protein was degraded via ubiquitination instead of autophagy. Inhibiting and activating the mTORC1 pathway can upregulate and downregulate ASNS ubiquitination, respectively, and the mTORC1 pathway can reverse the regulatory effects of TLK2 on ASNS. Furthermore, TLK2 was found to regulate the mRNA expression of ASNS. TLK2 directly interacted with ATF4, a transcription factor of ASNS, and promoted its expression. The kinase inhibitor fostamatinib significantly inhibited the proliferative, invasive, and migratory capabilities of GC cells by inhibiting TLK2 activity. Altogether, this study reveals a novel functional relationship between TLK2 and the mTORC1/ASNS axis in GC. Therefore, TLK2 may serve as a potential therapeutic target for GC.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
All data relevant to the study are included in the article or uploaded as online Supplementary materials. Source data will be made available upon direct request to the corresponding author.
References
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424.
Smyth EC, Nilsson M, Grabsch HI, van Grieken NC, Lordick F. Gastric cancer. Lancet. 2020;396:635–48.
Yu J, Zhang Y, Leung LH, Liu L, Yang F, Yao X. Efficacy and safety of angiogenesis inhibiors in advanced gastric cancer: a systematic review and meta-analysis. J Hematol Oncol. 2016;9:111.
WARBURG O. On respiratory impairment in cancer cells. Science. 1956;124:269–70.
Jing F, Hu X, Cao Y, Xu M, Wang Y, Jing Y, et al. Discriminating gastric cancer and gastric ulcer using human plasma amino acid metabolic profile. IUBMB Life. 2018;70:553–62.
Reis LMD, Adamoski D, Ornitz Oliveira Souza R, Rodrigues Ascenção CF, Sousa de Oliveira KR, Corrêa-da-Silva F, et al. Dual inhibition of glutaminase and carnitine palmitoyltransferase decreases growth and migration of glutaminase inhibition-resistant triple-negative breast cancer cells. J Biol Chem. 2019;294:9342–57.
Yamashita AS, da Costa Rosa M, Stumpo V, Rais R, Slusher BS, Riggins GJ. The glutamine antagonist prodrug JHU-083 slows malignant glioma growth and disrupts mTOR signaling. Neurooncol Adv. 2020;3:vdaa149.
Bruinsma W, van den Berg J, Aprelia M, Medema RH. Tousled-like kinase 2 regulates recovery from a DNA damage-induced G2 arrest. EMBO Rep. 2016;17:659–70.
Lee SB, Segura-Bayona S, Villamor-Payà M, Saredi G, Todd MAM, Attolini CS, et al. Tousled-like kinases stabilize replication forks and show synthetic lethality with checkpoint and PARP inhibitors. Sci Adv. 2018;4:eaat4985.
Khalil MI, Singh V, King J, De Benedetti A. TLK1-mediated MK5-S354 phosphorylation drives prostate cancer cell motility and may signify distinct pathologies. Mol Oncol. 2022;16:2537–57.
Li J, Huang C, Zou Y, Ye J, Yu J, Gui Y. CircTLK1 promotes the proliferation and metastasis of renal cell carcinoma by sponging miR-136-5p. Mol Cancer. 2020;19:103.
Lu Y, Liu Y, Zhang K, Jiang L. Circular RNA TLK1 exerts oncogenic functions in hepatocellular carcinoma by acting as a ceRNA of miR-138-5p. J Oncol. 2022;2022:2415836.
Singh V, Bhoir S, Chikhale RV, Hussain J, Dwyer D, Bryce RA, et al. Generation of phenothiazine with potent Anti-TLK1 activity for prostate cancer therapy. iScience. 2020;23:101474.
Kim JA, Tan Y, Wang X, Cao X, Veeraraghavan J, Liang Y, et al. Comprehensive functional analysis of the tousled-like kinase 2 frequently amplified in aggressive luminal breast cancers. Nat Commun. 2016;7:12991.
Xu Y, Lv F, Zhu X, Wu Y, Shen X. Loss of asparagine synthetase suppresses the growth of human lung cancer cells by arresting cell cycle at G0/G1 phase. Cancer Gene Ther. 2016;23:287–94.
Lorenzi PL, Llamas J, Gunsior M, Ozbun L, Reinhold WC, Varma S, et al. Asparagine synthetase is a predictive biomarker of L-asparaginase activity in ovarian cancer cell lines. Mol Cancer Ther. 2008;7:3123–8.
Yu Q, Wang X, Wang L, Zheng J, Wang J, Wang B. Knockdown of asparagine synthetase (ASNS) suppresses cell proliferation and inhibits tumor growth in gastric cancer cells. Scand J Gastroenterol. 2016;51:1220–6.
Cormerais Y, Vučetić M, Parks SK, Pouyssegur J. Amino acid transporters are a vital focal point in the control of mTORC1 signaling and cancer. Int J Mol Sci. 2020;22:23.
Liu GY, Sabatini DM. mTOR at the nexus of nutrition, growth, ageing and disease. Nat Rev Mol Cell Biol. 2020;21:183–203.
Seibert M, Kurrle N, Schnütgen F, Serve H. Amino acid sensory complex proteins in mTORC1 and macroautophagy regulation. Matrix Biol. 2021;100-101:65–83.
Chen J, Ou Y, Yang Y, Li W, Xu Y, Xie Y, et al. KLHL22 activates amino-acid-dependent mTORC1 signalling to promote tumorigenesis and ageing. Nature .2018;557:585–9.
Tabe Y, Lorenzi PL, Konopleva M. Amino acid metabolism in hematologic malignancies and the era of targeted therapy. Blood. 2019;134:1014–23.
Altman BJ, Stine ZE, Dang CV. From Krebs to clinic: glutamine metabolism to cancer therapy. Nat Rev Cancer. 2016;16:619–34.
Chandrashekar DS, Karthikeyan SK, Korla PK, Patel H, Shovon AR, Athar M, et al. UALCAN: an update to the integrated cancer data analysis platform. Neoplasia. 2022;25:18–27.
Lánczky A, Győrffy B. Web-based survival analysis tool tailored for medical research (KMplot): development and Implementation. J Med Internet Res. 2021;23:e27633.
Wishart DS, Feunang YD, Guo AC, Lo EJ, Marcu A, Grant JR, et al. DrugBank 5.0: a major update to the DrugBank database for 2018. Nucleic Acids Res. 2018;46:D1074–D1082.
Deng L, Yao P, Li L, Ji F, Zhao S, Xu C, et al. p53-mediated control of aspartate-asparagine homeostasis dictates LKB1 activity and modulates cell survival. Nat Commun. 2020;11:1755.
Yang H, Wen Y, Zhang M, Liu Q, Zhang H, Zhang J, et al. MTORC1 coordinates the autophagy and apoptosis signaling in articular chondrocytes in osteoarthritic temporomandibular joint. Autophagy. 2020;16:271–88.
Wang M, Lu Y, Wang H, Wu Y, Xu X, Li Y. High ATF4 expression is associated with poor prognosis, amino acid metabolism, and autophagy in gastric cancer. Front Oncol. 2021;11:740120.
Hong X, Huang H, Qiu X, Ding Z, Feng X, Zhu Y, et al. Targeting posttranslational modifications of RIOK1 inhibits the progression of colorectal and gastric cancers. Elife 2018;7:e29511.
Gwinn DM, Lee AG, Briones-Martin-Del-Campo M, Conn CS, Simpson DR, Scott AI, et al. Oncogenic KRAS regulates amino acid homeostasis and asparagine biosynthesis via ATF4 and alters sensitivity to L-Asparaginase. Cancer Cell. 2018;33:91–107.e6.
van der Mijn JC, Chen Q, Laursen KB, Khani F, Wang X, Dorsaint P, et al. Transcriptional and metabolic remodeling in clear cell renal cell carcinoma caused by ATF4 activation and the integrated stress response (ISR). Mol Carcinog. 2022;61:851–64.
Bussel J, Arnold DM, Grossbard E, Mayer J, Treliński J, Homenda W, et al. Fostamatinib for the treatment of adult persistent and chronic immune thrombocytopenia: results of two phase 3, randomized, placebo-controlled trials. Am J Hematol. 2018;93:921–30.
Wang J, Wang L, Chen S, Peng H, Xiao L, Du E, et al. PKMYT1 is associated with prostate cancer malignancy and may serve as a therapeutic target. Gene. 2020;744:144608.
Salarikia SR, Kashkooli M, Taghipour MJ, Malekpour M, Negahdaripour M. Identification of hub pathways and drug candidates in gastric cancer through systems biology. Sci Rep. 2022;12:9099.
Poh AR, Dwyer AR, Eissmann MF, Chand AL, Baloyan D, Boon L, et al. Inhibition of the SRC Kinase HCK impairs STAT3-dependent gastric tumor growth in mice. Cancer Immunol Res. 2020;8:428–35.
Lui GYL, Grandori C, Kemp CJ. CDK12: an emerging therapeutic target for cancer. J Clin Pathol. 2018;71:957–62.
Wei R, Xiao Y, Song Y, Yuan H, Luo J, Xu W. FAT4 regulates the EMT and autophagy in colorectal cancer cells in part via the PI3K-AKT signaling axis. J Exp Clin Cancer Res. 2019;38:112.
Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74.
Li Z, Zhang H. Reprogramming of glucose, fatty acid and amino acid metabolism for cancer progression. Cell Mol Life Sci. 2016;73:377–92.
Feng X, Ma D, Zhao J, Song Y, Zhu Y, Zhou Q, et al. UHMK1 promotes gastric cancer progression through reprogramming nucleotide metabolism. EMBO J. 2020;39:e102541.
Zhou Q, Lin M, Feng X, Ma F, Zhu Y, Liu X, et al. Targeting CLK3 inhibits the progression of cholangiocarcinoma by reprogramming nucleotide metabolism. J Exp Med. 2020;217:e20191779.
Park JH, Pyun WY, Park HW. Cancer metabolism: phenotype, signaling and therapeutic targets. Cells. 2020;9:2308.
Acknowledgements
We thank Bullet Edits Limited for the linguistic editing and proofreading of the manuscript.
Funding
This research was funded by the Key Programs of the Educational Commission of Anhui Province (the scientific research project chaired by Mingliang Wang from May 2023 to April 2025 and the project number has not yet been given) and the National Natural Science Foundation (grant no. 81874063).
Author information
Authors and Affiliations
Contributions
MW and YL designed this study. MW and FM wrote the manuscript. MW, JL, QY, HW, XX and YL performed the experiments. JL, YZ, SZ and RS assisted with cell culture and in vivo experiments. XY, DL, YW and ZZ were responsible for clinical sample collection. All authors have read and approved the final version of this manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
All procedures involving human participants were reviewed and approved by the Ethics Committee of Anhui Medical University (ethical code: 20180323). All patients/participants provided written informed consent to participate in this study.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, M., Li, J., Yang, X. et al. Targeting TLK2 inhibits the progression of gastric cancer by reprogramming amino acid metabolism through the mTOR/ASNS axis. Cancer Gene Ther 30, 1485–1497 (2023). https://doi.org/10.1038/s41417-023-00653-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41417-023-00653-8